DNA Double-Strand Breaks Affect Chromosomal Rearrangements during Methotrexate-Mediated Gene Amplification in Chinese Hamster Ovary Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. G-Banding and Karyotype Analysis
2.3. Immunocytochemistry of Phosphorylated Histone 2A Variant X (γH2AX)
3. Results
3.1. Bleomycin-Mediated DSBs Lead to Substantially Increased Chromosomal Rearrangements
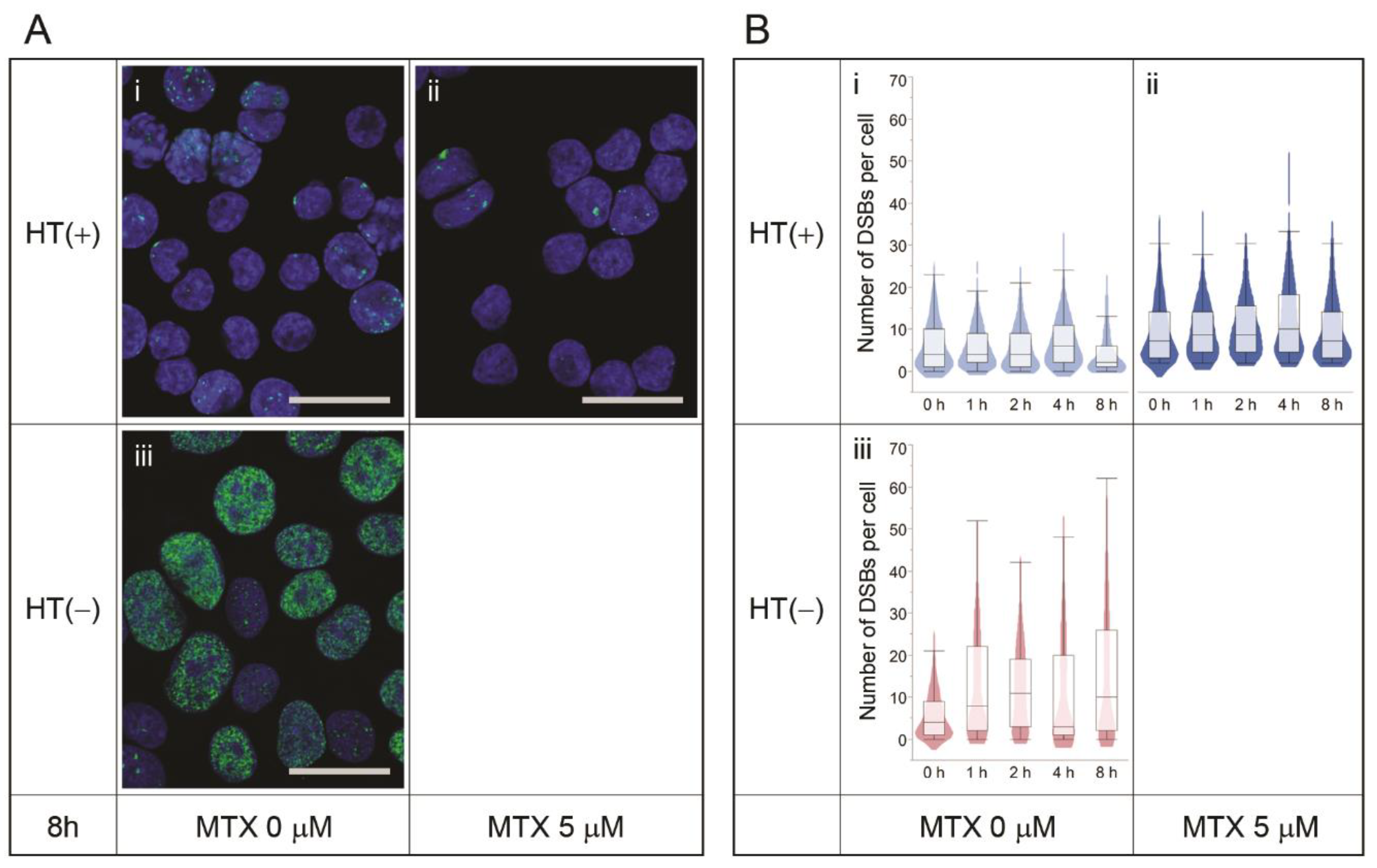
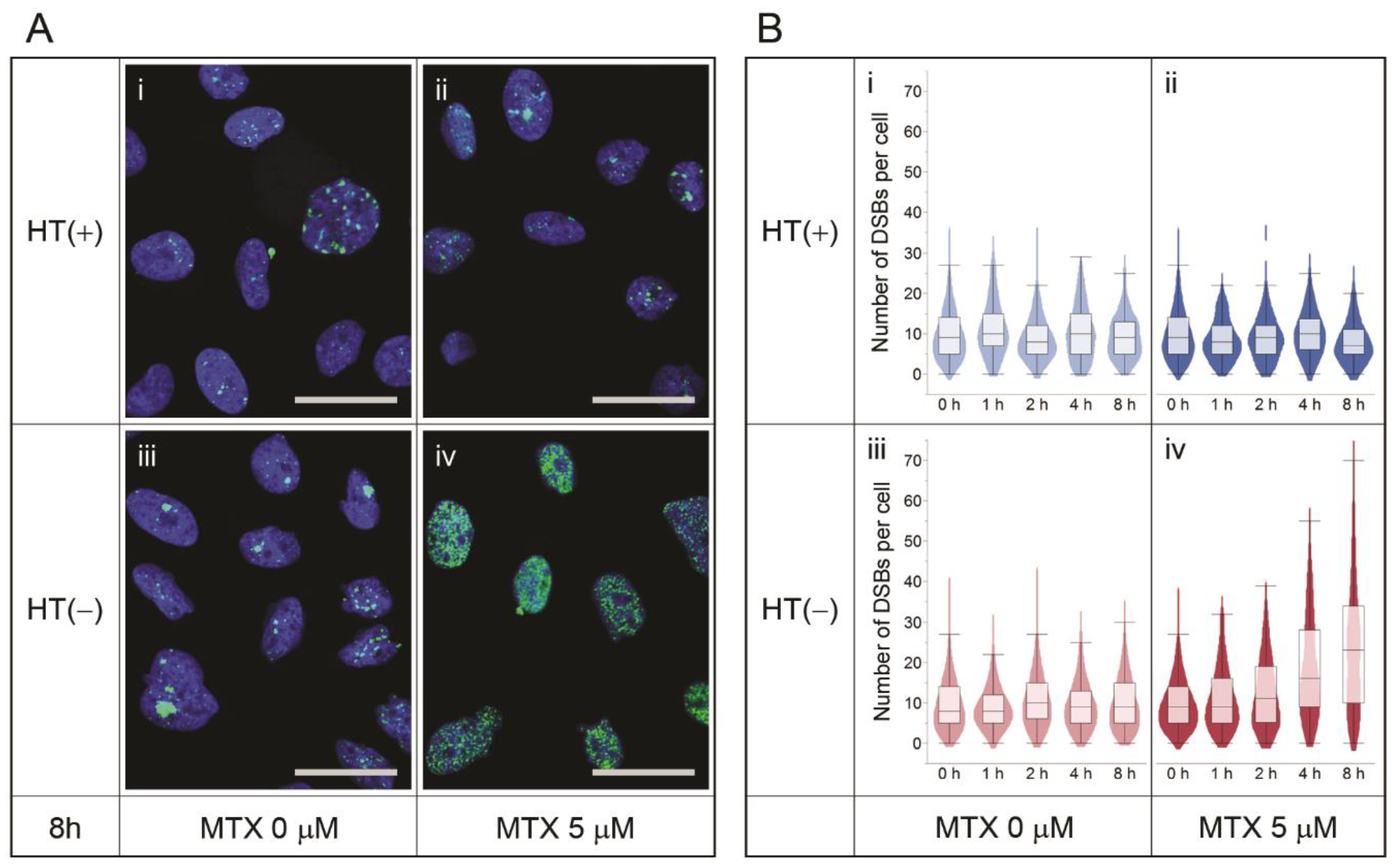
3.2. MTX Treatment Does Not Induce DSBs Per Se
3.3. Nucleotide Shortage Induces DSB Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noh, S.M.; Sathyamurthy, M.; Lee, G.M. Development of Recombinant Chinese Hamster Ovary Cell Lines for Therapeutic Protein Production. Curr. Opin. Chem. Eng. 2013, 2, 391–397. [Google Scholar] [CrossRef]
- Hamaker, N.K.; Lee, K.H. Site-Specific Integration Ushers in a New Era of Precise CHO Cell Line Engineering. Curr. Opin. Chem. Eng. 2018, 22, 152–160. [Google Scholar] [CrossRef]
- Cayley, P.J.; Dunn, S.M.; King, R.W. Kinetics of Substrate, Coenzyme, and Inhibitor Binding to Escherichia Coli Dihydrofolate Reductase. Biochemistry 1981, 20, 874–879. [Google Scholar] [CrossRef]
- Rajagopalan, P.R.; Zhang, Z.; McCourt, L.; Dwyer, M.; Benkovic, S.J.; Hammes, G.G. Interaction of Dihydrofolate Reductase with Methotrexate: Ensemble and Single-Molecule Kinetics. Proc. Ntal. Acad. Sci. USA 2002, 99, 13481–13486. [Google Scholar] [CrossRef] [Green Version]
- Flintoff, W.F.; Weber, M.K.; Nagainis, C.R.; Essani, A.K.; Robertson, D.; Salser, W. Overproduction of Dihydrofolate Reductase and Gene Amplification in Methotrexate-Resistant Chinese Hamster Ovary Cells. Mol. Cell. Biol. 1982, 2, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Kingston, R.E.; Kaufman, R.J.; Bebbington, C.R.; Rolfe, M.R. Amplification using CHO Cell Expression Vectors. Curr. Protoc. Mol. Biol. 2002, 60, 16.23.1–16.23.13. [Google Scholar] [CrossRef]
- Vishwanathan, N.; Le, H.; Jacob, N.M.; Tsao, Y.; Ng, S.; Loo, B.; Liu, Z.; Kantardjieff, A.; Hu, W. Transcriptome Dynamics of Transgene Amplification in Chinese Hamster Ovary Cells. Biotechnol. Bioeng. 2014, 111, 518–528. [Google Scholar] [CrossRef]
