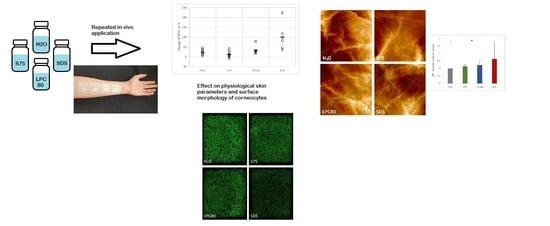
Changes in Skin Barrier Function after Repeated Exposition to Phospholipid-Based Surfactants and Sodium Dodecyl Sulfate In Vivo and Corneocyte Surface Analysis by Atomic Force Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study Design
2.3. Effect of Surfactants on Skin Parameters
2.3.1. Transepidermal Water Loss (TEWL)
2.3.2. Skin Surface pH
2.3.3. Skin Hydration
2.3.4. Capacitive Contact Imaging (Skin Permittivity)
2.3.5. Confocal Raman Spectroscopy (CRS)
2.4. Calculation of Skin Parameter Changes
2.5. Effect of Surfactants on Corneocyte Surface Morphology
2.5.1. Sampling of the SC by Tape Stripping
2.5.2. Analysis of Corneocyte Surface by Atomic Force Microscopy (AFM)
2.6. Statistical Analysis
3. Results
3.1. Effect of Surfactants on Skin In Vivo
Effect of the Surfactants on Physiological Skin Parameters
3.2. Effect of Surfactants on Corneocyte Surface Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Smeden, J.; Janssens, M.; Gooris, G.S.; Bouwstra, J.A. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Tettey, K.E.; Yarovoy, Y.; Lee, D. Effects of anionic surfactants on the water permeability of a model stratum corneum lipid membrane. Langmuir 2014, 30, 220–226. [Google Scholar] [CrossRef]
- Elias, P.M. Epidermal Lipids, Barrier Function, and Desquamation. J. Investig. Dermatol. 1983, 80, S44–S49. [Google Scholar] [CrossRef]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef] [PubMed]
- Boncheva, M.; Damien, F.; Normand, V. Molecular organization of the lipid matrix in intact Stratum corneum using ATR-FTIR spectroscopy. Biochim. Biophys. Acta Biomembr. 2008, 1778, 1344–1355. [Google Scholar] [CrossRef]
- Antunes, E.; Cavaco-Paulo, A. Stratum corneum lipid matrix with unusual packing: A molecular dynamics study. Colloids Surf. B Biointerfaces 2020, 190, 110928. [Google Scholar] [CrossRef] [PubMed]
- Tfayli, A.; Guillard, E.; Manfait, M.; Baillet-Guffroy, A. Raman spectroscopy: Feasibility of in vivo survey of stratum corneum lipids, effect of natural aging. Eur. J. Dermatol. 2012, 22, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Hadgraft, J. Skin, the final frontier. Int. J. Pharm. 2001, 224, 1–18. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Moore, D.J.; Subramanyan, K.; Misra, M.; Meyer, F. Cleansing without compromise: The impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol. Ther. 2004, 17, 16–25. [Google Scholar] [CrossRef]
- Walters, R.M.; Mao, G.; Gunn, E.T.; Hornby, S. Cleansing Formulations That Respect Skin Barrier Integrity. Dermatol. Res. Pract. 2012, 2012, 495917. [Google Scholar] [CrossRef]
- Seweryn, A. Interactions between surfactants and the skin—Theory and practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef]
- Mao, G.; Flach, C.R.; Mendelsohn, R.; Walters, R.M. Imaging the distribution of sodium dodecyl sulfate in skin by confocal raman and infrared microspectroscopy. Pharm. Res. 2012, 29, 2189–2201. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, C.M.; Jakasa, I.; Verberk, M.M.; Kezic, S. Variation in barrier impairment and inflammation of human skin as determined by sodium lauryl sulphate penetration rate. Br. J. Dermatol. 2006, 154, 651–657. [Google Scholar] [CrossRef]
