Multicompartmental Lipopolyplex as Vehicle for Antigens and Genes Delivery in Vaccine Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Reagents
2.1.2. Plasmids
2.1.3. Animals
2.2. Methods
2.2.1. Proteins Extraction and Quantitation
2.2.2. Preparation of Polyplexes
2.2.3. Preparation of Liposomes
2.2.4. Lipids Quantification
2.2.5. Preparation of Lipopolyplexes
2.2.6. Electrophoretic Lipopolyplex Characterization
2.2.7. Vaccination Assays
2.2.8. Obtaining Plasma and Titration of Specific IgG Against TMP
2.2.9. GD3 Antigen Serology Assays
2.2.10. Electron Microscopy
2.2.11. Tumor Volume Measurement and Mice Survival Rate
2.2.12. Expression of mGM-CSF in B16 Murine Melanoma Cells after Transfection with pMok-GMCSF or p2F-GMCSF
2.2.13. Statistical Analyses
3. Results
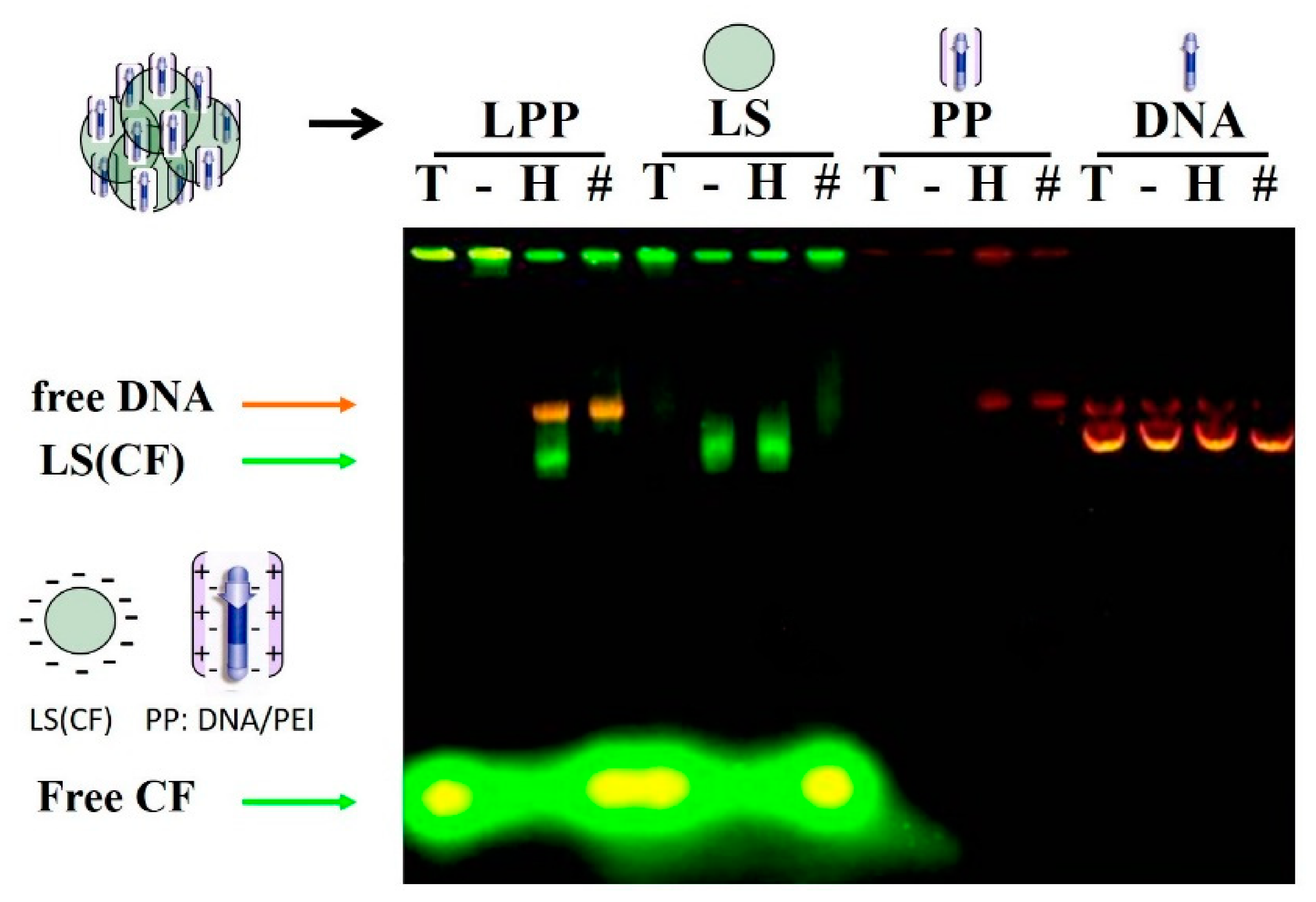
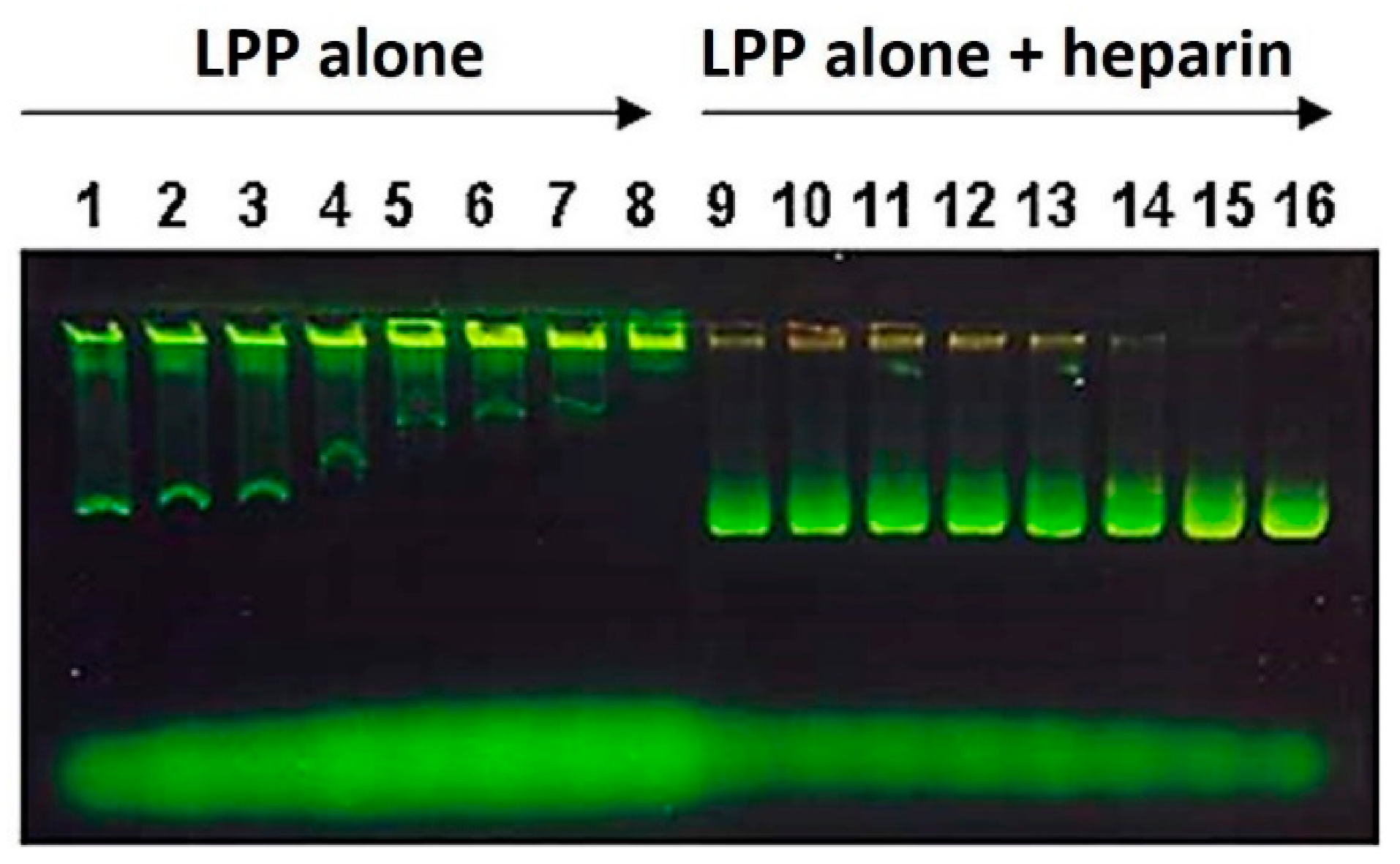
3.1. Lipopolyplex Preparation Method and Characterization
3.2. Optimization of Lipopolyplex Preparation
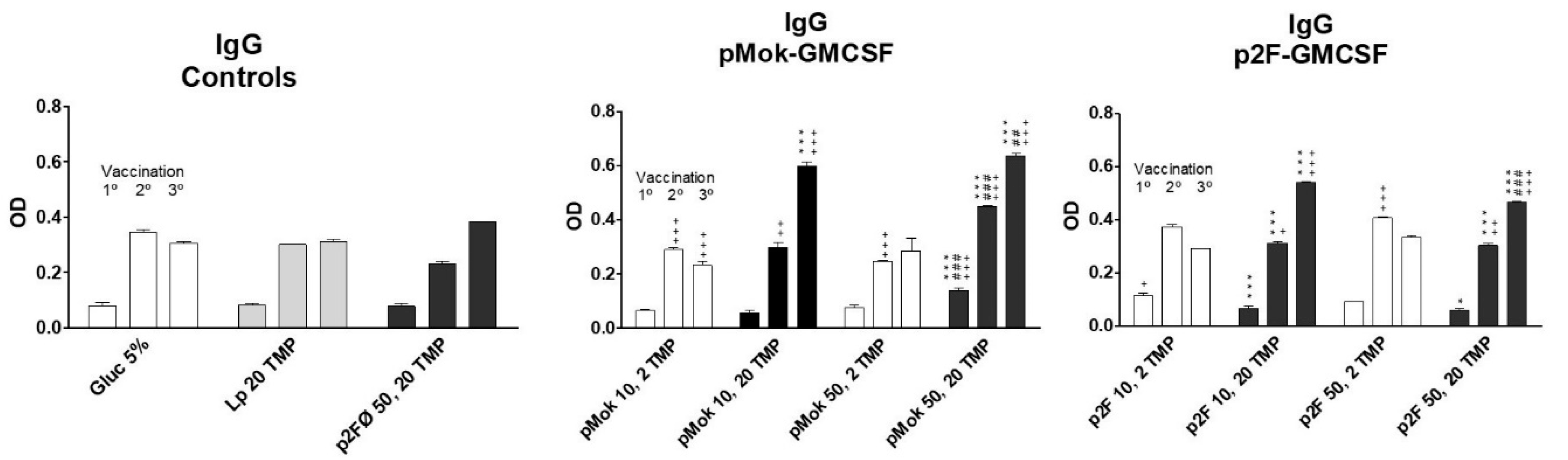
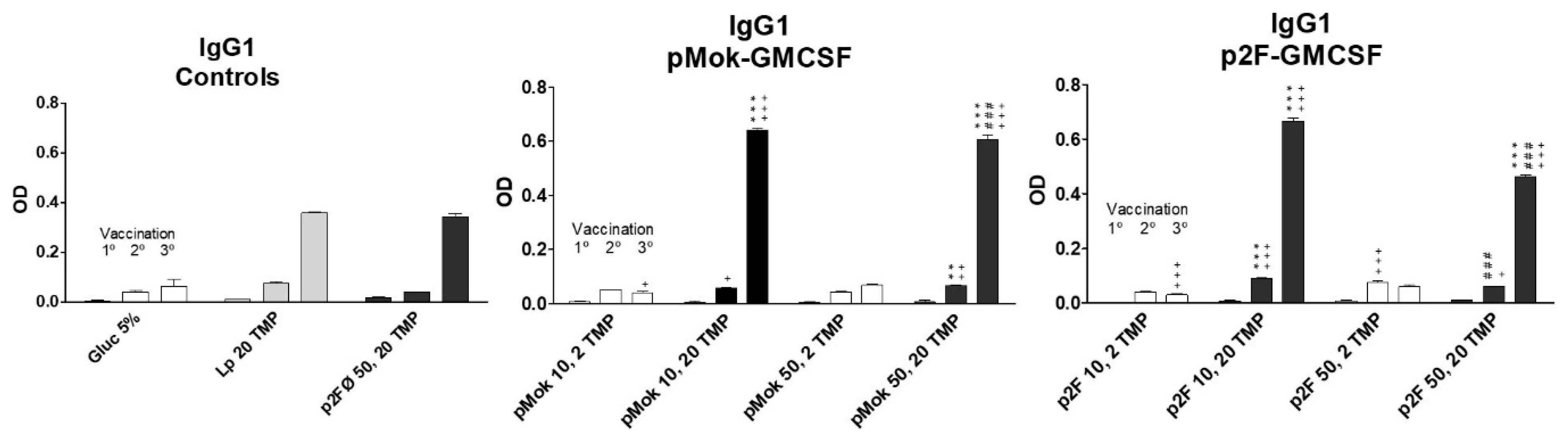
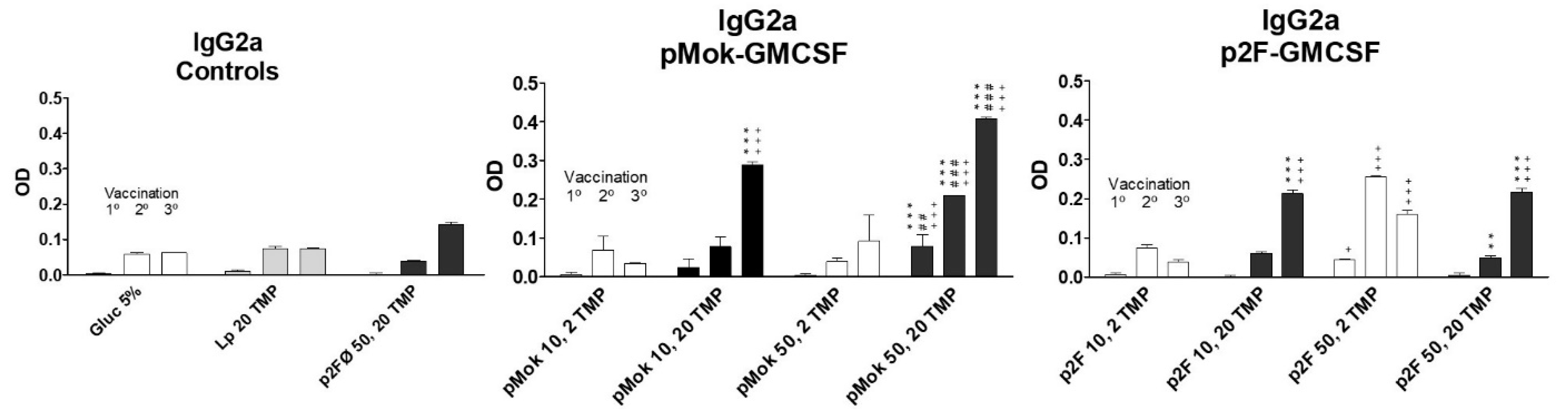
3.3. Vaccination With Hidrosoluble Proteins
3.4. Vaccination with Lipidic Antigen GD3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rezaee, M.; Oskuee, R.K.; Nassirli, H.; Malaekeh-Nikouei, B. Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems. J. Control. Release 2016, 236, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Ture, N.; Gaud, R.; Trotta, F. Lipid- and polymer-based plexes as therapeutic carriers for bioactive molecules. Int. J. Pharm. 2019, 558, 250–260. [Google Scholar] [CrossRef]
