Significance of Crosslinking Approaches in the Development of Next Generation Hydrogels for Corneal Tissue Engineering
Abstract
1. Introduction
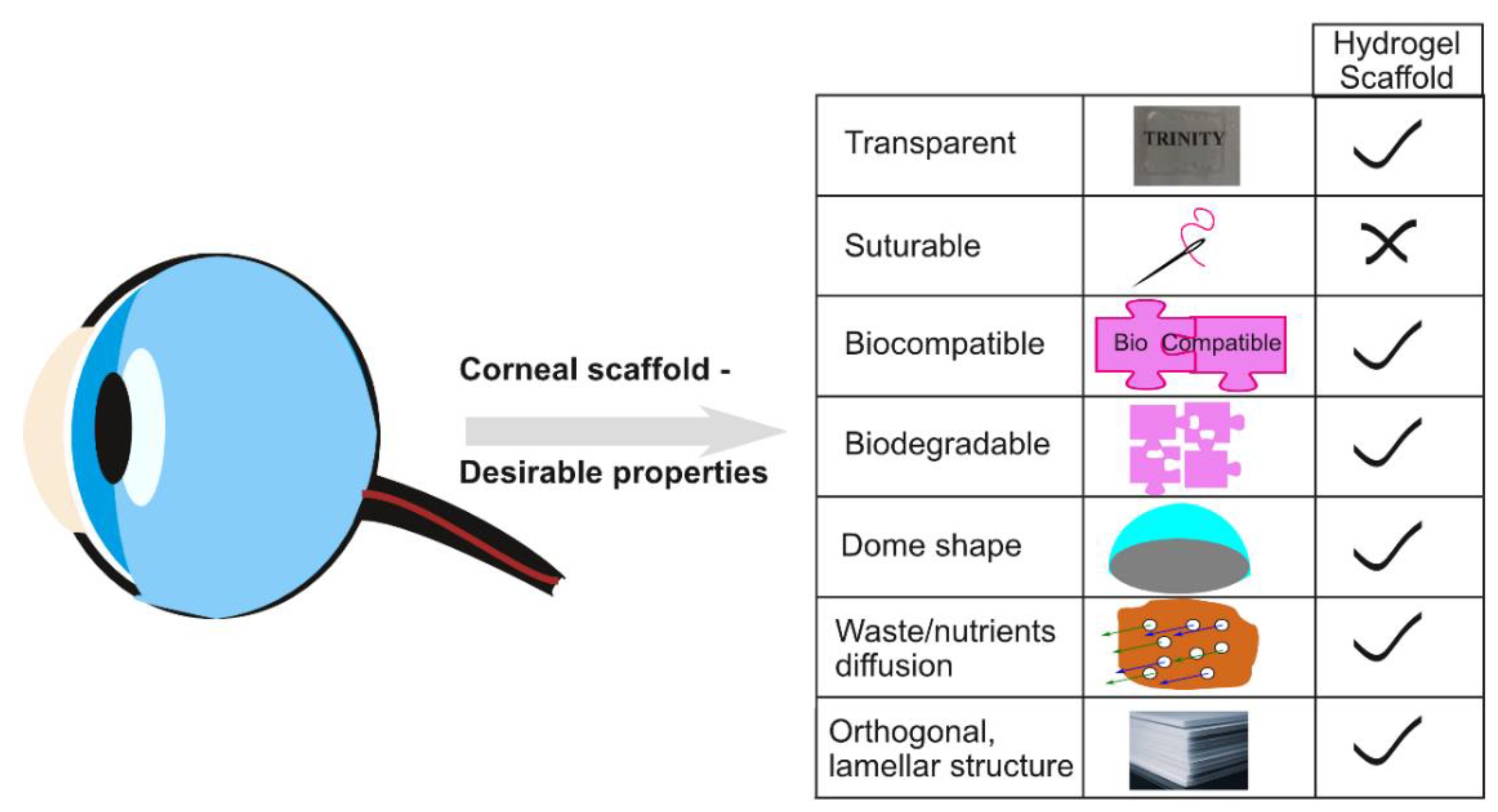
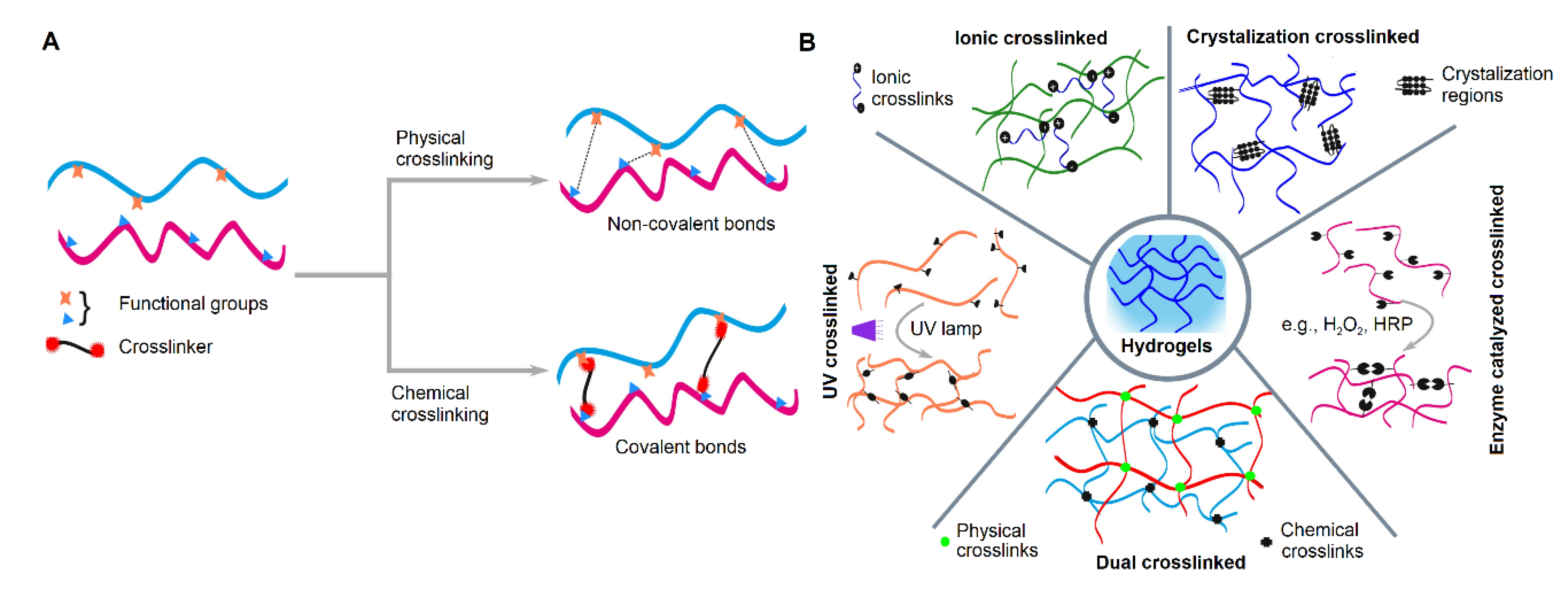
2. Crosslinking in Hydrogel Fabrication for Corneal Regeneration
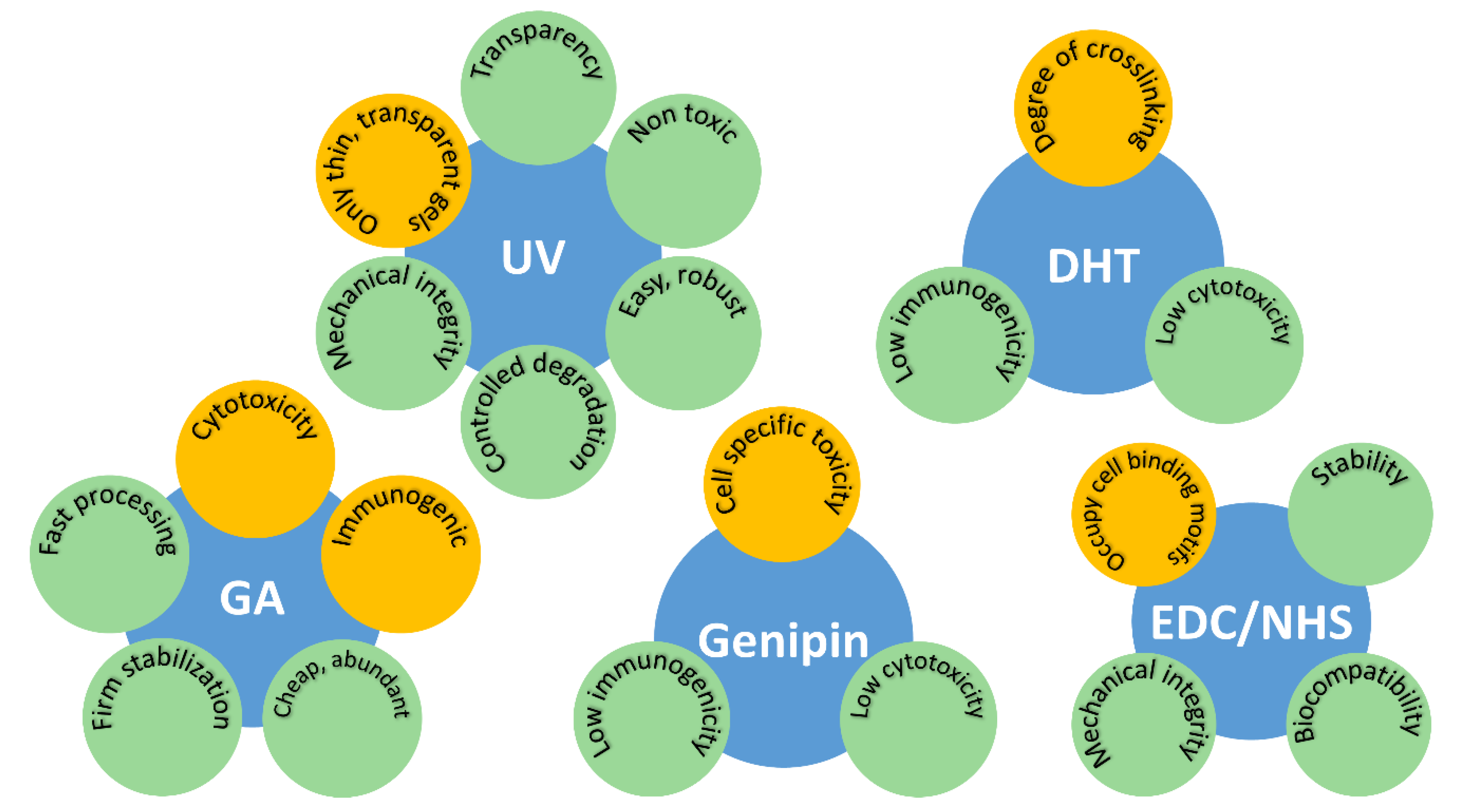
2.1. Dehydrothermal Treatment (DHT)
2.2. Ultra-Violet (UV) Irradiation
2.3. Crosslinking Using Chemical Additives
2.3.1. Glutaraldehyde (GA)
2.3.2. 1,4-Butanediol Diglycidyl Ether (BDDGE)
2.3.3. Genipin
2.3.4. Ethyl-3-[3-dimethylaminopropyl] Carbodiimide Hydrochloride (EDC) and N-hydroxy-succinimide (NHS)
2.4. Other Approaches
2.5. Comparative Studies
3. Crosslinking Strategies for Injectable Hydrogel
3.1. Gelation and Formulation
3.2. The Injectable Hydrogels in Treatment
4. Impact of Crosslinkers on Hydrogel Characteristics
4.1. Mechanical Characteristics
4.2. Degradation and Structural Properties
4.3. Toxicity and Biocompatibility
5. Challenges and Future Perspective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar] [PubMed]
- Thompson, R.W.; Price, M.O.; Bowers, P.J.; Price, F.W. Long-term graft survival after penetrating keratoplasty. Ophthalmology 2003, 110, 1396–1402. [Google Scholar] [CrossRef]
- Shimazaki, J.; Shinozaki, N.; Shimmura, S.; Holland, E.J.; Tsubota, K. Efficacy and Safety of International Donor Sharing: A Single-Center, Case-Controlled Study on Corneal Transplantation. Transplantation 2004, 78, 216–220. [Google Scholar] [CrossRef]
- Cao, K.Y.; Dorrepaal, S.J.; Seamone, C.; Slomovic, A.R. Demographics of corneal transplantation in Canada in 2004. Can. J. Ophthalmol. 2006, 41, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Culla, B.; Kolovou, P.E. Keratoprosthesis: A Review of Recent Advances in the Field. J. Funct. Biomater. 2016, 7, 13. [Google Scholar] [CrossRef]
- Dua, H.S.; Gomes, J.A.; King, A.J.; Maharajan, V. The amniotic membrane in ophthalmology. Surv. Ophthalmol. 2004, 49, 51–77. [Google Scholar] [CrossRef]
- Shimazaki, J.; Shinozaki, N.; Tsubota, K. Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblepharon. Br. J. Ophthalmol. 1998, 82, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C.; Espana, E.M.; Kawakita, T.; Di Pascuale, M.A.; Li, W.; He, H.; Liu, T.-S.; Cho, T.-H.; Gao, Y.-Y.; Yeh, L.-K.; et al. How Does Amniotic Membrane Work? Ocul. Surf. 2004, 2, 177–187. [Google Scholar] [CrossRef]
- Connon, C.J.; Doutch, J.; Chen, B.; Hopkinson, A.; Mehta, J.S.; Nakamura, T.; Kinoshita, S.; Meek, K.M. The variation in transparency of amniotic membrane used in ocular surface regeneration. Br. J. Ophthalmol. 2009, 94, 1057–1061. [Google Scholar] [CrossRef]
- Shortt, A.J.; Secker, G.A.; Rajan, M.S.; Meligonis, G.; Dart, J.K.; Tuft, S.J.; Daniels, J.T. Ex Vivo Expansion and Transplantation of Limbal Epithelial Stem Cells. Ophthalmology 2008, 115, 1989–1997. [Google Scholar] [CrossRef]
- Dua, H.S.; Rahman, I.; Miri, A.; Said, D.G. Variations in amniotic membrane: Relevance for clinical applications. Br. J. Ophthalmol. 2010, 94, 963–964. [Google Scholar] [CrossRef][Green Version]
- Wilson, S.L.; Sidney, L.E.; Dunphy, S.E.; Rose, J.B.; Hopkinson, A. Keeping an Eye on Decellularized Corneas: A Review of Methods, Characterization and Applications. J. Funct. Biomater. 2013, 4, 114–161. [Google Scholar] [CrossRef]
- Lynch, A.P.; Ahearne, M. Strategies for developing decellularized corneal scaffolds. Exp. Eye Res. 2013, 108, 42–47. [Google Scholar] [CrossRef]
- Lynch, A.P.; Wilson, S.L.; Ahearne, M. Dextran Preserves Native Corneal Structure during Decellularization. Tissue Eng. Part C Methods 2016, 22, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Qiu, P.; Wu, F.; Zhang, W.; Teng, W.; Qin, Z.; Li, C.; Zhou, J.; Fang, Z.; Tang, Q.; et al. Construction of a Corneal Stromal Equivalent with SMILE-Derived Lenticules and Fibrin Glue. Sci. Rep. 2016, 6, 33848. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Fernández-Pérez, J.; Masterton, S.; Madden, P.W.; Bhattacharjee, P. Designing Scaffolds for Corneal Regeneration. Adv. Funct. Mater. 2020, 30, 10. [Google Scholar] [CrossRef]
- Fernández-Pérez, J.; Kador, K.E.; Lynch, A.P.; Ahearne, M. Characterization of extracellular matrix modified poly(ε-caprolactone) electrospun scaffolds with differing fiber orientations for corneal stroma regeneration. Mater. Sci. Eng. C 2020, 108, 110415. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Z.; Liu, Y.; Wang, L.; Jiang, Z.; Li, T.; Zhang, W.; Liang, Y. Carboxymethyl chitosan/gelatin/hyaluronic acid blended-membranes as epithelia transplanting scaffold for corneal wound healing. Carbohydr. Polym. 2018, 192, 240–250. [Google Scholar] [CrossRef]
- Wu, Z.; Kong, B.; Liu, R.; Sun, W.; Mi, S. Engineering of Corneal Tissue through an Aligned PVA/Collagen Composite Nanofibrous Electrospun Scaffold. Nanomaterials 2018, 8, 124. [Google Scholar] [CrossRef]