- Lémery, E.; Briançon, S.; Chevalier, Y.; Bordes, C.; Oddos, T.; Gohier, A.; Bolzinger, M.A. Skin toxicity of surfactants: Structure/toxicity relationships. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 166–179. [Google Scholar] [CrossRef]
- Törmä, H.; Lindberg, M.; Berne, B. Skin barrier disruption by sodium lauryl sulfate-exposure alters the expressions of involucrin, transglutaminase 1, profilaggrin, and kallikreins during the repair phase in human skin in vivo. J. Investig. Dermatol. 2008, 128, 1212–1219. [Google Scholar] [CrossRef]
- Buraczewska, I.; Berne, B.; Lindberg, M.; Lodén, M.; Törmä, H. Long-term treatment with moisturizers affects the mRNA levels of genes involved in keratinocyte differentiation and desquamation. Arch. Dermatol. Res. 2009, 301, 175–181. [Google Scholar] [CrossRef]
- Tavss, E.A.; Eigen, E.; Kligman, A.M. Anionic skin irritation and anionic pH rise of bovine serum albumin. J. Soc. Cosmet. Chem. 1988, 39, 267–272. [Google Scholar]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Exploratory analysis of global cosmetic industry: Major players, technology and market trends. Technovation 2005, 25, 1263–1272. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Li, T.S.; Su, H.L.; Lee, P.C.; Wang, H.M.D. Transdermal Delivery Systems of Natural Products Applied to Skin Therapy and Care. Molecules 2020, 25, 5051. [Google Scholar] [CrossRef]
- Wilbey, R.A. Emulsifiers in Food Technology. Int. J. Dairy Technol. 2006, 59, 52–53. [Google Scholar] [CrossRef]
- Klang, V.; Valenta, C. Lecithin-based nanoemulsions. J. Drug Deliv. Sci. Technol. 2011, 21, 55–76. [Google Scholar] [CrossRef]
- Hoeller, S.; Klang, V.; Valenta, C. Skin-compatible lecithin drug delivery systems for fluconazole: Effect of phosphatidylethanolamine and oleic acid on skin permeation. J. Pharm. Pharmacol. 2008, 60, 587–591. [Google Scholar] [CrossRef]
- van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [PubMed]
- van Hoogevest, P.; Fahr, A. Phospholipids in Cosmetic Carriers. In Nanocosmetics; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 95–140. [Google Scholar]
- Vater, C.; Adamovic, A.; Ruttensteiner, L.; Steiner, K.; Tajpara, P.; Klang, V.; Elbe-Bürger, A.; Wirth, M.; Valenta, C. Cytotoxicity of lecithin-based nanoemulsions on human skin cells and ex vivo skin permeation: Comparison to conventional surfactant types. Int. J. Pharm. 2019, 566, 383–390. [Google Scholar] [CrossRef]
- Vater, C.; Hlawaty, V.; Werdenits, P.; Anna Cichoń, M.; Klang, V.; Elbe-Bürger Funding, A.; Wirth, M.; Valenta Funding, C. Effects of lecithin-based nanoemulsions on skin: Short-time cytotoxicity MTT and BrdU studies, skin penetration of surfactants and additives and the delivery of curcumin. Int. J. Pharm. 2020, 580. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, M.; Ettl, H.; Holper, E.; Valenta, C. Influence of the composition of monoacyl phosphatidylcholine based microemulsions on the dermal delivery of flufenamic acid. Int. J. Pharm. 2014, 475, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, A.; Lademann, J.; Richter, H.; Darvin, M.E.; Schanzer, S.; Thiede, G.; Sterry, W.; Vergou, T.; Hauser, M. In vivo investigations on the penetration of various oils and their influence on the skin barrier. Ski. Res. Technol. 2012, 18, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Binder, L.; Klang, V.; Sheikh Rezaei, S.; Neuer, O.; Zhang, Z.; Lunter, D.J.; Wolzt, M.; Valenta, C. Topical application of highly concentrated water-in-oil emulsions: Physiological skin parameters and skin penetration in vivo—A pilot study. Int. J. Pharm. 2019, 571, 118694. [Google Scholar] [CrossRef]