- Bofinger, R.; Zaw-Thin, M.; Mitchell, N.J.; Patrick, P.S.; Stowe, C.; Gomez-Ramirez, A.; Hailes, H.C.; Kalber, T.L.; Tabor, A.B. Development of lipopolyplexes for gene delivery: A comparison of the effects of differing modes of targeting peptide display on the structure and transfection activities of lipopolyplexes. J. Pept. Sci. 2018, 24, e3131. [Google Scholar] [CrossRef]
- Echen, W.; Eli, H.; Eliu, Z.; Eyuan, W. Lipopolyplex for Therapeutic Gene Delivery and Its Application for the Treatment of Parkinson’s Disease. Front. Aging Neurosci. 2016, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Paris, J.L.; Coelho, F.; Teixeira, A.; Diéguez, L.; Silva, B.F.B.; Abalde-Cela, S. In Vitro Evaluation of Lipopolyplexes for Gene Transfection: Comparing 2D, 3D and Microdroplet-Enabled Cell Culture. Molecules 2020, 25, 3277. [Google Scholar] [CrossRef]
- Li, L.; Song, H.; Wang, G.; He, B.; Li, C.; Lai, Y.; Xu, X.; Gu, Z. Cationic lipid-coated PEI/DNA polyplexes with improved efficiency and reduced cytotoxicity for gene delivery into mesenchymal stem cells. Int. J. Nanomed. 2012, 7, 4637–4648. [Google Scholar] [CrossRef] [Green Version]
- Fornaguera, C.; García-Celma, M.J. Personalized Nanomedicine: A Revolution at the Nanoscale. J. Pers. Med. 2017, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Hua, S.; De Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Moret, I.; Peris, J.E.; Guillem, V.M.; Benet, M.; Revert, F.; Dası́, F.; Crespo, A.; Aliño, S.F.; Guillem, V.M. Stability of PEI–DNA and DOTAP–DNA complexes: Effect of alkaline pH, heparin and serum. J. Control. Release 2001, 76, 169–181. [Google Scholar] [CrossRef]