- Ruberti, J.W.; Zieske, J.D. Prelude to corneal tissue engineering—Gaining control of collagen organization. Prog. Retin. Eye Res. 2008, 27, 549–577. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, J.; Bai, S. Biocompatibility of injectable hydrogel from decellularized human adipose tissue in vitro and in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1684–1694. [Google Scholar] [CrossRef]
- Pollinger, K.; Hennig, R.; Ohlmann, A.; Fuchshofer, R.; Wenzel, R.; Breunig, M.; Tessmar, J.; Tamm, E.R.; Goepferich, A. Ligand-functionalized nanoparticles target endothelial cells in retinal capillaries after systemic application. Proc. Natl. Acad. Sci. USA 2013, 110, 6115–6120. [Google Scholar] [CrossRef]
- Luschmann, C.; Tessmar, J.; Schoeberl, S.; Strauss, O.; Framme, C.; Luschmann, K.; Goepferich, A. Developing an in situ nanosuspension: A novel approach towards the efficient administration of poorly soluble drugs at the anterior eye. Eur. J. Pharm. Sci. 2013, 50, 385–392. [Google Scholar] [CrossRef]
- Lee, S.S.; Hughes, P.; Ross, A.D.; Robinson, M.R. Biodegradable Implants for Sustained Drug Release in the Eye. Pharm. Res. 2010, 27, 2043–2053. [Google Scholar] [CrossRef]
- Lin, C.-C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for Protein Delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef]
- Van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Atyabi, F.; Bakhshandeh, H.; Soleimani, M.; Hosseini, S.S.; Hashemi, H.; Shabani, I.; Shafiee, A.; Nejad, A.H.B.; Erfan, M.; Dinarvand, R. Poly (ε-caprolactone) nanofibrous ring surrounding a polyvinyl alcohol hydrogel for the development of a biocompatible two-part artificial cornea. Int. J. Nanomed. 2011, 6, 1509–1515. [Google Scholar] [CrossRef]
- Ahearne, M.; Liu, K.-K.; El Haj, A.J.; Then, K.Y.; Rauz, S.; Yang, Y. Online Monitoring of the Mechanical Behavior of Collagen Hydrogels: Influence of Corneal Fibroblasts on Elastic Modulus. Tissue Eng. Part C Methods 2010, 16, 319–327. [Google Scholar] [CrossRef]
- Gostynska, N.; Krishnakumar, G.S.; Campodoni, E.; Panseri, S.; Montesi, M.; Sprio, S.; Kon, E.; Marcacci, M.; Tampieri, A.; Sandri, M. 3D porous collagen scaffolds reinforced by glycation with ribose for tissue engineering application. Biomed. Mater. 2017, 12, 055002. [Google Scholar] [CrossRef]
- Ruini, F.; Tonda-Turo, C.; Chiono, V.; Ciardelli, G. Chitosan membranes for tissue engineering: Comparison of different crosslinkers. Biomed. Mater. 2015, 10, 65002. [Google Scholar] [CrossRef]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. Part A 2009, 89, 363–369. [Google Scholar] [CrossRef]
- Gomes, S.; Rodrigues, G.; Martins, G.; Henriques, C.; Silva, J. In vitro evaluation of crosslinked electrospun fish gelatin scaffolds. Mater. Sci. Eng. C 2013, 33, 1219–1227. [Google Scholar] [CrossRef]
- Ratanavaraporn, J.; Rangkupan, R.; Jeeratawatchai, H.; Kanokpanont, S.; Damrongsakkul, S. Influences of physical and chemical crosslinking techniques on electrospun type A and B gelatin fiber mats. Int. J. Biol. Macromol. 2010, 47, 431–438. [Google Scholar] [CrossRef]
- Hori, K.; Sotozono, C.; Hamuro, J.; Yamasaki, K.; Kimura, Y.; Ozeki, M.; Tabata, Y.; Kinoshita, S. Controlled-release of epidermal growth factor from cationized gelatin hydrogel enhances corneal epithelial wound healing. J. Control. Release 2007, 118, 169–176. [Google Scholar] [CrossRef]
- Watanabe, R.; Hayashi, R.; Kimura, Y.; Tanaka, Y.; Kageyama, T.; Hara, S.; Tabata, Y.; Nishida, K. A Novel Gelatin Hydrogel Carrier Sheet for Corneal Endothelial Transplantation. Tissue Eng. Part A 2011, 17, 2213–2219. [Google Scholar] [CrossRef]
- Weadock, K.S.; Miller, E.J.; Bellincampi, L.D.; Zawadsky, J.P.; Dunn, M.G. Physical crosslinking of collagen fibers: Comparison of ultraviolet irradiation and dehydrothermal treatment. J. Biomed. Mater. Res. 1995, 29, 1373–1379. [Google Scholar] [CrossRef]
- Germain, L.; Auger, F.A.; Grandbois, E.; Guignard, R.; Giasson, M.; Boisjoly, H.; Guérin, S.L. Reconstructed Human Cornea Produced in vitro by Tissue Engineering. Pathobiology 1999, 67, 140–147. [Google Scholar] [CrossRef]
- Lai, J.-Y. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci. Mater. Med. 2010, 21, 1899–1911. [Google Scholar] [CrossRef]
- Lew, D.-H.; Liu, P.H.-T.; Orgill, D.P. Optimization of UV cross-linking density for durable and nontoxic collagen GAG dermal substitute. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Bax, D.V.; Schuster, C.F.; Farndale, R.W.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds. J. Mater. Sci. Mater. Med. 2016, 27, 1–17. [Google Scholar] [CrossRef]
- Ahearne, M.; Yang, Y.; Then, K.Y.; Liu, K.-K. Non-destructive mechanical characterisation of UVA/riboflavin crosslinked collagen hydrogels. Br. J. Ophthalmol. 2007, 92, 268–271. [Google Scholar] [CrossRef]
- Ahearne, M.; Coyle, A. Application of UVA-riboflavin crosslinking to enhance the mechanical properties of extracellular matrix derived hydrogels. J. Mech. Behav. Biomed. Mater. 2016, 54, 259–267. [Google Scholar] [CrossRef]
