Are Nanobiosensors an Improved Solution for Diagnosis of Leishmania?
Abstract
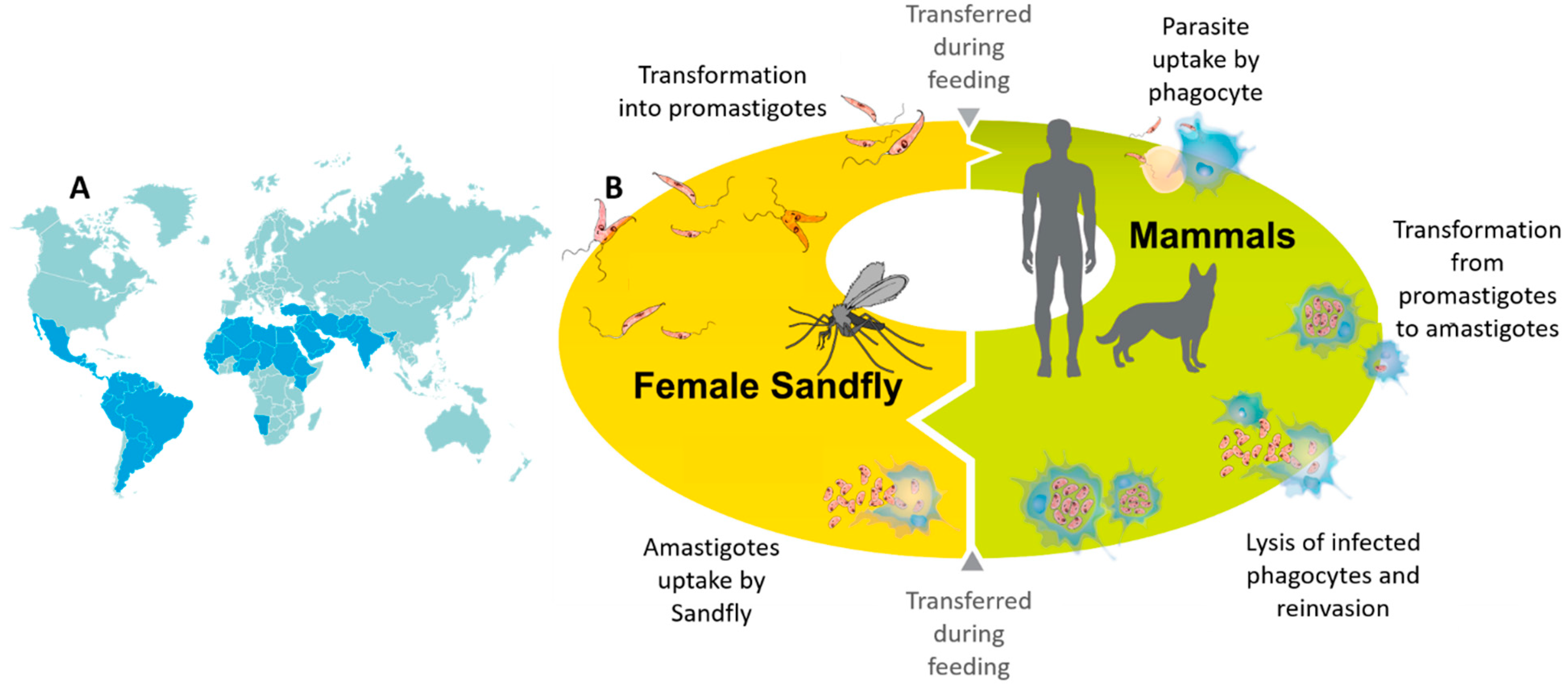
:1. Introduction