- Dunussi-Joannopoulos K, Dranoff G, Weinstein HJ, Ferrara JL, Bierer BE, Croop JM. Gene immunotherapy in murine acute myeloid leukemia: Granulocyte-macrophage colony-stimulating factor tumor cell vaccines elicit more potent antitumor immunity compared with B7 family and other cytokine vaccines. Blood 1998, 91, 222–230. [PubMed]
- Moret-Tatay, I.; Diaz, J.; Marco, F.M.; Crespo, A.; Aliño, S.F. Complete tumor prevention by engineered tumor cell vaccines employing nonviral vectors. Cancer Gene Ther. 2003, 10, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Moret-Tatay, I.; Sanmartín, I.; Marco, F.M.; Diaz, J.; Aliño, S.F. Nonviral therapeutic cell vaccine mediates potent antitumor effects. Vaccine 2006, 24, 3937–3945. [Google Scholar] [CrossRef]
- Borrello, I.; Pardoll, E. GM-CSF-based cellular vaccines: A review of the clinical experience. Cytokine Growth Factor Rev. 2002, 13, 185–193. [Google Scholar] [CrossRef]
- Serafini, P.; Carbley, R.; Noonan, K.A.; Tan, G.; Bronte, V.; Borrello, I. High-Dose Granulocyte-Macrophage Colony-Stimulating Factor-Producing Vaccines Impair the Immune Response through the Recruitment of Myeloid Suppressor Cells. Cancer Res. 2004, 64, 6337–6343. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.-S.; Weber, S.; Gan, J.; Rakhmilevich, A.L.; Mahvi, D.M. Granulocyte-macrophage colony-stimulating factor (GM-CSF) secreted by cDNA-transfected tumor cells induces a more potent antitumor response than exogenous GM-CSF. Cancer Gene Ther. 1999, 6, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliño, S.F.; Lejarreta, M.; Alfaro, J.; Iruarrizaga, A.; Bobadilla, M.; Blaya, C.; Crespo, J. Antimetastatic Effect of Immunization with Liposome-Encapsulated Tumor Cell-Membrane Proteins Obtained from Experimental Tumors. Immunopharmacol. Immunotoxicol. 1995, 17, 419–436. [Google Scholar] [CrossRef]