- Heo, J.; Koh, R.H.; Shim, W.; Kim, H.D.; Yim, H.-G.; Hwang, N.S. Riboflavin-induced photo-crosslinking of collagen hydrogel and its application in meniscus tissue engineering. Drug Deliv. Transl. Res. 2015, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Ohan, M.P.; Dunn, M.G. Glucose stabilizes collagen sterilized with gamma irradiation. J. Biomed. Mater. Res. Part A 2003, 67, 1188–1195. [Google Scholar] [CrossRef]
- Mi, S.; Khutoryanskiy, V.V.; Jones, R.R.; Zhu, X.; Hamley, I.W.; Connon, C.J. Photochemical cross-linking of plastically compressed collagen gel produces an optimal scaffold for corneal tissue engineering. J. Biomed. Mater. Res. Part A 2011, 99, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-a–induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Applegate, M.B.; Partlow, B.P.; Coburn, J.; Marelli, B.; Pirie, C.; Pineda, R.; Kaplan, D.L.; Omenetto, F.G. Photocrosslink-ing of silk fibroin using riboflavin for ocular prostheses. Adv. Mater. 2016, 28, 2417–2420. [Google Scholar]
- Bhattacharjee, P.; Fernández-Pérez, J.; Ahearne, M. Potential for combined delivery of riboflavin and all-trans retinoic acid, from silk fibroin for corneal bioengineering. Mater. Sci. Eng. C 2019, 105, 110093. [Google Scholar] [CrossRef]
- Li, L.; Lu, C.; Wang, L.; Chen, M.; White, J.F.; Hao, X.; McLean, K.M.; Chen, H.; Hughes, T.C. Gelatin-Based Photocurable Hydrogels for Corneal Wound Repair. ACS Appl. Mater. Interfaces 2018, 10, 13283–13292. [Google Scholar] [CrossRef]
- Rizwan, M.; Peh, G.S.; Ang, H.-P.; Lwin, N.C.; Adnan, K.; Mehta, J.S.; Tan, W.S.; Yim, E.K. Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications. Biomaterials 2017, 120, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.; Watanabe, K.; Zheng, L.L.; Kim, C.-Y.; Beck, S.E.; Huie, P.; Noolandi, J.; Cochran, J.R.; Ta, C.N.; Frank, C.W. Toward the development of an artificial cornea: Improved stability of interpenetrating polymer networks. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 8–17. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef]
- Yang, C.-F.; Yasukawa, T.; Kimura, H.; Miyamoto, H.; Honda, Y.; Tabata, Y.; Ikada, Y.; Ogura, Y. Experimental Corneal Neovascularization by Basic Fibroblast Growth Factor Incorporated into Gelatin Hydrogel. Ophthalmic Res. 2000, 32, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Doillon, C.J.; Watsky, M.A.; Hakim, M.; Wang, J.; Munger, R.; Laycock, N.; Osborne, R.; Griffith, M. A collagen-based scaf-fold for a tissue engineered human cornea: Physical and physiological properties. Int. J. Artif. Organs 2003, 26, 764–773. [Google Scholar] [CrossRef]
- Lu, P.-L.; Lai, J.-Y.; Ma, D.H.-K.; Hsiue, G.-H. Carbodiimide cross-linked hyaluronic acid hydrogels as cell sheet delivery vehicles: Characterization and interaction with corneal endothelial cells. J. Biomater. Sci. Polym. Ed. 2008, 19, 1–18. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Ma, D.H.-K.; Cheng, H.-Y.; Sun, C.-C.; Huang, S.-J.; Li, Y.-T.; Hsiue, G.-H. Ocular Biocompatibility of Carbodiimide Cross-Linked Hyaluronic Acid Hydrogels for Cell Sheet Delivery Carriers. J. Biomater. Sci. Polym. Ed. 2010, 21, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Bentley, E.; Murphy, C.J.; Li, F.; Carlsson, D.J.; Griffith, M. Biosynthetic Corneal Substitute Implantation in Dogs. Cornea 2010, 29, 910–916. [Google Scholar] [CrossRef]
- Kitagawa, K.; Okabe, M.; Yanagisawa, S.; Zhang, X.-Y.; Nikaido, T.; Hayashi, A. Use of a hyperdried cross-linked amniotic membrane as initial therapy for corneal perforations. Jpn. J. Ophthalmol. 2011, 55, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Calles, J.; Tártara, L.; López-García, A.; Diebold, Y.; Palma, S.; Vallés, E. Novel bioadhesive hyaluronan–itaconic acid crosslinked films for ocular therapy. Int. J. Pharm. 2013, 455, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-Y.; Ma, D.H.-K. Glutaraldehyde cross-linking of amniotic membranes affects their nanofibrous structures and limbal epithelial cell culture characteristics. Int. J. Nanomed. 2013, 8, 4157–4168. [Google Scholar] [CrossRef] [PubMed]
- Nam, E.; Fujita, N.; Morita, M.; Tsuzuki, K.; Lin, H.Y.; Chung, C.S.; Nakagawa, T.; Nishimura, R. Comparison of the canine corneal epithelial cell sheets cultivated from limbal stem cells on canine amniotic membrane, atelocollagen gel, and temperature-responsive culture dish. Veter Ophthalmol. 2014, 18, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Yang, X.; Pan, W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017, 155, 208–217. [Google Scholar] [CrossRef]
- Rafat, M.; Griffith, M.; Hakim, M.; Muzakare, L.; Li, F.; Khulbe, K.; Matsuura, T. Plasma surface modification and characterization of collagen-based artificial cornea for enhanced epithelialization. J. Appl. Polym. Sci. 2007, 106, 2056–2064. [Google Scholar] [CrossRef]
