Chronicles of Nanoerythrosomes: An Erythrocyte-Based Biomimetic Smart Drug Delivery System as a Therapeutic and Diagnostic Tool in Cancer Therapy
Abstract
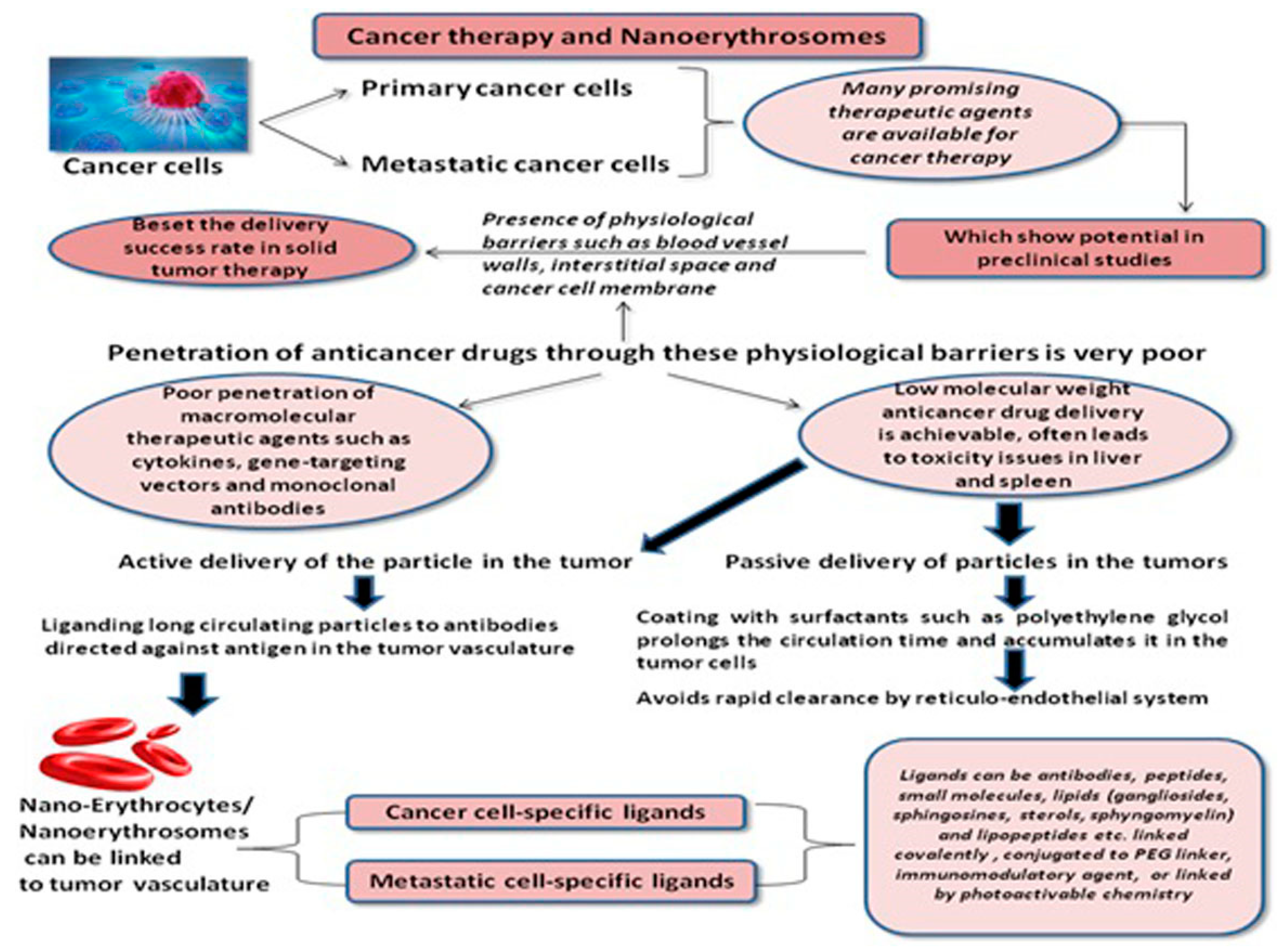
:1. Introduction
2. Fabrication of NERs
2.1. Dilutional Hemolysis and Resealing Method
2.2. Preswell Dilutional Hemolysis Method
2.3. Hypotonic Dialysis Method
2.4. Use of Red Cell Loader
2.5. Isotonic Osmotic Lysis
2.6. Membrane Perturbation Technique
2.7. Lipid Fusion Technique
3. NERs as an Efficient Drug Delivery Tool
4. Applications of NERs in Cancer Therapy and Diagnosis
4.1. NERs in Cancer Therapy
4.2. NERs in Immunotherapy
4.3. NERs in Cancer Imaging and Diagnostics
4.4. NERs in Cancer Combination Therapy
4.5. NERs in Glioma Therapy
4.6. NERs in Overcoming Drug Resistance
5. Applications of NERs in Non-Cancer Therapies
6. Biosensing Applications of RBC-Mediated Carriers Systems
7. Recent Patents on NERs for Cancer Therapy
8. Recent Clinical Trials on Anticancer Drug-Loaded NERs
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lutz, H.; Hu, S.; Dinh, P.-U.; Cheng, K. Cells and cell derivatives as drug carriers for targeted delivery. Med. Drug Discov. 2019, 3, 100014. [Google Scholar] [CrossRef]
- Han, X.; Wang, C.; Liu, Z. Red Blood Cells as Smart Delivery Systems. Bioconjugate Chem. 2018, 29, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Su, J.; Liu, G.; Chen, J.; Zhang, X.; Zhang, R.; Jiang, M.; Qiu, M. Advances of blood cell-based drug delivery systems. Eur. J. Pharm. Sci. 2017, 96, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Kharazi, A.Z.; Zargar, S.M.; Hafshejani, D.K.; Eskandarinia, A.; Rafienia, M. A review of controlled drug delivery systems based on cells and cell membranes. J. Med. Signals Sensors 2019, 9, 181–189. [Google Scholar] [CrossRef]
- Millán, C.G.; Gandarillas, C.I.C.; Marinero, M.L.S.; Lanao, J.M. Cell-based drug-delivery platforms. Ther. Deliv. 2012, 3, 25–41. [Google Scholar] [CrossRef]
- Pierige, F.; Serafini, S.; Rossi, L.; Magnani, M. Cell-based drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Tajerzadeh, H. Carrier Erythrocytes: An Overview. Drug Deliv. 2003, 10, 9–20. [Google Scholar] [CrossRef]
- Hamidi, M.; Zarrin, A.; Foroozesh, M.; Mohammadi-Samani, S. Applications of carrier erythrocytes in delivery of biophar-maceuticals. J. Control Rel. 2007, 118, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Serafini, S.; Pierigé, F.; Antonelli, A.; Cerasi, A.; Fraternale, A.; Chiarantini, L.; Magnani, M. Erythrocyte-based drug delivery. Expert Opin. Drug Deliv. 2005, 2, 311–322. [Google Scholar] [CrossRef]
- Magnani, M.; Pierigè, F.; Rossi, L. Erythrocytes as a novel delivery vehicle for biologics: From enzymes to nucleic acid-based therapeutics. Ther. Deliv. 2012, 3, 405–414. [Google Scholar] [CrossRef]
- Zarrin, A.; Foroozesh, M.; Hamidi, M. Carrier erythrocytes: Recent advances, present status, current trends and future horizons. Expert Opin. Drug Deliv. 2014, 11, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Zarei, N.; Zarrin, A.; Mohammadi-Samani, S. Preparation and in vitro characterization of carrier erythrocytes for vaccine delivery. Int. J. Pharm. 2007, 338, 70–78. [Google Scholar] [CrossRef]
- Villa, C.H.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Red blood cells: Supercarriers for drugs, biologicals, and nanopar-ticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. 2016, 106, 88–103. [Google Scholar] [CrossRef] [Green Version]
- Millán, C.G.; Marinero, M.L.S.; Castañeda, A.Z.; Lanao, J.M. Drug, enzyme and peptide delivery using erythrocytes as carriers. J. Control. Release 2004, 95, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, L.A.; Pivovarov, Y.I.; Kurilskaya, T.E.; Sergeeva, A.S. Modern state of problem of delivery of medicines with use of erythrocytes as cell-carriers. Patol. Fiziol. Eksperimental’naia Ter. 2016, 60, 88–94. [Google Scholar]
- Bourgeaux, V.; Lanao, J.M.; Bax, B.E.; Godfrin, Y. Drug-loaded erythrocytes: On the road toward marketing approval. Drug Des. Dev. Ther. 2016, 10, 665–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Verma, M.; Jha, K.K. Resealed erythrocytes as a carrier for drug targeting: A review. Pharm. Innov. 2012, 1, 8–16. [Google Scholar]
- Nangare, K.A.; Powar, S.D.; Payghan, S.A. Nanoerythrosomes: Engineered erythrocytes as a novel carrier for the targeted drug delivery. Asian J. Pharm. 2016, 10, S223–S233. [Google Scholar]