- Weidle, U.H.; Buckel, P.; Wienberg, J. Amplified Expression Constructs for Human Tissue-Type Plasminogen Activator in Chinese Hamster Ovary Cells: Instability in the Absence of Selective Pressure. Gene 1988, 66, 193–203. [Google Scholar] [CrossRef]
- Trask, B.J.; Hamlin, J.L. Early Dihydrofolate Reductase Gene Amplification Events in CHO Cells Usually Occur on the Same Chromosome Arm as the Original Locus. Genes Dev. 1989, 3, 1913–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamlin, J.L. Amplification of the Dihydrofolate Reductase Gene in Methotrexate-Resistant Chinese Hamster Cells. Mutat. Res. 1992, 276, 179–187. [Google Scholar] [CrossRef]
- Ma, C.; Martin, S.; Trask, B.; Hamlin, J.L. Sister Chromatid Fusion Initiates Amplification of the Dihydrofolate Reductase Gene in Chinese Hamster Cells. Genes Dev. 1993, 7, 605–620. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Takagi, Y.; Yamatani, M.; Honda, K.; Asakawa, S.; Shimizu, N.; Omasa, T.; Ohtake, H. Identification and Analysis of Specific Chromosomal Region Adjacent to Exogenous Dhfr-Amplified Region in Chinese Hamster Ovary Cell Genome. J. Biosci. Bioeng. 2010, 109, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Flintoff, W.F.; Livingston, E.; Duff, C.; Worton, R.G. Moderate-Level Gene Amplification in Methotrexate-Resistant Chinese Hamster Ovary Cells is Accompanied by Chromosomal Translocations at Or Near the Site of the Amplified DHFR Gene. Mol. Cell. Biol. 1984, 4, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Lee, G.M. Cytogenetic Analysis of Chimeric Antibody-producing CHO Cells in the Course of Dihydrofolate Reductase-mediated Gene Amplification and their Stability in the Absence of Selective Pressure. Biotechnol. Bioeng. 1999, 64, 741–749. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Nakanishi, F.; Ogura, Y.; Oi, D.; Omasa, T.; Katakura, Y.; Kishimoto, M.; Suga, K. Amplified Gene Location in Chromosomal DNA Affected Recombinant Protein Production and Stability of Amplified Genes. Biotechnol. Prog. 2000, 16, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.A.; O’Brien, S.A.; Zhao, L.; Fu, H.; Vishwanathan, N.; Hu, W. Recurring Genomic Structural Variation Leads to Clonal Instability and Loss of Productivity. Biotechnol. Bioeng. 