- Cheresh, D.A.; Honsik, C.J.; Staffileno, L.K.; Jung, G.; Reisfeld, R.A. Disialoganglioside GD3 on human melanoma serves as a relevant target antigen for monoclonal antibody-mediated tumor cytolysis. Proc. Natl. Acad. Sci. USA 1985, 82, 5155–5159. [Google Scholar] [CrossRef] [Green Version]
- Ravindranath, M.H.; Irie, R.F. Gangliosides as antigens of human melanoma. Cancer Treat. Res. 1988, 43, 17–43. [Google Scholar] [CrossRef]
- Bordier, C. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 1981, 256, 1604–1607. [Google Scholar] [CrossRef]
- Stewart, J.C.M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef]
- De Ilarduya, C.T.; García, L.; Duzgunes, N. Liposomes and lipopolymeric carriers for gene delivery. J. Microencapsul. 2010, 27, 602–608. [Google Scholar] [CrossRef]
- Massó, O.; Aliño, S.F.; Lejarreta, M.; Blasco, F.; Piulats, J. Specific serological response by active immunization with GD3-bearing liposomes. J. Pharmacol. Exp. Ther. 1996, 278. [Google Scholar]
- Lin, C.; Zhong, Z.; Lok, M.C.; Jiang, X.; Hennink, W.E.; Feijen, J.; Engbersen, J.F.J. Novel Bioreducible Poly(amido amine)s for Highly Efficient Gene Delivery. Bioconjugate Chem. 2007, 18, 138–145. [Google Scholar] [CrossRef]
- Yadav, M.R.; Kumar, M.; Murumkar, P.R.; Hazari, P.P.; Mishra, A.K. Gemini Amphiphile-Based Lipoplexes for Efficient Gene Delivery: Synthesis, Formulation Development, Characterization, Gene Transfection, and Biodistribution Studies. ACS Omega 2018, 3, 11802–11816. [Google Scholar] [CrossRef]
- De Ilarduya, C.T.; Sun, Y.; Düzgüneş, N. Gene delivery by lipoplexes and polyplexes. Eur. J. Pharm. Sci. 2010, 40, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Gillies, G.T.; Allison, S.W.; Tissue, B.M. Positive pressure infusion of fluorescent nanoparticles as a probe of the structure of brain phantom gelatins. Nanotechnology 2002, 13, 484–486. [Google Scholar] [CrossRef]