- Wadhwa, R.; Aggarwal, T.; Thapliayl, N.; Kumar, A.; Priya; Yadav, P.; Kumari, V.; Reddy, B.S.C.; Chandra, P.; Maurya, P.K. Red blood cells as an efficient in vitro model for evaluating the efficacy of metallic nanoparticles. 3 Biotech. 2019, 9, E279. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, H.-H.; Ping, Y.; Gao, J.-Q. Fabrication of Cell-Derived Biomimetic Drug Delivery System. Nanofabrication 2019, 5, 1–18. [Google Scholar] [CrossRef]
- Dale, G.; Villacorte, D.; Beutler, E. High-yield entrapment of proteins into erythrocytes. Biochem. Med. 1977, 18, 220–225. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Zhang, S.; Qin, J.; Wang, J. Cell membrane-based nanoparticles: A new biomimetic platform for tumor diagnosis and treatment. Acta Pharm. Sin. B 2018, 8, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Sushnitha, M.; Evangelopoulos, M.; Tasciotti, E.; Taraballi, F. Cell Membrane-Based Biomimetic Nanoparticles and the Immune System: Immunomodulatory Interactions to Therapeutic Applications. Front. Bioeng. Biotechnol. 2020, 8, 627. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Yu, X.; You, B.; Wu, Y.; Wang, R.; Han, L.; Wang, Y.; Gao, S.; Yuan, Y. Macrophage-cancer hybrid membrane-coated nanoparticles for targeting lung metastasis in breast cancer therapy. J. Nanobiotechnology 2020, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Wu, H.C.; Hoang, D.; Bentley, W.E.; D’Souza, W.D.; Raghavan, S.R. Colloidal properties of nanoerythrosomes derived from bovine red blood cells. Langmuir 2016, 32, 171–179. [Google Scholar] [CrossRef]
- Vijayan, V.; Uthaman, S.; Park, I.-K. Cell Membrane Coated Nanoparticles: An Emerging Biomimetic Nanoplatform for Targeted Bioimaging and Therapy. Adv. Exp. Med. Biol. 2018, 1064, 45–59. [Google Scholar] [CrossRef]
- Bhateria, M.; Rachumallu, R.; Singh, R.; Bhatta, R.S. Erythrocytes-based synthetic delivery systems: Transition from conven-tional to novel engineering strategies. Expert Opin. Drug Deliv. 2014, 11, 1219–1236. [Google Scholar] [CrossRef] [PubMed]
- Poonia, N.; Kharb, R.; Lather, V.; Pandita, D. Nanostructured lipid carriers: Versatile oral delivery vehicle. Futur. Sci. OA 2016, 2, FSO135. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Zang, Y.; Qu, J.; Tang, M.; Zhang, T. The toxicity of metallic nanoparticles on liver: The subcellular damages, mechanisms, and outcomes. Int. J. Nanomed. 2019, 14, 8787–8804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esenaliev, R.O. Radiation and Nanoparticles for Enhancement of Drug Delivery in Solid Tumors. U.S. Patent 6165440, 26 December 2000. [Google Scholar]
- Xue, X.; Liang, X.-J. Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin. J. Cancer 2012, 31, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Lejeune, A.; Moorjani, M.; Gicquaud, C.; Lacroix, J.; Poyet, P.; Gaudreault, R. Nanoerythrosome, a new derivative of eryth-rocyte ghost: Preparation and antineoplastic potential as drug carrier for daunorubicin. Anticancer Res. 1994, 14, 915–919. [Google Scholar] [PubMed]
- Moorjani, M.; Lejeune, A.; Gicquaud, C.; Lacroix, J.; Poyet, P.; Gaudreault, R.C. Nanoerythrosomes, a new derivative of erythrocyte ghost II: Identification of the mechanism of action. Anticancer. Res. 1996, 16, 2831–2836. [Google Scholar] [PubMed]
- Lejeune, A.; Poyet, P.; Gaudreault, R.C.; Gicquaud, C. Nanoerythrosomes, a new derivative of erythrocyte ghost: III. Is phagocytosis involved in the mechanism of action? Anticancer. Res. 1997, 17, 3599–3603. [Google Scholar] [PubMed]
- Désilets, J.; Lejeune, A.; Mercer, J.; Gicquaud, C. Nanoerythrosomes, a new derivative of erythrocyte ghost: IV. Fate of reinjected nanoerythrosomes. Anticancer. Res. 2001, 21, 1741–1747. [Google Scholar]
- Chambers, E.; Mitragotri, S. Prolonged circulation of large polymeric nanoparticles by non-covalent adsorption on erythrocytes. J. Control. Release 2004, 100, 111–119. [Google Scholar] [CrossRef]
- Chambers, E.; Mitragotri, S. Long circulating nanoparticles via adhesion on red blood cells: Mechanism and extended circula-tion. Exp. Biol. Med. 2007, 232, 958–966. [Google Scholar]
- Hirlekar, R.S.; Patel, P.D.; Dand, N.; Kadam, V.J. Drug Loaded Erythrocytes: As Novel Drug Delivery System. Curr. Pharm. Des. 2008, 14, 63–70. [Google Scholar] [CrossRef]