2019, 116, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Derouazi, M.; Martinet, D.; Schmutz, N.B.; Flaction, R.; Wicht, M.; Bertschinger, M.; Hacker, D.L.; Beckmann, J.S.; Wurm, F.M. Genetic Characterization of CHO Production Host DG44 and Derivative Recombinant Cell Lines. Biochem. Biophys. Res. Commun. 2006, 340, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.Y.; Lee, K.H. A Framework to Quantify Karyotype Variation Associated with CHO Cell Line Instability at a Single-cell Level. Biotechnol. Bioeng. 2017, 114, 1045–1053. [Google Scholar] [CrossRef]
- Baik, J.Y.; Lee, K.H. Growth Rate Changes in CHO Host Cells Are Associated with Karyotypic Heterogeneity. Biotechnol. J. 2018, 13, e1700230. [Google Scholar] [CrossRef]
- Grav, L.M.; Sergeeva, D.; Lee, J.S.; Marin de Mas, I.; Lewis, N.E.; Andersen, M.R.; Nielsen, L.K.; Lee, G.M.; Kildegaard, H.F. Minimizing Clonal Variation during Mammalian Cell Line Engineering for Improved Systems Biology Data Generation. ACS Synth. Biol. 2018, 7, 2148–2159. [Google Scholar] [CrossRef]
- Vcelar, S.; Melcher, M.; Auer, N.; Hrdina, A.; Puklowski, A.; Leisch, F.; Jadhav, V.; Wenger, T.; Baumann, M.; Borth, N. Changes in Chromosome Counts and Patterns in CHO Cell Lines upon Generation of Recombinant Cell Lines and Subcloning. Biotechnol. J. 2018, 13, 1700495. [Google Scholar] [CrossRef]
- Dahodwala, H.; Lee, K.H. The Fickle CHO: A Review of the Causes, Implications, and Potential Alleviation of the CHO Cell Line Instability Problem. Curr. Opin. Biotechnol. 2019, 60, 128–137. [Google Scholar] [CrossRef]
- Fann, C.H.; Guirgis, F.; Chen, G.; Lao, M.S.; Piret, J.M. Limitations to the Amplification and Stability of Human Tissue-type Plasminogen Activator Expression by Chinese Hamster Ovary Cells. Biotechnol. Bioeng. 2000, 69, 204–212. [Google Scholar] [CrossRef]