2. Current Diagnostic Methods and Limitations
2.1. Parasitological Tests
2.2. Immunological Tests
2.3. Molecular Tests
3. Emerging Nanomaterial-Based Detection Technologies
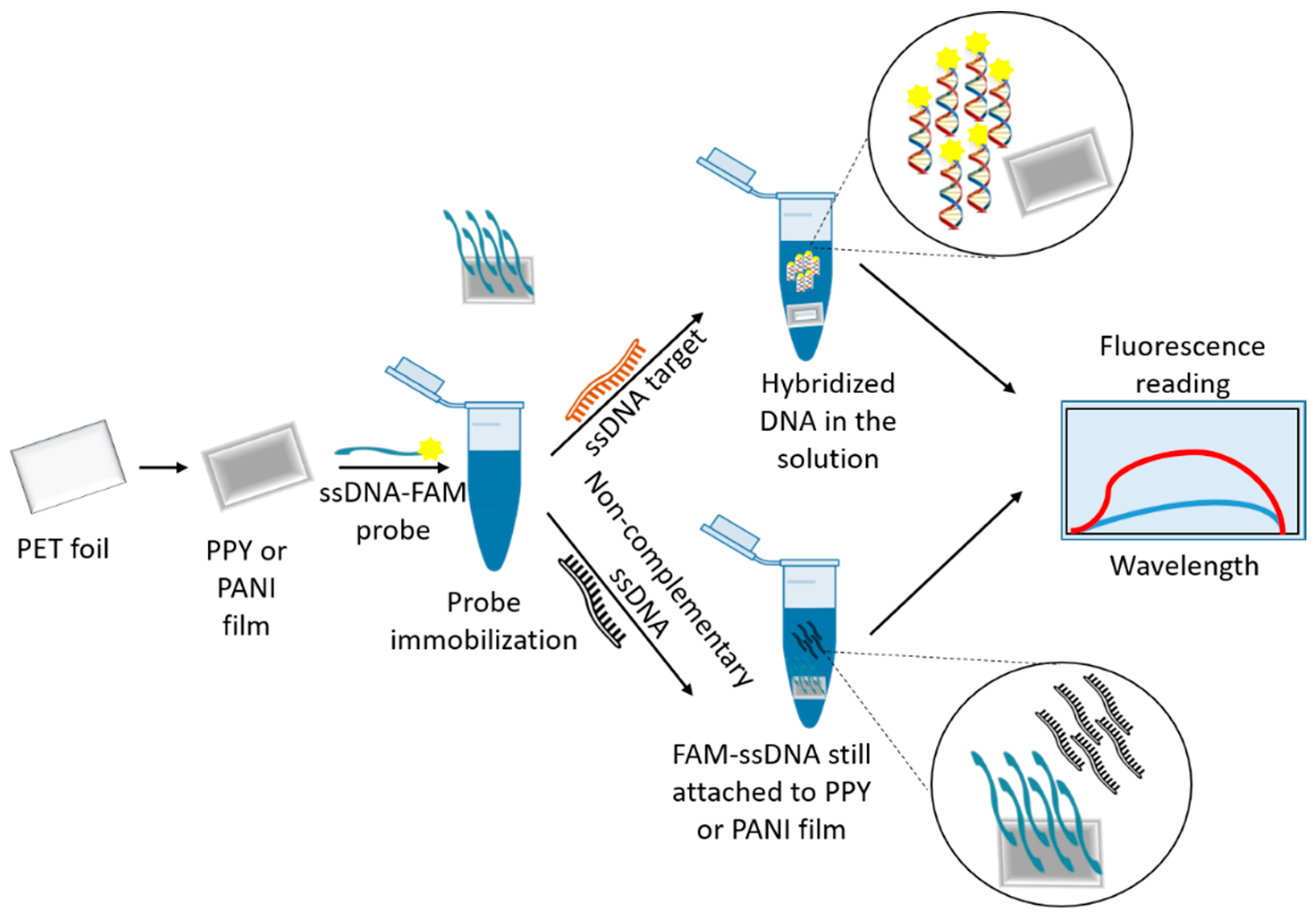
3.1. Nanomaterials as Fluorescence Quenchers
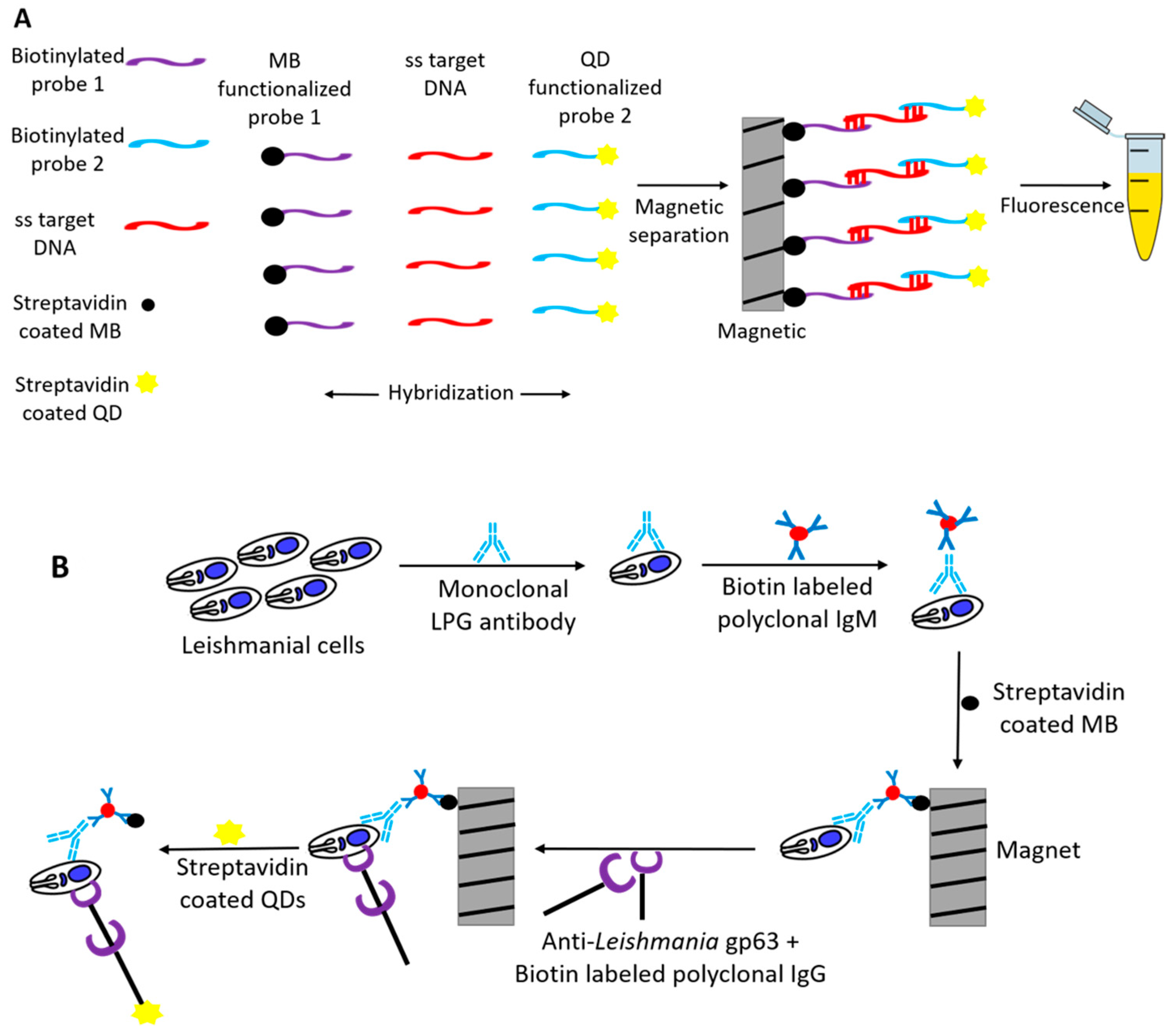