- Nicoletti, A.; Fiorini, M.; Paolillo, J.; Dolcini, L.; Sandri, M.; Pressato, D. Effects of different crosslinking conditions on the chemical–physical properties of a novel bio-inspired composite scaffold stabilised with 1,4-butanediol diglycidyl ether (BDDGE). J. Mater. Sci. Mater. Med. 2012, 24, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, W.; Han, B.; Yang, L. Study on a hydroxypropyl chitosan–gelatin based scaffold for corneal stroma tissue engineering. Appl. Surf. Sci. 2009, 255, 8701–8705. [Google Scholar] [CrossRef]
- Koh, L.B.; Islam, M.M.; Mitra, D.; Noel, C.W.; Merrett, K.; Odorcic, S.; Fagerholm, P.; Jackson, W.B.; Liedberg, B.; Phopase, J.; et al. Epoxy Cross-Linked Collagen and Collagen-Laminin Peptide Hydrogels as Corneal Substitutes. J. Funct. Biomater. 2013, 4, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.F.; Ng, Y.-F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Gentile, P.; Saracino, S.; Chiono, V.; Nandagiri, V.; Muzio, G.; Canuto, R.; Ciardelli, G. Comparative analysis of gelatin scaffolds crosslinked by genipin and silane coupling agent. Int. J. Biol. Macromol. 2011, 49, 700–706. [Google Scholar] [CrossRef]
- Madhavan, K.; Belchenko, D.; Motta, A.; Tan, W. Evaluation of composition and crosslinking effects on collagen-based composite constructs. Acta Biomater. 2010, 6, 1413–1422. [Google Scholar] [CrossRef]
- Fessel, G.; Cadby, J.; Wunderli, S.; van Weeren, R.; Snedeker, J.G. Dose- and time-dependent effects of genipin crosslinking on cell viability and tissue mechanics—Toward clinical application for tendon repair. Acta Biomater. 2014, 10, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Grolik, M.; Szczubiałka, K.; Wowra, B.; Dobrowolski, D.; Orzechowska-Wylęgała, B.; Wylęgała, E.; Nowakowska, M. Hydrogel membranes based on genipin-cross-linked chitosan blends for corneal epithelium tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Cheng, C.-Y.; Wang, N.-K.; Tan, H.-Y.; Tsai, Y.-J.; Hsiao, C.-H.; Ma, D.H.-K.; Yeh, L.-K. Characterization of the modified chitosan membrane cross-linked with genipin for the cultured corneal epithelial cells. Colloids Surf. B Biointerfaces 2015, 126, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, R.; Li, J.; Wang, Y.; Song, Y.; Tan, G.; Liu, D.; Liu, W.; Yang, X.; Pan, H.; et al. A hybrid genipin-crosslinked dual-sensitive hydrogel/nanostructured lipid carrier ocular drug delivery platform. Asian J. Pharm. Sci. 2019, 14, 423–434. [Google Scholar] [CrossRef]
- Nam, K.; Kimura, T.; Kishida, A. Controlling Coupling Reaction of EDC and NHS for Preparation of Collagen Gels Using Ethanol/Water Co-Solvents. Macromol. Biosci. 2008, 8, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Cammarata, C.R.; Hughes, M.E.; Ofner, C.M. Carbodiimide Induced Cross-Linking, Ligand Addition, and Degradation in Gelatin. Mol. Pharm. 2015, 12, 783–793. [Google Scholar] [CrossRef]
- Bax, D.V.; Davidenko, N.; Gullberg, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental insight into the effect of carbodiimide crosslinking on cellular recognition of collagen-based scaffolds. Acta Biomater. 2017, 49, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Damink, L.O.; Dijkstra, P.J.; Van Luyn, M.; Van Wachem, P.; Nieuwenhuis, P.; Feijen, J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials 1996, 17, 765–773. [Google Scholar] [CrossRef]
- Pieper, J.; Hafmans, T.; Veerkamp, J.; Van Kuppevelt, T. Development of tailor-made collagen–glycosaminoglycan matrices: EDC/NHS crosslinking, and ultrastructural aspects. Biomaterials 2000, 21, 581–593. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.; Bax, D.; Raynal, N.; Farndale, R.; Best, S.; Cameron, R. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef]
- Liu, W.; Merrett, K.; Griffith, M.; Fagerholm, P.; Dravida, S.; Heyne, B.; Scaiano, J.C.; Watsky, M.A.; Shinozaki, N.; Lagali, N.; et al. Recombinant human collagen for tissue engineered corneal substitutes. Biomaterials 2008, 29, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Rafat, M.; Li, F.; Fagerholm, P.; Lagali, N.S.; Watsky, M.A.; Munger, R.; Matsuura, T.; Griffith, M. PEG-stabilized carbodiimide crosslinked collagen–chitosan hydrogels for corneal tissue engineering. Biomaterials 2008, 29, 3960–3972. [Google Scholar] [CrossRef]
- Liu, W.; Deng, C.; McLaughlin, C.R.; Fagerholm, P.; Lagali, N.S.; Heyne, B.; Scaiano, J.C.; Watsky, M.A.; Kato, Y.; Munger, R.; et al. Collagen–phosphorylcholine interpenetrating network hydrogels as corneal substitutes. Biomaterials 2009, 30, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.H.-K.; Lai, J.-Y.; Cheng, H.-Y.; Tsai, C.-C.; Yeh, L.-K. Carbodiimide cross-linked amniotic membranes for cultivation of limbal epithelial cells. Biomaterials 2010, 31, 6647–6658. [Google Scholar] [CrossRef]
- Lai, J.-Y. Hyaluronic acid concentration-mediated changes in structure and function of porous carriers for corneal endothelial cell sheet delivery. Mater. Sci. Eng. C 2016, 59, 411–419. [Google Scholar] [CrossRef]