- Kim, M.; O’Callaghan, P.M.; Droms, K.A.; James, D.C. A Mechanistic Understanding of Production Instability in CHO Cell Lines Expressing Recombinant Monoclonal Antibodies. Biotechnol. Bioeng. 2011, 108, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, N.S.; Ryu, C.J.; Hong, H.J.; Lee, G.M. Characterization of Chimeric Antibody Producing CHO Cells in the Course of Dihydrofolate Reductase-mediated Gene Amplification and their Stability in the Absence of Selective Pressure. Biotechnol. Bioeng. 1998, 58, 73–84. [Google Scholar] [CrossRef]
- Pallavicini, M.G.; DeTeresa, P.S.; Rosette, C.; Gray, J.W.; Wurm, F.M. Effects of Methotrexate on Transfected DNA Stability in Mammalian Cells. Mol. Cell. Biol. 1990, 10, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, K.K.; Jackson, S.P. DNA Double-Strand Breaks: Signaling, Repair and the Cancer Connection. Nat. Genet. 2001, 27, 247–254. [Google Scholar] [CrossRef]
- Morgan, W.F.; Corcoran, J.; Hartmann, A.; Kaplan, M.I.; Limoli, C.L.; Ponnaiya, B. DNA Double-Strand Breaks, Chromosomal Rearrangements, and Genomic Instability. Mutat. Res 1998, 404, 125–128. [Google Scholar] [CrossRef]
- Tounekti, O.; Pron, G.; Belehradek, J.; Mir, L.M. Bleomycin, an Apoptosis-Mimetic Drug that Induces Two Types of Cell Death Depending on the Number of Molecules Internalized. Cancer Res. 1993, 53, 5462–5469. [Google Scholar] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Natarajan, A.T. Analysis of bleomycin-induced chromosomal aberrations in Chinese hamster primary embryonic cells by FISH using arm-specific painting probes. Mutagenesis 1999, 14, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newell, A.H.; Hemphill, A.; Akkari, Y.M.; Hejna, J.; Moses, R.E.; Olson, S.B. Loss of Homologous Recombination Or Non-Homologous End-Joining Leads to Radial Formation Following DNA Interstrand Crosslink Damage. Cytogenet. Genome Res. 2008, 121, 174–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA Double-Stranded Breaks Induce Histone H2AX Phosphorylation on Serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [Green Version]