- Walde, P.; Ichikawa, S. Enzymes inside lipid vesicles: Preparation, reactivity and applications. Biomol. Eng. 2001, 18, 143–177. [Google Scholar] [CrossRef]
- Tsai, J.T.; Furstoss, K.J.; Michnick, T.; Sloane, D.L.; Paul, R.W. Quantitative physical characterization of lipid‒polycation‒DNA lipopolyplexes. Biotechnol. Appl. Biochem. 2002, 36, 13–20. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Ho, P.Y.; Tu, M.-J.; Jilek, J.L.; Chen, Q.-X.; Zeng, S.; Yu, A.-M. Lipidation of polyethylenimine-based polyplex increases serum stability of bioengineered RNAi agents and offers more consistent tumoral gene knockdown in vivo. Int. J. Pharm. 2018, 547, 537–544. [Google Scholar] [CrossRef]
- García, L.; Buñuales, M.; Düzgüneş, N.; De Ilarduya, C.T. Serum-resistant lipopolyplexes for gene delivery to liver tumour cells. Eur. J. Pharm. Biopharm. 2007, 67, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.W.; Kim, J.-D.; Park, K. Polycation gene delivery systems: Escape from endosomes to cytosol. J. Pharm. Pharmacol. 2003, 55, 721–734. [Google Scholar] [CrossRef] [Green Version]
- Guevara, M.L.; Jilesen, Z.; Stojdl, D.; Persano, S. Codelivery of mRNA with α-Galactosylceramide Using a New Lipopolyplex Formulation Induces a Strong Antitumor Response upon Intravenous Administration. ACS Omega 2019, 4, 13015–13026. [Google Scholar] [CrossRef] [Green Version]