- Muzykantov, V.R. Drug delivery by red blood cells: Vascular carriers designed by mother nature. Expert Opin. Drug Deliv. 2010, 7, 403–427. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanopar-ticles as a biomimetic delivery platform. Proc. Nat. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, R.H.; Hu, C.M.J.; Chen, K.N.H.; Luk, B.T.; Carpenter, C.W.; Gao, W.; Li, S.; Zhang, D.E.; Lu, W.; Zhang, L. Li-pid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles. Nanoscale 2013, 5, 8884–8888. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Sun, X.; Cheng, L.; Yin, S.; Yang, G.; Li, Y.; Liu, Z. Multifunctional theranostic red blood cells for magnet-ic-field-enhanced in vivo combination therapy of cancer. Adv. Mater. 2014, 26, 4794–4802. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Liu, J.; Li, Y.; Wang, H.; Ge, S.; Yuan, A.; Hu, Y.; Wu, J. Oxygen self-enriched nanoparticles functionalized with erythrocyte membranes for long circulation and enhanced phototherapy. Acta Biomater. 2017, 59, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Niu, Y.; Ding, Y.; Wang, Y.; Zhao, J.; Leng, W.; Qin, L. Formulation and Drug Loading Features of Nano-Erythrocytes. Nanoscale Res. Lett. 2017, 12, 202. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Chen, J.; Wang, Y.; Ji, H.; Peng, R.; Jin, L.; Wu, W. Advances in refunctionalization of erythrocyte-based nanomedicine for enhancing cancer-targeted drug delivery. Theranostics 2019, 9, 6885–6900. [Google Scholar] [CrossRef]
- Malhotra, S.; Dumoga, S.; Sirohi, P.; Singh, N. Red Blood Cells-Derived Vesicles for Delivery of Lipophilic Drug Camptothecin. ACS Appl. Mater. Interfaces 2019, 11, 22141–22151. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Harisa, G.I.; Badran, M.M.; Alghamdi, K.M.; Kumar, A.; Salem-Bekhit, M.M.; Ahmad, S.F.; Alanazi, F.K. Nano-erythrocyte membrane-chaperoned 5-fluorouracil liposomes as biomimetic delivery platforms to target hepatocellular carcinoma cell lines. Artif. Cells, Nanomed. Biotechnol. 2019, 47, 989–996. [Google Scholar] [CrossRef]
- Han, X.; Shen, S.; Fan, Q.; Chen, G.; Archibong, E.; Dotti, G.; Liu, Z.; Gu, Z.; Wang, C. Red blood cell–derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Sci. Adv. 2019, 5, eaaw6870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deák, R.; Mihály, J.; Szigyártó, I.C.; Beke-Somfai, T.; Turiák, L.; Drahos, L.; Wacha, A.; Bóta, A.; Varga, Z. Nanoerythrosomes tailoring: Lipid induced protein scaffolding in ghost membrane derived vesicles. Mater. Sci. Eng. C 2020, 109, 110428. [Google Scholar] [CrossRef]
- Buss, N.; Yasa, O.; Alapan, Y.; Akolpoglu, M.B.; Sitti, M. Nanoerythrosome-functionalized biohybrid microswimmers. APL Bioeng. 2020, 4, 026103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Wang, D.; Song, Q.; Wu, T.; Zhuang, X.; Bao, Y.; Kong, M.; Qi, Y.; Tan, S.; Zhang, Z. Erythrocyte Membrane-Enveloped Polymeric Nanoparticles as Nanovaccine for Induction of Antitumor Immunity against Melanoma. ACS Nano 2015, 9, 6918–6933. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Lv, P.; Chen, Z.; Ni, D.; Zhang, L.; Yue, H.; Yue, Z.; Wei, W.; Ma, G. Programmed co-delivery of paclitaxel and dox-orubicin boosted by camouflaging with erythrocyte membrane. Nanoscale 2015, 7, 4020–4030. [Google Scholar] [CrossRef]
- Qi, H.; Liu, C.; Long, L.; Ren, Y.; Zhang, S.; Chang, X.; Qian, X.; Jia, H.; Zhao, J.; Sun, J.; et al. Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS Nano 2016, 10, 3323–3333. [Google Scholar] [CrossRef]
- Sun, H.; Su, J.; Meng, Q.; Yin, Q.; Chen, L.; Gu, W.; Zhang, P.; Zhang, Z.; Yu, H.; Wang, S.; et al. Cancer-Cell-Biomimetic Nanoparticles for Targeted Therapy of Homotypic Tumors. Adv. Mater. 2016, 28, 9581–9588. [Google Scholar] [CrossRef]
- Zhang, H. Erythrocytes in nanomedicine: An optimal blend of natural and synthetic materials. Biomater. Sci. 2016, 4, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.F.; Qian, H.Q.; Zhang, D.H.; Huang, Y.; Sha, H.Z.; Liu, B.R. Evaluation for preparation and anticancer efficacy in vitro of drug-loaded nanoerythrosomes. Zhongguo Zhong Yao Za Zhi 2016, 41, 2093–2097. [Google Scholar]
- Sahoo, K.; Karumuri, S.; Koralege, R.S.H.; Flynn, N.H.; Hartson, S.; Liu, J.; Ramsey, J.D.; Kalkan, A.K.; Pope, C.; Ranjan, A. Molecular and Biocompatibility Characterization of Red Blood Cell Membrane Targeted and Cell-Penetrating-Peptide-Modified Polymeric Nanoparticles. Mol. Pharm. 2017, 14, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qian, H.; Yang, M.; Li, R.; Hu, J.; Li, L.; Yu, L.; Liu, B.; Qian, X. Gambogic acid-loaded biomimetic nanoparticles in colorectal cancer treatment. Int. J. Nanomed. 2017, 12, 1593–1605. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Han, X.; Xu, L.; Gao, M.; Xu, J.; Yang, R.; Liu, Z. Surface-Engineering of Red Blood Cells as Artificial Antigen Presenting Cells Promising for Cancer Immunotherapy. Small 2017, 13, 1701864. [Google Scholar] [CrossRef]