- Machková, L.; Švadlák, D.; Dolečková, I. A comprehensive in vivo study of Caucasian facial skin parameters on 442 women. Arch. Dermatol. Res. 2018, 310, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Luebberding, S.; Krueger, N.; Kerscher, M. Skin physiology in men and women: In vivo evaluation of 300 people including TEWL, SC hydration, sebum content and skin surface pH. Int. J. Cosmet. Sci. 2013, 35, 477–483. [Google Scholar] [CrossRef]
- Koppes, S.A.; Kemperman, P.; Van Tilburg, I.; Calkoen-Kwa, F.; Engebretsen, K.A.; Puppels, G.J.; Caspers, P.J.; Kezic, S. Determination of natural moisturizing factors in the skin: Raman microspectroscopy versus HPLC. Biomarkers 2017, 22, 502–507. [Google Scholar] [CrossRef]
- Soltanipoor, M.; Stilla, T.; Riethmüller, C.; Thyssen, J.P.; Sluiter, J.K.; Rustemeyer, T.; Fischer, T.W.; Kezic, S.; Angelova-Fischer, I. Specific barrier response profiles after experimentally induced skin irritation in vivo. Contact Dermat. 2018, 79, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.D.; Guy, R.H.; Gordeev, S.N. Mechanical tomography of human corneocytes with a nanoneedle. J. Investig. Dermatol. 2013, 133, 1565–1571. [Google Scholar] [CrossRef]
- Franz, J.; Beutel, M.; Gevers, K.; Kramer, A.; Thyssen, J.P.; Kezic, S.; Riethmüller, C. Nanoscale alterations of corneocytes indicate skin disease. Ski. Res. Technol. 2016, 22, 174–180. [Google Scholar] [CrossRef]
- Rüther, L.; Kezic, S.; Riethmüller, C. Corneocyte Nanotexture as Biomarker for Individual Susceptibility to Skin Irritants. Ann. Work Expo. Heal. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Riethmüller, C. Assessing the skin barrier via corneocyte morphometry. Exp. Dermatol. 2018, 27, 923–930. [Google Scholar] [CrossRef]
- Imhof, R.E.; De Jesus, M.E.P.; Xiao, P.; Ciortea, L.I.; Berg, E.P. Closed-chamber transepidermal water loss measurement: Microclimate, calibration and performance. Int. J. Cosmet. Sci. 2009, 31, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, V. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, 117–128. [Google Scholar] [CrossRef]
- Parra, J.L.; Paye, M. EEMCO Guidance for the in vivo Assessment of Skin Surface pH. Skin Pharmacol. Physiol. 2003, 16, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Plessis, J.D.; Stefaniak, A.; Eloff, F.; John, S.; Agner, T.; Chou, T.; Nixon, R.; Steiner, M.; Franken, A.; Kudla, I.; et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: Part 2. transepidermal water loss and skin hydration. Ski. Res. Technol. 2013, 19, 265–278. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, X.; Chirikhina, E.; Bontozoglou, C.; Xiao, P. Measurement of Skin Hydration with a Permittivity Contact Imaging System. Present. IFSCC Technol. Showc. Poster 2015, 1, 1–5. [Google Scholar]
- Caspers, P.J.; Lucassen, G.W.; Carter, E.A.; Bruining, H.A.; Puppels, G.J. In vivo confocal raman microspectroscopy of the skin: Noninvasive determination of molecular concentration profiles. J. Investig. Dermatol. 2001, 116, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Isac, L.; Thoelking, G.; Schwab, A.; Oberleithner, H.; Riethmuller, C. Endothelial f-actin depolymerization enables leukocyte transmigration. Anal. Bioanal. Chem. 2011, 399, 2351–2358. [Google Scholar] [CrossRef]