- Mah, L.-J.; El-Osta, A.; Karagiannis, T.C. γH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coquelle, A.; Pipiras, E.; Toledo, F.; Buttin, G.; Debatisse, M. Expression of Fragile Sites Triggers Intrachromosomal Mammalian Gene Amplification and Sets Boundaries to Early Amplicons. Cell 1997, 89, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, R.J.; Schimke, R.T. Amplification and Loss of Dihydrofolate Reductase Genes in a Chinese Hamster Ovary Cell Line. Mol. Cell. Biol. 1981, 1, 1069–1076. [Google Scholar] [CrossRef] [Green Version]
- Nunberg, J.H.; Kaufman, R.J.; Schimke, R.T.; Urlaub, G.; Chasin, L.A. Amplified Dihydrofolate Reductase Genes are Localized to a Homogeneously Staining Region of a Single Chromosome in a Methotrexate-Resistant Chinese Hamster Ovary Cell Line. Proc. Natl. Acad. Sci. USA 1978, 75, 5553–5556. [Google Scholar] [CrossRef] [Green Version]
- Beck, H.; Nähse-Kumpf, V.; Larsen, M.S.Y.; O’Hanlon, K.A.; Patzke, S.; Holmberg, C.; Mejlvang, J.; Groth, A.; Nielsen, O.; Syljuåsen, R.G. Cyclin-Dependent Kinase Suppression by WEE1 Kinase Protects the Genome through Control of Replication Initiation and Nucleotide Consumption. Mol. Cell. Biol. 2012, 32, 4226–4236. [Google Scholar] [CrossRef] [Green Version]
- Zeman, M.K.; Cimprich, K.A. Causes and Consequences of Replication Stress. Nat. Cell Biol. 2014, 16, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmon, B.; Sedat, J. Cell-by-Cell Dissection of Gene Expression and Chromosomal Interactions Reveals Consequences of Nuclear Reorganization. PLoS Biol. 2005, 3, e67. [Google Scholar] [CrossRef] [Green Version]
- Mitelman, F.; Johansson, B.; Mertens, F. Fusion Genes and Rearranged Genes as a Linear Function of Chromosome Aberrations in Cancer. Nat. Genet. 2004, 36, 331–334. [Google Scholar] [CrossRef]
- Stranger, B.E.; Forrest, M.S.; Dunning, M.; Ingle, C.E.; Beazley, C.; Thorne, N.; Redon, R.; Bird, C.P.; De Grassi, A.; Lee, C. Relative Impact of Nucleotide and Copy Number Variation on Gene Expression Phenotypes. Science 2007, 315, 848–853. [Google Scholar] [CrossRef] [Green Version]