- Rao, L.; Meng, Q.F.; Bu, L.; Cai, B.; Huang, Q.; Sun, Z.J.; Zhang, W.F.; Li, A.; Shi-Guo, S.S.; Liu, W.; et al. Erythrocyte membrane-coated upconversion nanoparticles with minimal protein adsorption for enhanced tumor imaging. ACS Appl. Mater. Interf. 2017, 9, 2159–2168. [Google Scholar] [CrossRef]
- Rao, L.; Cai, B.; Bu, L.-L.; Liao, Q.-Q.; Guo, S.-S.; Zhao, X.-Z.; Dong, W.-F.; Liu, W. Microfluidic Electroporation-Facilitated Synthesis of Erythrocyte Membrane-Coated Magnetic Nanoparticles for Enhanced Imaging-Guided Cancer Therapy. ACS Nano 2017, 11, 3496–3505. [Google Scholar] [CrossRef] [PubMed]
- Narain, A.; Asawa, S.; Chhabria, V.; Patil-Sen, Y. Cell membrane coated nanoparticles: Next-generation therapeutics. Nanomedicine 2017, 12, 2677–2692. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Luo, Q.; Li, Y.; Lin, X.; Fan, L.; Zhang, Y.; Liu, J.; Liu, X. Fabrication of Red Blood Cell-Based Multimodal Theranostic Probes for Second Near-Infrared Window Fluorescence Imaging-Guided Tumor Surgery and Photodynamic Therapy. Theranostics 2019, 9, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dong, H.; Li, M.; Cao, Y.; Yang, F.; Zhang, K.; Dai, W.; Wang, C.; Zhang, X. Erythrocyte-cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photother-mal/chemotherapy of melanoma. ACS Nano 2018, 12, 5241–5252. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Wang, F.; Fan, Z.; Zhu, Q.; Tian, H.; Zhang, Y.; Jiang, B.; Hou, Z.; Li, Y.; Su, G. Light/pH-Triggered biomimetic red blood cell membranes camouflaged small molecular drug assemblies for imaging-guided combinational chemo-photothermal therapy. ACS Appl. Mater. Interf. 2019, 11, 15262–15275. [Google Scholar] [CrossRef]
- Chen, H.; Sha, H.; Zhang, L.; Qian, H.; Chen, F.; Ding, N.; Ji, L.; Zhu, A.; Xu, Q.; Meng, F.; et al. Lipid insertion enables targeted functionalization of paclitaxel-loaded erythrocyte membrane nanosystem by tumor-penetrating bispecific recombinant protein. Int. J. Nanomed. 2018, 13, 5347–5359. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.-M.; Wu, L.; Suo, M.; Gao, S.; Xie, W.; Zan, M.-H.; Liu, A.; Chen, B.; Wu, W.-T.; Ji, L.-W.; et al. Engineered red blood cells for capturing circulating tumor cells with high performance. Nanoscale 2018, 10, 6014–6023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; He, D.; Zhou, Y.; Qin, L. Surface-modified nanoerythrocyte loading DOX for targeted liver cancer chem-otherapy. Mol. Pharm. 2018, 15, 5728–5740. [Google Scholar] [CrossRef]
- Liu, W.; Ruan, M.; Wang, Y.; Song, R.; Ji, X.; Xu, J.; Dai, J.; Xue, W. Light-triggered biomimetic nanoerythrocyte for tu-mor-targeted lung metastatic combination therapy of malignant melanoma. Small 2018, 14, E1801754. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, X.; Ruan, M.; Liu, W.; Song, R.; Dai, J.; Xue, W. Worm-Like Biomimetic Nanoerythrocyte Carrying siRNA for Melanoma Gene Therapy. Small 2018, 14, e1803002. [Google Scholar] [CrossRef]
- Villa, C.H.; Pan, D.C.; Zaitsev, S.; Cines, D.B.; Siegel, D.L.; Muzykantov, V.R. Delivery of drugs bound to erythrocytes: New avenues for an old intravascular carrier. Ther. Deliv. 2015, 6, 795–826. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, C.; Gao, M.; Hu, A.; Liu, Z. Remotely Controlled Red Blood Cell Carriers for Cancer Targeting and Near-Infrared Light-Triggered Drug Release in Combined Photothermal-Chemotherapy. Adv. Funct. Mater. 2015, 25, 2386–2394. [Google Scholar] [CrossRef]
- Luk, B.T.; Fang, R.H.; Hu, C.-M.J.; Copp, J.A.; Thamphiwatana, S.; Dehaini, D.; Gao, W.; Zhang, K.; Li, S.; Zhang, L. Safe and Immunocompatible Nanocarriers Cloaked in RBC Membranes for Drug Delivery to Treat Solid Tumors. Theranostics 2016, 6, 1004–1011. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Ye, X.; Wang, C.; Xing, C.; Miao, Q.; Xie, Z.; Chen, X.; Zhang, X.; Zhang, H.; Mei, L. Photothermal cancer immu-notherapy by erythrocyte membrane-coated black phosphorus formulation. J. Control Rel. 2019, 296, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, X.; Huang, R.; Jiang, J.; Wang, Y.; Zhang, J.; Jiang, H.; Xiang, X.; Chen, W.; Nie, X.; et al. Erythrocyte membrane camouflaged graphene oxide for tumor-targeted photothermal-chemotherapy. Carbon 2019, 146, 660–670. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, Y.; Guo, R.; Yao, X.; Sung, S.; Pang, Z.; Yang, W. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials 2019, 192, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Liang, M.; Wang, Y.; Cui, L.; Gao, C.; Chu, X.; Liu, Q.; Feng, Y.; Gong, W.; Yang, M.; et al. Dual-Modified Novel Biomimetic Nanocarriers Improve Targeting and Therapeutic Efficacy in Glioma. ACS Appl. Mater. Interfaces 2018, 11, 1841–1854. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Ran, D.; Lu, L.; Zhan, C.; Ruan, H.; Hu, X.; Xie, C.; Jiang, K.; Li, J.; Zhou, J.; et al. Ligand-Modified Cell Membrane Enables the Targeted Delivery of Drug Nanocrystals to Glioma. ACS Nano 2019, 13, 5591–5601. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Shipunova, V.O.; Babenyshev, A.V.; Balalaeva, I.V.; Nikitin, P.I.; Deyev, S.M.; Nikitin, M.P. Nanoparticle-based drug delivery via RBC-hitchhiking for the inhibition of lung metastases growth. Nanoscale 2019, 11, 1636–1646. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, J.; Pan, H.; Shi, J.; Wang, J.; Chen, L. Preparation and evaluation of long circulating erythrocyte mem-brane-cloaked anti-cancer drug delivery system. Drug Deliv. Transl. Res. 2020, 10, 1278–1287. [Google Scholar] [CrossRef]
- Zhong, P.; Chen, X.; Guo, R.; Chen, X.; Chen, Z.; Wei, C.; Li, Y.; Wang, W.; Zhou, Y.; Qin, L. Folic acid-modified nanoeryth-rocyte for codelivery of paclitaxel and tariquidar to overcome breast cancer multidrug resistance. Mol. Pharm. 2020, 17, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Sun, J.; Hao, W.; Chen, M.; Wang, Y.; Xu, F.; Gao, C. Dual-Target Peptide-Modified Erythrocyte Membrane-Enveloped PLGA Nanoparticles for the Treatment of Glioma. Front. Oncol. 2020, 10, 563938. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Harisa, G.I.; Alomrani, A.H.; Alanazi, F.K.; Badran, M.M. Improved pharmacokinetic and biodistribution of 5-fluorouracil loaded biomimetic nanoerythrocytes decorated nanocarriers for liver cancer treatment. Colloids Surf. B: Biointerfaces 2021, 197, 111380. [Google Scholar] [CrossRef]
- Fornasier, M.; Porcheddu, A.; Casu, A.; Raghavan, S.R.; Jönsson, P.; Schillén, K.; Murgia, S. Surface-modified nanoerythro-somes for potential optical imaging diagnostics. J. Coll. Interf. Sci. 2021, 582, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Xu, J.-H.; Cai, B.; Liu, H.; Li, M.; Jia, Y.; Xiao, L.; Guo, S.-S.; Liu, W.; Zhao, X.-Z. Synthetic nanoparticles camouflaged with biomimetic erythrocyte membranes for reduced reticuloendothelial system uptake. Nanotechnology 2016, 27, 085106. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, F.; Gui, L.; He, Q.; Yao, Y.; Chen, H. The potential of biomimetic nanoparticles for tumor-targeted drug delivery. Nanomedicine 2018, 13, 2099–2118. [Google Scholar] [CrossRef]
- Li, H.; Jin, K.; Luo, M.; Wang, X.; Zhu, X.; Liu, X.; Jiang, T.; Zhang, Q.; Wang, S.; Pang, Z. Size Dependency of Circulation and Biodistribution of Biomimetic Nanoparticles: Red Blood Cell Membrane-Coated Nanoparticles. Cells 2019, 8, 881. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, J.; Jain, N.K. Biodegradable long circulating cellular carrier for antimalarial drug pyrimethamine. Artif. Cells Nanomed. Biotechnol. 2013, 41, 309–314. [Google Scholar] [CrossRef]
- Agnihotri, J.; Saraf, S.; Singh, S.; Bigoniya, P. Development and evaluation of anti-malarial bio-conjugates: Artesunate-loaded nanoerythrosomes. Drug Deliv. Transl. Res. 2015, 5, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, K.; Qin, X.; Li, T.; Qiu, J.; Yin, T.; Huang, J.; McGinty, S.; Pontrelli, G.; Ren, J.; et al. Biomimetic Nanotherapies: Red Blood Cell Based Core–Shell Structured Nanocomplexes for Atherosclerosis Management. Adv. Sci. 2019, 6, 1900172. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Patel, B.; Nahar, K.; Ahsan, F. Cell permeable peptide conjugated nanoerythrosomes of fasudil prolong pulmonary arterial vasodilation in PAH rats. Eur. J. Pharm. Biopharm. 2014, 88, 1046–1055. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Patel, B.; Ahsan, F. Nano-Engineered Erythrocyte Ghosts as Inhalational Carriers for Delivery of Fasudil: Preparation and Characterization. Pharm. Res. 2014, 31, 1553–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahid, J.; Nahar, K.; Raut, S.; Keshavarz, A.; Ahsan, F. Fasudil and DETA NANOate, loaded in peptide-modified liposomal carrier, slow PAH progression upon pulmonary delivery. Mol. Pharm. 2018, 15, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Koleva, L.; Bovt, E.; Ataullakhanov, F.; Sinauridze, E. Erythrocytes as Carriers: From Drug Delivery to Biosensors. Pharmaceutics 2020, 12, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, R.; Avsievich, T.; Popov, A.; Bykov, A.; Meglinski, I. In vivo nano-biosensing element of red blood cell-mediated delivery. Biosens. Bioelectron. 2021, 175, 112845. [Google Scholar] [CrossRef]