- Simonsen, A.B.; Ruge, I.F.; Quaade, A.S.; Johansen, J.D.; Thyssen, J.P.; Zachariae, C. Increased occurrence of hand eczema in young children following the Danish hand hygiene recommendations during the COVID-19 pandemic. Contact Dermat. 2020, 144–152. [Google Scholar] [CrossRef]
- Wolf, M.; Klang, V.; Stojcic, T.; Fuchs, C.; Wolzt, M.; Valenta, C. NLC versus nanoemulsions: Effect on physiological skin parameters during regular in vivo application and impact on drug penetration. Int. J. Pharm. 2018, 549, 343–351. [Google Scholar] [CrossRef]
- Kottner, J.; Lichterfeld, A.; Blume-Peytavi, U. Transepidermal water loss in young and aged healthy humans: A systematic review and meta-analysis. Arch. Dermatol. Res. 2013, 305, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, A.; Conti, A.; Seidenari, S. In vivo assessment of n-alkyl-sulfate-induced skin irritation by means of non-invasive methods. Ski. Res. Technol. 1998, 4, 192–195. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; de Graaff, A.; Gooris, G.S.; Nijsse, J.; Wiechers, J.W.; van Aelst, A.C. Water distribution and related morphology in human stratum corneum at different hydration levels. J. Investig. Dermatol. 2003, 120, 750–758. [Google Scholar] [CrossRef]
- Lodén, M.; Olsson, H.; Axéll, T.; Linde, Y.W. Friction, capacitance and transepidermal water loss (TEWL) in dry atopic and normal skin. Br. J. Dermatol. 1992, 126, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Falcone, D.; Uzunbajakava, N.E.; Varghese, B.; De Aquino Santos, G.R.; Richters, R.J.H.; Van De Kerkhof, P.C.M.; Van Erp, P.E.J. Microspectroscopic Confocal Raman and Macroscopic Biophysical Measurements in the in vivo Assessment of the Skin Barrier: Perspective for Dermatology and Cosmetic Sciences. Skin Pharmacol. Physiol. 2015, 28, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V.; Scott, I.R.; Harding, C.R.; Bowser, P.A. Stratrum Corneum Moisturization at the Molecular Level. J. Investig. Dermatol. 1994, 103, 731–740. [Google Scholar] [CrossRef]
- Kezic, S.; O’Regan, G.M.; Yau, N.; Sandilands, A.; Chen, H.; Campbell, L.E.; Kroboth, K.; Watson, R.; Rowland, M.; Irwin McLean, W.H.; et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy Eur. J. Allergy Clin. Immunol. 2011, 66, 934–940. [Google Scholar] [CrossRef]
- Binder, L.; SheikhRezaei, S.; Baierl, A.; Gruber, L.; Wolzt, M.; Valenta, C. Confocal Raman spectroscopy: In vivo measurement of physiological skin parameters—A pilot study. J. Dermatol. Sci. 2017, 88, 280–288. [Google Scholar] [CrossRef]
- Richters, R.J.H.; Falcone, D.; Uzunbajakava, N.E.; Varghese, B.; Caspers, P.J.; Puppels, G.J.; Van Erp, P.E.J.; Van De Kerkhof, P.C.M. Sensitive Skin: Assessment of the Skin Barrier Using Confocal Raman Microspectroscopy. Skin Pharmacol. Physiol. 2017, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pany, A.; Wohlgenannt, M.; Klopprogge, S.; Wolzt, M.; Heuser, T.; Kotisch, H.; Valenta, C.; Klang, V. Effect of hydroxypropyl-β-cyclodextrin in fluid and semi-solid submicron emulsions on physiological skin parameters during regular in vivo application. Int. J. Cosmet. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Koppes, S.A.; Ljubojević Hadžavdić, S.; Jakasa, I.; Franceschi, N.; Riethmüller, C.; Jurakić Tončic, R.; Marinovic, B.; Raj, N.; Rawlings, A.V.; Voegeli, R.; et al. Effect of allergens and irritants on levels of natural moisturizing factor and corneocyte morphology. Contact Dermat. 