- Persano, S.; Guevara, M.L.; Li, Z.; Mai, J.; Ferrari, M.; Pompa, P.P.; Shen, H. Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials 2017, 125, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Cui, W.; Prasad, P.; Touve, M.A.; Gianneschi, N.C.; Mager, J.; Thayumanavan, S. Bait-and-Switch Supramolecular Strategy To Generate Noncationic RNA–Polymer Complexes for RNA Delivery. Biomacromolecules 2018, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Lezoualc’H, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, S.; Zhang, Y.; Maji, S.; Greiner, A. PDMAEMA based gene delivery materials. Mater. Today 2012, 15, 388–393. [Google Scholar] [CrossRef]
- Herrero, M.J.; Botella, R.; Dasí, F.; Algás, R.; Sánchez, M.; Aliño, S.F. Antigens and Cytokine Genes in Antitumor Vaccines. Ann. N. Y. Acad. Sci. 2006, 1091, 412–424. [Google Scholar] [CrossRef]
- Selby, M.J.; Engelhardt, J.J.; Quigley, M.; Henning, K.A.; Chen, T.; Srinivasan, M.; Korman, A.J. Anti-CTLA-4 Antibodies of IgG2a Isotype Enhance Antitumor Activity through Reduction of Intratumoral Regulatory T Cells. Cancer Immunol. Res. 2013, 1, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Vaccine Group | Type | Lipid Composition | Plasmid | GM-CSF (µg) | TMP (µg) |
|---|---|---|---|---|---|
| 5% glucose | - | - | - | - | |
| TMP20 | liposome | PC:CH:DP | - | - | 20 |
| p2F50/TMP20 | lipopolyplex | PC:CH:DP | p2F | - | 20 |
| p2FGM10/TMP2 | lipopolyplex | SM:CH:DP | p2F | 10 | 2 |
| p2FGM10/TMP20 | lipopolyplex | SM:CH:DP | p2F | 10 | 20 |
| p2FGM50/TMP2 | lipopolyplex | SM:CH:DP | p2F | 50 | 2 |
| p2FGM50/TMP20 | lipopolyplex | SM:CH:DP | p2F | 50 | 20 |
| pMokGM10/TMP2 | lipopolyplex | PC:CH:DP | pMOK | 10 | 2 |
| pMokGM10/TMP20 | lipopolyplex | PC:CH:DP | pMOK | 10 | 20 |
| pMokGM50/TMP2 | lipopolyplex | PC:CH:DP | pMOK | 50 | 2 |