- Lai, J.Y.; Cheng, H.Y.; Ma, D.H.K. Investigation of overrun-processed porous hyaluronic acid carriers in corneal endo-thelial tissue engineering. PLoS ONE 2015, 10, e0136067. [Google Scholar] [CrossRef]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of cross-linked collagen–gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef]
- Duan, X.; Sheardown, H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: Mechanical properties and corneal epithelial cell interactions. Biomaterials 2006, 27, 4608–4617. [Google Scholar] [CrossRef]
- Myung, D.; Koh, W.; Bakri, A.; Zhang, F.; Marshall, A.; Ko, J.; Noolandi, J.; Carrasco, M.; Cochran, J.R.; Frank, C.W.; et al. Design and fabrication of an artificial cornea based on a photolithographically patterned hydrogel construct. Biomed. Microdevices 2007, 9, 911–922. [Google Scholar] [CrossRef]
- Farooqui, N.; Myung, D.; Koh, W.; Masek, R.; Dalal, M.; Carrasco, M.R.; Noolandi, J.; Frank, C.W.; Ta, C.N. Histological processing of ph-sensitive hydrogels used in corneal implant applications. J. Histotechnol. 2007, 30, 157–163. [Google Scholar] [CrossRef]
- Myung, D.; Farooqui, N.; Zheng, L.L.; Koh, W.; Gupta, S.; Bakri, A.; Noolandi, J.; Cochran, J.R.; Frank, C.W.; Ta, C.N. Bioactive interpenetrating polymer network hydrogels that support corneal epithelial wound healing. J. Biomed. Mater. Res. Part A 2009, 90, 70–81. [Google Scholar] [CrossRef]
- Merrett, K.; Liu, W.; Mitra, D.; Camm, K.D.; McLaughlin, C.R.; Liu, Y.; Watsky, M.A.; Li, F.; Griffith, M.; Fogg, D.E. Syn-thetic neoglycopolymer-recombinant human collagen hybrids as biomimetic crosslinking agents in corneal tissue engineering. Biomaterials 2009, 30, 5403. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, W.; Han, B.; Yang, C.; Ma, Q.; Song, F.; Bi, Q. An in situ formed biodegradable hydrogel for reconstruction of the corneal endothelium. Colloids Surf. B Biointerfaces 2011, 82, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Nam, S.H.; Koh, W.-G. Preparation of collagen-immobilized poly(ethylene glycol)/poly(2-hydroxyethyl methacrylate) interpenetrating network hydrogels for potential application of artificial cornea. J. Appl. Polym. Sci. 2011, 123, 637–645. [Google Scholar] [CrossRef]
- Ozcelik, B.; Brown, K.D.; Blencowe, A.; Daniell, M.; Stevens, G.W.; Qiao, G.G. Ultrathin chitosan–poly(ethylene glycol) hydrogel films for corneal tissue engineering. Acta Biomater. 2013, 9, 6594–6605. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Sun, J.; Hong, J.; Wang, W.; Wei, A.; Le, Q.; Xu, J. T-style keratoprosthesis based on surface-modified poly (2-hydroxyethyl methacrylate) hydrogel for cornea repairs. Mater. Sci. Eng. C 2015, 50, 274–285. [Google Scholar] [CrossRef]
- Lei, L.; Li, X.; Xiong, T.; Yu, J.; Yu, X.; Song, Z.; Li, X. Covalently Cross-Linked Chitosan Hydrogel Sheet for Topical Ophthalmic Delivery of Levofloxacin. J. Biomed. Nanotechnol. 2018, 14, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Sani, E.S.; Kheirkhah, A.; Rana, D.; Sun, Z.M.; Foulsham, W.; Sheikhi, A.; Khademhosseini, A.; Dana, R.; Annabi, N. Su-tureless repair of corneal injuries using naturally derived bioadhesive hydrogels. Sci. Adv. 2019, 5, eaav1281. [Google Scholar] [CrossRef]
- Lai, J.-Y. Biocompatibility of Genipin and Glutaraldehyde Cross-Linked Chitosan Materials in the Anterior Chamber of the Eye. Int. J. Mol. Sci. 2012, 13, 10970–10985. [Google Scholar] [CrossRef]
- Ahn, J.-I.; Kuffova, L.; Merrett, K.; Mitra, D.; Forrester, J.V.; Li, F.; Griffith, M. Crosslinked collagen hydrogels as corneal implants: Effects of sterically bulky vs. non-bulky carbodiimides as crosslinkers. Acta Biomater. 2013, 9, 7796–7805. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Han, Z. Injectable hydrogels for ophthalmic applications. J. Control. Release 2017, 268, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, D.J.; Dutta, D.; Stabenfeldt, S.E.; Vernon, B.L. Injectable hydrogels. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 881–903. [Google Scholar] [CrossRef]
- Li, Y.L.; Rodrigues, J.; Tomas, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical ap-plications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Yang, J.-A.; Yeom, J.; Hwang, B.W.; Hoffman, A.S.; Hahn, S.K. In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 2014, 39, 1973–1986. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Ulijn, R.V.; Bibi, N.; Jayawarna, V.; Thornton, P.D.; Todd, S.J.; Mart, R.J.; Smith, A.M.; Gough, J.E. Bioresponsive hydro-gels. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Xinming, L.; Yingde, C.; Lloyd, A.W.; Mikhalovsky, S.V.; Sandeman, S.R.; Howel, C.A.; Liewen, L. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Contact Lens Anterior Eye 2008, 31, 57–64. [Google Scholar] [CrossRef]
- Chien, Y.; Liao, Y.W.; Liu, D.M.; Lin, H.L.; Chen, S.J.; Chen, H.L.; Peng, C.H.; Liang, C.M.; Mou, C.Y.; Chiou, H. Corneal repair by human corneal keratocyte-reprogrammed ipscs and amphiphatic carboxymethyl-hexanoyl chitosan hy-drogel. Biomaterials 2012, 33, 8003–8016. [Google Scholar] [CrossRef]