2017, 76, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Kroll, L.M.; Hoffman, D.R.; Cunningham, C.; Koenig, D.W. Impact of Stratum Corneum Damage on Natural Moisturizing Factor (NMF) in the Skin. In Treatment of Dry Skin Syndrome: The Art and Science of Moisturizers; Lodén, M., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 441–451. ISBN 978-3-642-27606-4. [Google Scholar]
- Egawa, M.; Sato, Y. In vivo evaluation of two forms of urea in the skin by Raman spectroscopy after application of urea-containing cream. Ski. Res. Technol. 2015, 21, 259–264. [Google Scholar] [CrossRef]
- Fulmer, A.W.; Kramer, G.J. Stratum corneum lipid abnormalities in surfactant-induced dry scaly skin. J. Investig. Dermatol. 1986, 86, 598–602. [Google Scholar] [CrossRef]
- Crowther, J.M.; Sieg, A.; Blenkiron, P.; Marcott, C.; Matts, P.J.; Kaczvinsky, J.R.; Rawlings, A.V. Measuring the effects of topical moisturizers on changes in stratum corneum thickness, water gradients and hydration in vivo. Br. J. Dermatol. 2008, 159, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Egawa, M.; Tagami, H. Comparison of the depth profiles of water and water-binding substances in the stratum corneum determined in vivo by Raman spectroscopy between the cheek and volar forearm skin: Effects of age, seasonal changes and artificial forced hydration. Br. J. Dermatol. 2008, 158, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ogawa-Fuse, C.; Morisaki, N.; Shima, K.; Hotta, M.; Sugata, K.; Ichihashi, T.; Oguri, M.; Yoshida, O.; Fujimura, T. Impact of water exposure on skin barrier permeability and ultrastructure. Contact Dermat. 2019, 80, 228–233. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vater, C.; Apanovic, A.; Riethmüller, C.; Litschauer, B.; Wolzt, M.; Valenta, C.; Klang, V. Changes in Skin Barrier Function after Repeated Exposition to Phospholipid-Based Surfactants and Sodium Dodecyl Sulfate In Vivo and Corneocyte Surface Analysis by Atomic Force Microscopy. Pharmaceutics 2021, 13, 436. https://doi.org/10.3390/pharmaceutics13040436
Vater C, Apanovic A, Riethmüller C, Litschauer B, Wolzt M, Valenta C, Klang V. Changes in Skin Barrier Function after Repeated Exposition to Phospholipid-Based Surfactants and Sodium Dodecyl Sulfate In Vivo and Corneocyte Surface Analysis by Atomic Force Microscopy. Pharmaceutics. 2021; 13(4):436. https://doi.org/10.3390/pharmaceutics13040436
Chicago/Turabian StyleVater, Claudia, Alexandra Apanovic, Christoph Riethmüller, Brigitte Litschauer, Michael Wolzt, Claudia Valenta, and Victoria Klang. 2021. "Changes in Skin Barrier Function after Repeated Exposition to Phospholipid-Based Surfactants and Sodium Dodecyl Sulfate In Vivo and Corneocyte Surface Analysis by Atomic Force Microscopy" Pharmaceutics 13, no. 4: 436. https://doi.org/10.3390/pharmaceutics13040436
APA StyleVater, C., Apanovic, A., Riethmüller, C., Litschauer, B., Wolzt, M., Valenta, C., & Klang, V. (2021). Changes in Skin Barrier Function after Repeated Exposition to Phospholipid-Based Surfactants and Sodium Dodecyl Sulfate In Vivo and Corneocyte Surface Analysis by Atomic Force Microscopy. Pharmaceutics, 13(4), 436. https://doi.org/10.3390/pharmaceutics13040436