| pMokGM50/TMP20 | lipopolyplex | PC:CH:DP | pMOK | 50 | 20 |
| Vaccine Group | Type | Lipidic Vesicle | Plasmid | GM-CSF (µg) | GD3 (µmol) |
|---|---|---|---|---|---|
| 5% glucose solution | - | - | - | - | - |
| MLV(SM:CH:GD3) | liposome | Multilamellar | - | - | 0.03 |
| MLV(SM:CH:DP:GD3) | liposome | Multilamellar | - | - | 0.03 |
| MLV(SM:CH:GD3)/pMok | lipopolyplex | Multilamellar | pMok | 50 | 0.03 |
| SUV(SM:CH:GD3)/pMok | lipopolyplex | Small unilamellar | pMok | 50 | 0.03 |
| MLV(SM:CH:DP:GD3)/pMok | lipopolyplex | Multilamellar | pMok | 50 | 0.03 |
| SUV(SM:CH:DP:GD3)/pMok | lipopolyplex | Small unilamellar | pMok | 50 | 0.03 |
| TREATMENT GROUP | ||||||||
|---|---|---|---|---|---|---|---|---|
| CONTROL | LIPOSOMES | LIPOPOLYPLEX | ||||||
| SM:CH:GD3 | SM:CH:DP:GD3 | SM:CH:GD3/pMok | SM:CH:DPGD3/pMok | |||||
| VACCINATION | Ig Type | 5% Glucose | MLV | MLV | MLV | SUV | MLV | SUV |
| 1° VACCINATION | IgM | 0 | 1:25 | 1:50 | 1:25 | 1:25 | 0 | 1:100 |
| IgG | 0 | 0 | 1:100 | 1:50 | 1:100 | 1:50 | 1:100 | |
| 2° VACCINATION | IgM | 0 | 1:50 | 1:200 | 1:25 | 1:25 | 1:50 | 1:200 |
| IgG | 0 | 1:50 | 1:200 | 1:100 | 1:100 | 1:200 | 1:200 | |
| 3° VACCINATION | IgM | 0 | 1:50 | 1:100 | 1:100 | 1:50 | 1:50 | 1:100 |
| IgG | 0 | 1:100 | 1:200 | 1:100 | 1:200 | 1:200 | 1:800 | |
| TREATMENT GROUP | |||||||
|---|---|---|---|---|---|---|---|
| CONTROL | LIPOSOMES | LIPOPOLYPLEX | |||||
| SM:CH:GD3 | SM:CH:DP:GD3 | SM:CH:GD3/pMok | SM:CH:DPGD3/pMok | ||||
| Ig Type | 5% Glucose | MLV | MLV | MLV | SUV | MLV | SUV |
| IgM | 0 | 1:50 | 1:100 | 1:100 | 1:50 | 1:50 | 1:100 |
| Total IgG | 0 | 1:100 | 1:200 | 1:100 | 1:200 | 1:200 | 1:800 |
| IgG1 | 0 | 1:25 | 1:25 | 1:25 | 1:25 | 1:25 | 1:25 |