- Garagorri, N.; Fermanian, S.; Thibault, R.; Ambrose, W.M.; Schein, O.D.; Chakravarti, S.; Elisseeff, J. Keratocyte behavior in three-dimensional photopolymerizable poly(ethylene glycol) hydrogels. Acta Biomater. 2008, 4, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, C.D.; Simpson, F.C.; Haagdorens, M.; Samarawickrama, C.; Hunter, D.; Buznyk, O.; Fagerholm, P.; Ljunggren, M.K.; Lewis, P.; Pintelon, I.; et al. LiQD Cornea: Pro-regeneration collagen mimetics as patches and alternatives to corneal transplantation. Sci. Adv. 2020, 6, eaba2187. [Google Scholar] [CrossRef] [PubMed]
- Anumolu, S.S.; DeSantis, A.S.; Menjoge, A.R.; Hahn, R.A.; Beloni, J.A.; Gordon, M.K.; Sinko, P.J. Doxycycline loaded poly(ethylene glycol) hydrogels for healing vesicant-induced ocular wounds. Biomaterials 2010, 31, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Pratoomsoot, C.; Tanioka, H.; Hori, K.; Kawasaki, S.; Kinoshita, S.; Tighe, P.J.; Dua, H.; Shakesheff, K.M.; Rose, F.R.A. A thermoreversible hydrogel as a biosynthetic bandage for corneal wound repair. Biomaterials 2008, 29, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Fanesi, G.; Abrami, M.; Zecchin, F.; Giassi, I.; Dal Ferro, E.; Boisen, A.; Grassi, G.; Bertoncin, P.; Grassi, M.; Marizza, P. Com-bined used of rheology and lf-nmr for the characterization of pvp-alginates gels containing liposomes. Pharm. Res. 2018, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Yang, Y.; El Haj, A.J.; Then, K.Y.; Liu, K.-K. Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. J. R. Soc. Interface 2005, 2, 455–463. [Google Scholar] [CrossRef]
- Wen, J.H.; Vincent, L.G.; Fuhrmann, A.; Choi, Y.S.; Hribar, K.C.; Taylor-Weiner, H.; Chen, S.; Engler, A.J. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 2014, 13, 979–987. [Google Scholar] [CrossRef]
- Ahearne, M.; Wilson, S.L.; Liu, K.-K.; Rauz, S.; El Haj, A.J.; Yang, Y. Influence of cell and collagen concentration on the cell–matrix mechanical relationship in a corneal stroma wound healing model. Exp. Eye Res. 2010, 91, 584–591. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Kawazoe, N.; Chen, G. Fabrication of highly crosslinked gelatin hydrogel and its influence on chondro-cyte proliferation and phenotype. Polymers 2017, 9, 309. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenviron-ments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef]
- Bryant, S.J.; Bender, R.J.; Durand, K.L.; Anseth, K.S. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: Engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol. Bioeng. 2004, 86, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional hydrogels with tunable structures and properties for tissue engi-neering applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A. The swollen polymer network hypothesis: Quantitative models of hydrogel swelling, stiffness, and solute transport. Prog. Polym. Sci. 2020, 105, 101243. [Google Scholar] [CrossRef]
- Dai, W.; Barbari, T. Hydrogel membranes with mesh size asymmetry based on the gradient crosslinking of poly(vinyl alcohol). J. Membr. Sci. 1999, 156, 67–79. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Aimetti, A.A.; Machen, A.J.; Anseth, K.S. Poly(ethylene glycol) hydrogels formed by thiol-ene photopolymerization for enzyme-responsive protein delivery. Biomaterials 2009, 30, 6048–6054. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Lam, J.; Trachtenberg, J.E.; Lee, E.J.; Seyednejad, H.; van den Beucken, J.J.J.P.; Tabata, Y.; Wong, M.E.; Jansen, J.A.; Mikos, A.G.; et al. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials 2014, 35, 8829–8839. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayi, E.; Ananta, M.; Binkowski, M.; Streeter, I.; Lu, Z.; Cui, Z.F.; Brown, R.A.; Mudera, V. Mechanisms of structure generation during plastic compression of nanofibrillar collagen hydrogel scaffolds: Towards engineering of collagen. J. Tissue Eng. Regen. Med. 2010, 5, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Cheema, U.; Brown, R.A. Rapid Fabrication of Living Tissue Models by Collagen Plastic Compression: Understanding Three-Dimensional Cell Matrix Repair In Vitro. Adv. Wound Care 2013, 2, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Torbet, J.; Ronzière, M.C. Magnetic alignment of collagen during self-assembly. Biochem. J. 1984, 219, 1057–1059. [Google Scholar] [CrossRef] [PubMed]
- Torbet, J.; Malbouyres, M.; Builles, N.; Justin, V.; Roulet, M.; Damour, O.; Oldberg, A.; Ruggiero, F.; Hulmes, D.J. Orthogo-nal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Biomaterials 2007, 28, 4268–4276. [Google Scholar] [CrossRef]
- Panteli, P.A.; Patrickios, C.S. Multiply Interpenetrating Polymer Networks: Preparation, Mechanical Properties, and Applications. Gels 2019, 5, 36. [Google Scholar] [CrossRef]