- López, S.C.B.; Meissner, K.E. Characterization of carrier erythrocytes for biosensing applications. J. Biomed. Opt. 2017, 22, 91510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.W.; Yoo, J.Y. Three-dimensional focusing of red blood cells in microchannel flows for bio-sensing applications. Biosens. Bioelectron. 2009, 24, 3677–3682. [Google Scholar] [CrossRef]
- Milanick, M.A.; Ritter, S.; Meissner, K. Engineering erythrocytes to be erythrosensors: First steps. Blood Cells Mol. Dis. 2011, 47, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ritter, S.C.; Milanick, M.A.; Meissner, K.E. Encapsulation of FITC to monitor extracellular pH: A step towards the development of red blood cells as circulating blood analyte biosensors. Biomed. Opt. Express 2011, 2, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, Y.; Liu, J.; Yang, X.; Huang, J.; Li, L.; Wan, L.; Wang, K. Red blood cell membrane-mediated fusion of hy-drophobic quantum dots with living cell membranes for cell imaging. J. Mater. Chem. B. 2016, 4, 4191–4197. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, R.C.; Claude, G.; Patrick, P. Nanoerythrocytes Bioactive Agent Carrier. U.S. Patent 5653999, 5 August 1997. [Google Scholar]
- Joyce, T. Polyethyleneglycol Conjugated Nanoerythrosomes, Method of Making Same and Use Thereof. Patent No. WO1998011919A3, 4 June 1998. [Google Scholar]
- Muzykantov, V.; Brener, J.; Myerson, J. Methods and Compositions for Drug Delivery. U.S. Patent 20180243440, 30 August 2018. [Google Scholar]
- Hyde, R.A.; Ishikawa, M.Y.; Jung, E.K.Y.; William, G.; Langer, A.A.L.; Levien, E.C.; Tegreene, R.A.; Weaver, C.T.; Whitmer, T.A.; Wood, C., Jr.; et al. Biological Targeting Compositions and Methods of Using the Same. U.S. Patent 8211656, 7 March 2012. [Google Scholar]
- Dmitri, S.; Guixin, S. Use of Human Erythrocytes for Prevention and Treatment of Cancer Dissemination and Growth. U.S. Patent 20130202625, 8 August 2013. [Google Scholar]
- Ahn, B.C.; Son, S.H.; Prakash, G. Composition for Material Delivery, Including Exosome Mimetics Derived from red Blood Cells, and Use Thereof. U.S. Patent 20200138987, 7 May 2020. [Google Scholar]
- Green, J.J.; Meyer, R.A.; Ben-Akiva, E. Biomimetic Anisotropic Polymeric Particles with Naturally Derived Cell Membranes for enhanced DRUG Delivery. U.S. Patent 20200289666, 17 September 2020. [Google Scholar]
- Lee, T. Use of Nanoparticles Coated with red Blood Cell Membranes to Enable Blood Transfusion. U.S. Patent 20170367990, 8 October 2017. [Google Scholar]
- Lee, J.S.; Corcoran, T.E.; Kagan, V. Red Blood Cell Membrane-Derived Microparticles and Their Use for the Treatment of Lung Disease. U.S. Patent 10004764, 27 September 2018. [Google Scholar]
- Lee, T. Use of Nanoparticles Coated with Red Blood Cell Membranes to Treat Hemolytic Diseases and Disorders. U.S. Patent 20170095510, 6 April 2017. [Google Scholar]
- Rossi, L.; Pierigè, F.; Aliano, M.P.; Magnani, M. Ongoing Developments and Clinical Progress in Drug-Loaded Red Blood Cell Technologies. BioDrugs 2020, 34, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efficacy and Safety of L-Asparaginase Encapsulated in RBC Combined with Gemcitabine or FOLFOX in 2nd Line for Progressive Metastatic Pancreatic Carcinoma. NCT02195180. Available online: https://clinicaltrials.gov/ct2/show/NCT02195180 (accessed on 20 January 2021).
- Hammel, P.; Fabienne, P.; Mineur, L.; Metges, J.-P.; Andre, T.; De La Fouchardiere, C.; Louvet, C.; El Hajbi, F.; Faroux, R.; Guimbaud, R.; et al. Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: An open-label, randomized Phase IIb trial. Eur. J. Cancer 2020, 124, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Study of Eryaspase in Combination with Chemotherapy Versus Chemotherapy Alone as 2nd-line Treatment in PAC (Trybeca-1). NCT03665441. Available online: https://clinicaltrials.gov/ct2/show/NCT03665441 (accessed on 20 January 2021).