| IgG2a | 0 | 1:25 | 1:25 | 1:100 | 1:100 | 1:200 | 1:400 |
| IgG2b | 1:25 | 1:25 | 1:25 | 1:25 | 1:25 | 1:25 | 1:25 |
| IgG3 | 0 | 1:100 | 1:200 | 1:50 | 1:50 | 1:200 | 1:200 |
| TREATMENT GROUP | |||||||
|---|---|---|---|---|---|---|---|
| CONTROL | LIPOSOMES | LIPOPOLYPLEX | |||||
| SM:CH:GD3 | SM:CH:DP:GD3 | SM:CH:GD3/pMok | SM:CH:DPGD3/pMok | ||||
| Total IgG | 5% Glucose | MLV | MLV | MLV | SUV | MLV | SUV |
| GD3 | 0 | 1:100 | 1:200 | 1:100 | 1:200 | 1:200 | 1:800 |
| GM1 | - | - | - | - | - | - | - |
| GM2 | - | - | - | - | - | - | - |
| GM3 | - | - | - | - | - | - | - |
| GD1b | ± | ± | ± | ± | ± | ± | ± |
| GD2 | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanmartín, I.; Sendra, L.; Moret, I.; Herrero, M.J.; Aliño, S.F. Multicompartmental Lipopolyplex as Vehicle for Antigens and Genes Delivery in Vaccine Formulations. Pharmaceutics 2021, 13, 281. https://doi.org/10.3390/pharmaceutics13020281
Sanmartín I, Sendra L, Moret I, Herrero MJ, Aliño SF. Multicompartmental Lipopolyplex as Vehicle for Antigens and Genes Delivery in Vaccine Formulations. Pharmaceutics. 2021; 13(2):281. https://doi.org/10.3390/pharmaceutics13020281
Chicago/Turabian StyleSanmartín, Isaías, Luis Sendra, Inés Moret, María José Herrero, and Salvador F. Aliño. 2021. "Multicompartmental Lipopolyplex as Vehicle for Antigens and Genes Delivery in Vaccine Formulations" Pharmaceutics 13, no. 2: 281. https://doi.org/10.3390/pharmaceutics13020281
APA StyleSanmartín, I., Sendra, L., Moret, I., Herrero, M. J., & Aliño, S. F. (2021). Multicompartmental Lipopolyplex as Vehicle for Antigens and Genes Delivery in Vaccine Formulations. Pharmaceutics, 13(2), 281. https://doi.org/10.3390/pharmaceutics13020281