- Krishnakumar, G.S.; Sampath, S.; Muthusamy, S.; John, M.A. Importance of crosslinking strategies in designing smart biomaterials for bone tissue engineering: A systematic review. Mater. Sci. Eng. C 2019, 96, 941–954. [Google Scholar] [CrossRef]
- Martínez, A.; Blanco, M.; Davidenko, N.; Cameron, R. Tailoring chitosan/collagen scaffolds for tissue engineering: Effect of composition and different crosslinking agents on scaffold properties. Carbohydr. Polym. 2015, 132, 606–619. [Google Scholar] [CrossRef]
- Khor, E. Methods for the treatment of collagenous tissues for bioprostheses. Biomaterials 1997, 18, 95–105. [Google Scholar] [CrossRef]
- Casali, D.M.; Yost, M.J.; Matthews, M.A. Eliminating glutaraldehyde from crosslinked collagen films using supercritical CO2. J. Biomed. Mater. Res. Part A 2018, 106, 86–94. [Google Scholar] [CrossRef]
- Yamazaki, M.; Chiba, K.; Mohri, T.; Hatanaka, H. Activation of the mitogen-activated protein kinase cascade through nitric oxide synthesis as a mechanism of neuritogenic effect of genipin in PC12h cells. J. Neurochem. 2008, 79, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-C.; Kim, H.-G.; Lee, S.-A.; Lim, S.; Park, E.-H.; Kim, S.-J.; Lim, C.-J. Genipin-induced apoptosis in hepatoma cells is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of mitochondrial pathway. Biochem. Pharmacol. 2005, 70, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, S.; Goepferich, A.M.; Brandl, F.P. Hydrogels in ophthalmic applications. Eur. J. Pharm. Biopharm. 2015, 95, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Hammer, N.; Brandl, F.P.; Kirchhof, S.; Messmann, V.; Goepferich, A.M. Protein Compatibility of Selected Cross-linking Reactions for Hydrogels. Macromol. Biosci. 2015, 15, 405–413. [Google Scholar] [CrossRef]
- Zhang, B.; Xue, Q.; Li, J.; Ma, L.; Yao, Y.; Ye, H.; Cui, Z.; Yang, H. 3d bioprinting for artificial cornea: Challenges and perspectives. Med. Eng. Phys. 2019, 71, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.F.D.; Rohde, M.; Ross, M.; Anvari, P.; Blaeser, A.; Vogt, M.; Panfil, C.; Yam, G.H.; Mehta, J.S.; Fischer, H.; et al. Corneal bioprinting utilizing collagen-based bioinks and primary human keratocytes. J. Biomed. Mater. Res. Part A 2019, 107, 1945–1953. [Google Scholar] [CrossRef] [PubMed]

| Paper | Biomaterial | Crosslinkers | Fabrication Method | Cell Study | In Vivo Study |
|---|---|---|---|---|---|
| Glutaraldehyde (GA) | |||||
| [57] | Gelatin | 10% GA at 4 °C for 14 h | Lyophilization | - | Pigmented rabbits |
| [58] | Collagen I + chondroitin sulphate | GA conc. (0.02, 0.04, 0.06 and 0.08%) | Air-lifted and maintained at air-liquid interfaces | Keratocytes ± corneal epithelial and endothelial cells | - |
| [67] | Collagen + poly(ethylene oxide dialdehyde) | GA | Air drying and argon plasma surface modification | Human epithelial cells | - |
| [59] | Hyaluronic acid | 100 mM GA at 25 °C for 2 days | Solution casting and air-drying | Corneal endothelial cells | - |
| [60] | Hyaluronic acid | 100 mM GA at 25 °C for 2 days | Solution casting and air-drying | - | New Zealand white rabbits |
| [41] | Gelatin | 50 mM GA at 25 °C for 80 min | Solution casting and air-drying | Rat iris pigment epithelial cells | New Zealand white rabbits |
| [61] | Collagen, copolymers of collagen and TERP | 0.22% GA at room temperature for 7 days | Air drying | - | Adult laboratory beagles |
| [62] | Amniotic membrane | 0.1% GA and hyperdried | Far infrared rays and microwaves | - | Three eyes of three patients |
| [63] | Hyaluronic acid + itaconic acid + PEGDE | GA under acidic pH | Air drying | Human corneal epithelial cell line | New Zealand white rabbits |
| [64] | Amniotic membrane (AM) | 0.05 mmol GA per mg AM | Air drying | Limbal epithelial cells | - |
| [65] | Canine AM + atelocollagen | 0.1% GA | Air drying | Canine corneal epithelial cells | - |
| [66] | Carboxymethyl chitosan + poloxamer | 1% GA for 1 h at 50 °C | Air drying | Human corneal epithelial cells | - |
| 1,4-Butanediol diglycidyl ether (BDDGE) | |||||
| [69] | Chitosan + gelatin + chondroitin sulfate | 0.5% BDDGE | Lyophilization | Human and rabbit keratocytes | - |
| [70] | Porcine collagen type I | BDDGE at pH 11 | Air drying | Human corneal epithelial and rodent DRG cell | - |
| Genipin (GP) | |||||
| [75] | Chitosan + collagen, cellulose or elastin | GP (40 µL) | Air drying | Human corneal epithelial cells | - |
| [76] | Chitosan | 0.5–5.0 mM GP | Lyophilization | Human corneal epithelial cells | - |
| [77] | Carboxymethyl chitosan + poloxamer | 02–0.8% GP | Lyophilization | - | New Zealand rabbits (ex vivo) |
| Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) & N-hydroxy-succinimide (NHS) | |||||
| [87] | Amniotic membranes (AM) | 0–0.25 mmol EDC per mg AM EDC:NHS molar ratios = 5:1 | Immersion | Limbal epithelial cells | New Zealand white rabbits |
| [41] | Gelatin | 50 mM EDC | Solution casting and air-drying | Rat iris pigment epithelial cells | New Zealand white rabbits |