- Study of Eryaspase in Combination with Chemotherapy Versus Chemotherapy Alone as 1st Line Treatment of TNBC (TRYbeCA-2). NCT03674242. Available online: https://clinicaltrials.gov/ct2/show/NCT03674242 (accessed on 20 January 2021).
- Asparaginase Encapsulated in Erythrocytes for Patients with All and Hypersensitivity to PEG-Asparaginase. NCT03267030. Available online: https://clinicaltrials.gov/ct2/show/NCT03267030 (accessed on 20 January 2021).



| Name of The Anticancer Drug/Agents | Type of RBC-Based Nanoformulation | Type of Surface Modification/Functionalization/Ligands | Reported Applications | Ref. |
|---|---|---|---|---|
| DAU | NERs | DAU was covalently linked by glutraldehyde to the NERs | CDF1 leukemia tumor | [32] |
| DOX | RBC-Iron oxide NPs | Pre-coated with chlorine6 | For imaging-guided combined photodynamic and chemotherapy of cancers | [42] |
| ICG and Perfluorocarbon (PFC) | RBC membrane cloaked albumin NPs | -- | Ideal for clinical cancer phototherapy treatment | [43] |
| Sodium Transhione II A sulphonate | Drug loaded nano-RBCs | -- | Nanosystem was better than conventional injection in-vivo | [44] |
| Campothecin | RBC-membrane loaded nanovesicles | Labelled non-covalently with amphiphilic fluorophore | -- | [46] |
| 5-FU | Biomimetic nanoerythrocyte- membrane–chaperoned liposomes | -- | Hepatocellular carcinoma | [47] |
| Engineered E. coli sp. | Biohybrid microswimmers (RBC-NERs) | Conjugation of streptavidin-modified bacteria with biotin-modified-NERs using non-covalent streptavidin interaction | Targeted cargo delivery system | [50] |
| Antigenic peptide Hgp 10025-33 | Erythrocyte membrane enveloped PLGA-NPs | Mannose-inserted membrane structure was constructed to actively target antigen presenting cells in lymphatic organs | Cancer nanovaccine in cancer immunotherapy | [51] |
| Co-delivery of PAX and DOX | Magnetic O-Carboxy methyl chitosan NPs | Camouflaged with an Arg-Gly-Asp anchored ER-membrane | Better tumor uptake | [52] |
| PAX | Biomimetic polymeric NPs | -- | 4T1- breast cancer cell membrane | [54] |
| Curcumin (CUR) | NERs | -- | Enhanced antitumor activity | [56] |
| Gambogic acid | Biomimetic RBC-membrane coated PLGA NPs | -- | Colorectal cancer | [58] |
| FA | Upconversion NP coated with RBC-membranes | Surface-modified with ligands for active targeting of cancer cells | For in-vivo cancer imaging | [60] |
| DOX | Drug loaded RBC-membrane coated copper sulphide NPs | -- | 100% melanoma tumor growth inhibition rate | [64] |
| 10-Hydroxy Campothecin and ICG | Biomimetic RBC membrane nanovesicles | -- | Synergistic chemo-photothermal therapy | [65] |
| PAX | Encapsulated in human erythrocyte membrane | A phospholipid derivative was used for tumor targeting into ER-membrane derived nanovesicles | Gastric cancer | [66] |
| FA | Magnetic NPs coated on the surface of RBCs | Chemical conjugation and hydrophobic interactions between RBC-circulating tumor cells | Enhanced tumor targeting ability | [67] |
| DOX | NERs | Surface modified by FA and PEG | Enhanced tumor targeting ability in vivo in liver cancer | [68] |
| siRNA | Nanoworms, biomimetic NERs | - | Efficient siRNA therapy in vivo | [70] |
| DOX | Coencapsulated inside RBCs | Albumin bound NIR dye | Combinational photothermal and chemotherapy of cancer | [72] |
| DOX | RBC-cloaked membrane | -- | For the treatment of solid tumors | [73] |
| DOX | NPs of graphene oxide-DOX-RBC-membrane- ICG as photosensitizer | FA | Had excellent ability to evade RES | [75] |
| Vincristine | RBC-membrane coated solid lipid NPs | T7 and NGR peptide | Brain delivery for treatment of gliomas | [78] |
| Codelivery of PAX and Tariquidar | Nano-erythrocytes (NEs) | FA modified NEs | Breast cancer management | [81] |
| Euphorbia factor L1 | PLGA-NPs coated with ER-membrane | Dual–modified peptide ligands | Brain delivery for gliomas | [82] |
| 5-FU | FU –loaded chitosan-coated-PLGA –NPs-NE-chitosomes | -- | Liver targeting | [83] |
| Patent Number | Invention Title | Description of The Invention | Pharmaceutical Advantages | Ref. |
|---|---|---|---|---|
| US5653999 | NERs as bioactive carrier | A complex comprising of a bioactive agent coupled to vesicles derived from ERs. Prepared ERs had size less than 1 µm and substantially free of hemoglobin. | DAU–NERs conjugate had a higher antineoplastic activity than the free bioactive agent. | [102] |
| WO1998011919A3 | Polyethylene glycol conjugated NERs, method of making same and use thereof. | Long circulating NERs avoid rapid clearance by RES. | Prolonged circulation time. | [103] |
| US20040180094A1 | Activation agents on the surface of encapsulation vesicles | Target ligands can be synthetic, semi-synthetic and naturally occurring such as antibiotics, hormones, lectins, glycoproteins, peptides, amino acids, polypeptides, sugars, saccharides, carbohydrates, cofactors, bioactive agents, and genetic materials such as nucleotides and nucleosides, etc. | The present invention addressed drug resistance problems in vivo by attaching optimal target ligand to encapsulation vesicles. | [104] |
| US8211656 | Biological targeting composition and methods of using the same. | Targeted delivery of imaging agents, drugs, peptides, proteins, and pharmaceuticals using modified RBCs is described here. | Modified RBCs can carry a variety of therapeutic moieties for the treatment of various ailments including cancer. | [105] |