- Vcelar, S.; Jadhav, V.; Melcher, M.; Auer, N.; Hrdina, A.; Sagmeister, R.; Heffner, K.; Puklowski, A.; Betenbaugh, M.; Wenger, T. Karyotype Variation of CHO Host Cell Lines Over Time in Culture Characterized by Chromosome Counting and Chromosome Painting. Biotechnol. Bioeng. 2018, 115, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Girod, P.; Zahn-Zabal, M.; Mermod, N. Use of the Chicken Lysozyme 5′ Matrix Attachment Region to Generate High Producer CHO Cell Lines. Biotechnol. Bioeng. 2005, 91, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Benton, T.; Chen, T.; McEntee, M.; Fox, B.; King, D.; Crombie, R.; Thomas, T.C.; Bebbington, C. The use of UCOE Vectors in Combination with a Preadapted Serum Free, Suspension Cell Line Allows for Rapid Production of Large Quantities of Protein. Cytotechnology 2002, 38, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Hacker, D.L.; De Jesus, M.; Wurm, F.M. 25 Years of Recombinant Proteins from Reactor-Grown Cells—Where do we Go from here? Biotechnol. Adv. 2009, 27, 1023–1027. [Google Scholar] [CrossRef]



| Bleomycin Treatment (µg/mL) | Radial | Non-Radial | Overall Rearrangement | |
|---|---|---|---|---|
| Rearrangement | Normal | |||
| 1.56 | 30% (42/138) | 70% (96/138) | 76% | |
| 65% (15/23) | 35% (8/23) | |||
| 3.13 | 32% (42/132) | 68% (90/132) | 97% | |
| 95% (19/20) | 5% (1/20) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baik, J.Y.; Han, H.-J.; Lee, K.H. DNA Double-Strand Breaks Affect Chromosomal Rearrangements during Methotrexate-Mediated Gene Amplification in Chinese Hamster Ovary Cells. Pharmaceutics 2021, 13, 376. https://doi.org/10.3390/pharmaceutics13030376
Baik JY, Han H-J, Lee KH. DNA Double-Strand Breaks Affect Chromosomal Rearrangements during Methotrexate-Mediated Gene Amplification in Chinese Hamster Ovary Cells. Pharmaceutics. 2021; 13(3):376. https://doi.org/10.3390/pharmaceutics13030376
Chicago/Turabian StyleBaik, Jong Youn, Hye-Jin Han, and Kelvin H. Lee. 2021. "DNA Double-Strand Breaks Affect Chromosomal Rearrangements during Methotrexate-Mediated Gene Amplification in Chinese Hamster Ovary Cells" Pharmaceutics 13, no. 3: 376. https://doi.org/10.3390/pharmaceutics13030376
APA StyleBaik, J. Y., Han, H.-J., & Lee, K. H. (2021). DNA Double-Strand Breaks Affect Chromosomal Rearrangements during Methotrexate-Mediated Gene Amplification in Chinese Hamster Ovary Cells. Pharmaceutics, 13(3), 376. https://doi.org/10.3390/pharmaceutics13030376