| [88] | Hyaluronic acid | 10 mM EDC at 25 °C for 2 days | Lyophilization | Corneal endothelia | New Zealand white rabbits |
| [89] | Hyaluronic acid | 10 mM EDC | Lyophilization | Corneal endothelia | New Zealand white rabbits |
| [90] | Collagen I + gelatin (Col/Gel) | EDC:NHS:(Col/Gel) = 1:1:12 for 4 h | Lyophilization | Human mesenchymal stem cells | - |
| Other crosslinkers | |||||
| [91] | Type I collagen | Generation 2 polypropyleneimine octaamine dendrimers | Chemical crosslinking | Human corneal epithelial cells | - |
| [92,93] | PEG and PAAc double network hydrogel | 50% acrylic acid 1% v/v with respect to hydroxyl-2-methyl propiophenone and triethylene glycol dimethacrylate | Two-step sequential network formation technique | Primary corneal epithelial and fibroblast cells | New Zealand Red rabbits |
| [94] | Collagen coupled PEG/PAAc | 1% triethylene glycol dimethacrylate for 24 h at room temperature | UV- free radical polymerization | Rabbit corneal cell line | New Zealand Red rabbits |
| [85] | PEG-stabilized collagen + chitosan | Hybrid cross-linking system comprising of a long-range bi-functional cross-linker | Chemical crosslinking | Human corneal epithelial cells, and DRG | Yucatan porcine cornea and rat subcutaneous |
| [86] | Collagen–phosphorylcholine | PEG diacrylate initiated by ammonium persulphate or 0.5% Irgacure 2959 | Photopolymerization | Human corneal epithelial cell line and DRG | Mini-pigs and New Zealand white rabbits |
| [95] | Neoglycopolymer—recombinant collagen III | Carbohydrate-functionalized norbornenes | Tandem ring-open metathesis polymerization hydrogenation | Human corneal epithelial cells | - |
| [96] | Hydroxypropyl chitosan (HPCTS) | Sodium alginate dialdehyde (20 mg/mL) mixed equal volume with HPCTS | Self-cross-linking process of chitosan and oxidized alginate | Corneal endothelial cells | New Zealand rabbits |
| [97] | Collagen I-Immobilized PEG | 1% Triethylene glycol dimethacrylate and poly(2-hydroxyethyl methacrylate) | UV-initiated free radical polymerization | Human corneal epithelial cells | - |
| [98] | Chitosan + PEG | Diepoxy-PEG:cystamine (4:1 molar ratio) | Casting and chemical crosslinking for 24 h at 25 °C | Sheep endothelial cell | Ovine eyes (ex vivo) |
| [99] | Poly(2-hydroxyethyl methacrylate) | N, N′-methylenebis 0.5% acrylamide | Polymerization and molding processes | Rabbit corneal stromal cells | New Zealand rabbits |
| [100] | Levofloxacin loaded glycol chitosan | 4-arm polyethylene glycol with aldehyde end groups (4-arm PEG-CHO) | Chemical crosslinking | L-929 cells | - |
| [101] | GelCORE bioadhesive hydrogels | Photocrosslinking with visible light (450 to 550 nm) | Lyophilization, chemical and photo-crosslinking | Corneal fibroblast cells | New Zealand white rabbits |
| Paper | Biomaterial | Crosslinkers & Concentration | Results |
|---|---|---|---|
| [91] | Type I collagen | Generation 2 polypropyleneimine octaamine dendrimers: EDC: molar ratio 1:1 GA: 0.02%. | Dendrimer-crosslinked gel had no cellular toxicity and higher glucose permeability than natural human cornea and more transparent than GA/EDC crosslinked gels |
| [59] | Hyaluronic acid (HA) | EDC:100 mM GA:100 mM | EDC-HA was more transparent, smoother surface, faster degradation and lower toxicity than GA-HA |
| [60] | Hyaluronic acid (HA) | EDC:100 mM GA:100 mM | EDC-HA gel had no adverse inflammatory reaction GA-HA gel induced significant inflammatory cell infiltration and foreign body reaction observed |
| [41] | Gelatin | EDC:50 mM GA:50 mM | EDC-gelatin was biocompatible without causing toxicity GA-gelatin showed significant inflammatory reaction |
| [102] | Chitosan | 10 mM GA 10 mM Genipin (GP) | GP crosslinked implants were more biocompatible without providing significant intraocular inflammation |
| [103] | Recombinant human atelocollagen type III | EDC: 0.3 ME (Molar equivalent) CMC: 2.0 ME. | CMC crosslinked samples had comparable properties to EDC crosslinked hydrogels |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharjee, P.; Ahearne, M. Significance of Crosslinking Approaches in the Development of Next Generation Hydrogels for Corneal Tissue Engineering. Pharmaceutics 2021, 13, 319. https://doi.org/10.3390/pharmaceutics13030319
Bhattacharjee P, Ahearne M. Significance of Crosslinking Approaches in the Development of Next Generation Hydrogels for Corneal Tissue Engineering. Pharmaceutics. 2021; 13(3):319. https://doi.org/10.3390/pharmaceutics13030319
Chicago/Turabian StyleBhattacharjee, Promita, and Mark Ahearne. 2021. "Significance of Crosslinking Approaches in the Development of Next Generation Hydrogels for Corneal Tissue Engineering" Pharmaceutics 13, no. 3: 319. https://doi.org/10.3390/pharmaceutics13030319
APA StyleBhattacharjee, P., & Ahearne, M. (2021). Significance of Crosslinking Approaches in the Development of Next Generation Hydrogels for Corneal Tissue Engineering. Pharmaceutics, 13(3), 319. https://doi.org/10.3390/pharmaceutics13030319