| US20130202625 | Use of human erythrocytes for prevention and treatment of cancer dissemination and growth. | Cancer metastasis specially breast cancer metastasis can be prevented by blocking the circulation of metastatic cells and by blocking angiogenesis such as capturing endothelial progenitors that are recruited to the tumor, or by physically blocking of the capillaries of the tumor or the metastasis. | RBCs have potential for use as therapeutics as they are easily retrieved from a patient, non-immunogenic, and are biologically designed to navigate the microcirculation including tortuous tumor vasculature. | [106] |
| US20200138987 | Composition for material delivery including exosome mimetics derived from RBCs, and use thereof. | Exosome mimetics derived from RBCs are used for material delivery such as drug, radioactive material and fluorescent materials, etc. | Exosomes (small vesicles, 30nm-100nm) have drawn attention as new drug delivery carrier system for targeted delivery to a specific organ and can be used as imaging tools. | [107] |
| US20200289666 | Biomimetic anisotropic polymeric particles with naturally derived cell membranes for enhanced drug delivery. | Biomimetic particles can be used in the treatment of excessive bleeding, thalassemia, thrombopenia, cancer, infectious diseases, etc. | Particles comprised of polymeric core of defined shape, size, surface from naturally derived cell membranes such as RBCs, have application in drug delivery and cell engineering. | [108] |
| US20170367990 | Use of NPs coated with RBC membranes to enable blood transfusion. | An inner core of nanoparticle comprised of non-cellular compound and an outer surface comprise of cellular membrane derived from RBCs. | Suitable in blood transfusion for giving a blood-source with a mismatched type of blood, or potentially a mismatched blood type to a recipient. | [109] |
| US10596197 | Red blood cell membrane derived microparticles and their use for the treatment of lung diseases. | Treatment with RBCs-MPs to the lung through inhalational route promoted the expression of immune regulatory cytokines including IL-10 and reduced inflammatory responses and injury to the lungs. | Have remarkable potential as immuno-modulating agent for a number of lung disorders such as chronic-obstructive pulmonary disorder (COPD), bronchitis, acute lung injury, pulmonary fibrosis, etc. | [110] |
| US20170095510 | Use of NPs coated with red blood cell membranes to treat hemolytic diseases and disorders. | Hemolytic diseases are auto-immune disorders caused by an attack of said mammal RBCs by said mammal’s own body or in between pregnant mammal and fetus RBCs. | Invention will be employed in nano-engineering, molecular biology, etc. | [111] |
| Clinicaltrial.gov Identifier | NCT03674242 | NCT03665441 | NCT02195180 | NCT03267030 |
|---|---|---|---|---|
| Drug encapsulated in erythrocyte | Asparaginase encapsulated in erythrocytes (Eryaspase) | |||
| Eryaspase combined with other anti-cancer drugs | Eryaspase combined with gemcitabine or carboplatin | Eryaspase combined with either gemcitabine plus abraxane, or irinotecan-based therapy | Eryaspase combined with gemcitabine or 5-fluoro-uracil/oxaliplatin/leucovorin (FOLFOX) | Eryaspase combined with GRASPA |
| Purpose | Treatment | Treatment | Treatment | Treatment |
| Cancer type | Triple negative breast cancer | Pancreatic adenocarcinoma | Progressive metastatic pancreatic carcinoma | Acute lymphoblastic leukemia |
| Recruitment status | Recruiting | Active, not recruiting | Completed | Completed |
| Sponsor | ERYtech Pharma | ERYtech Pharma | ERYtech Pharma | Birgitte Klug Albertsen |
| Study-type | Interventional | Interventional | Interventional | Interventional |
| No of participants | 64 | 500 | 141 | 55 |
| Allocation | Randomized | Randomized | Randomized | N/A |
| Intervention model | Parallel assignment | Parallel assignment | Parallel assignment | Single group assignment |
| Masking | Open label | Open label | Open label | Open label |
| Phase | Phase 2/3 | Phase 3 | Phase 2 | Phase 2 |
| Start of the study | June 2019 | September 2018 | July 2014 | August 2017 |
| Completion of the study | October 2020 | October 2021 | November 2017 | October 2020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, S.; Alshehri, S.; Shoaib, A.; Ahsan, W.; Sultan, M.H.; Alqahtani, S.S.; Kazi, M.; Shakeel, F. Chronicles of Nanoerythrosomes: An Erythrocyte-Based Biomimetic Smart Drug Delivery System as a Therapeutic and Diagnostic Tool in Cancer Therapy. Pharmaceutics 2021, 13, 368. https://doi.org/10.3390/pharmaceutics13030368
Javed S, Alshehri S, Shoaib A, Ahsan W, Sultan MH, Alqahtani SS, Kazi M, Shakeel F. Chronicles of Nanoerythrosomes: An Erythrocyte-Based Biomimetic Smart Drug Delivery System as a Therapeutic and Diagnostic Tool in Cancer Therapy. Pharmaceutics. 2021; 13(3):368. https://doi.org/10.3390/pharmaceutics13030368
Chicago/Turabian StyleJaved, Shamama, Sultan Alshehri, Ambreen Shoaib, Waquar Ahsan, Muhammad Hadi Sultan, Saad Saeed Alqahtani, Mohsin Kazi, and Faiyaz Shakeel. 2021. "Chronicles of Nanoerythrosomes: An Erythrocyte-Based Biomimetic Smart Drug Delivery System as a Therapeutic and Diagnostic Tool in Cancer Therapy" Pharmaceutics 13, no. 3: 368. https://doi.org/10.3390/pharmaceutics13030368
APA StyleJaved, S., Alshehri, S., Shoaib, A., Ahsan, W., Sultan, M. H., Alqahtani, S. S., Kazi, M., & Shakeel, F. (2021). Chronicles of Nanoerythrosomes: An Erythrocyte-Based Biomimetic Smart Drug Delivery System as a Therapeutic and Diagnostic Tool in Cancer Therapy. Pharmaceutics, 13(3), 368. https://doi.org/10.3390/pharmaceutics13030368