3.2. Nanomaterial as Fluorophore
3.3. Use of Dendrimers
3.4. Surface Plasmon Coupling
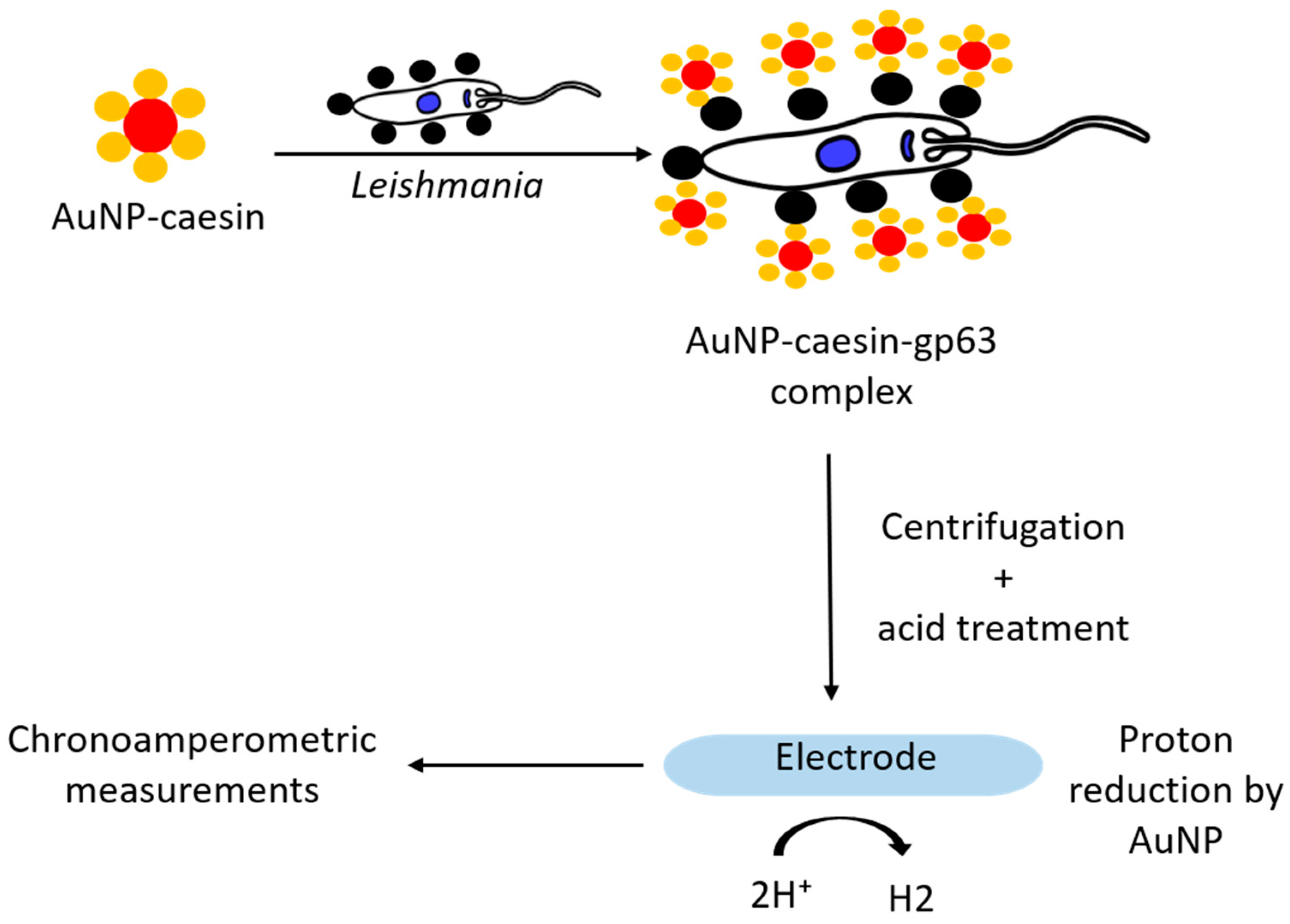
3.5. Nanomaterials as Reducing Agents
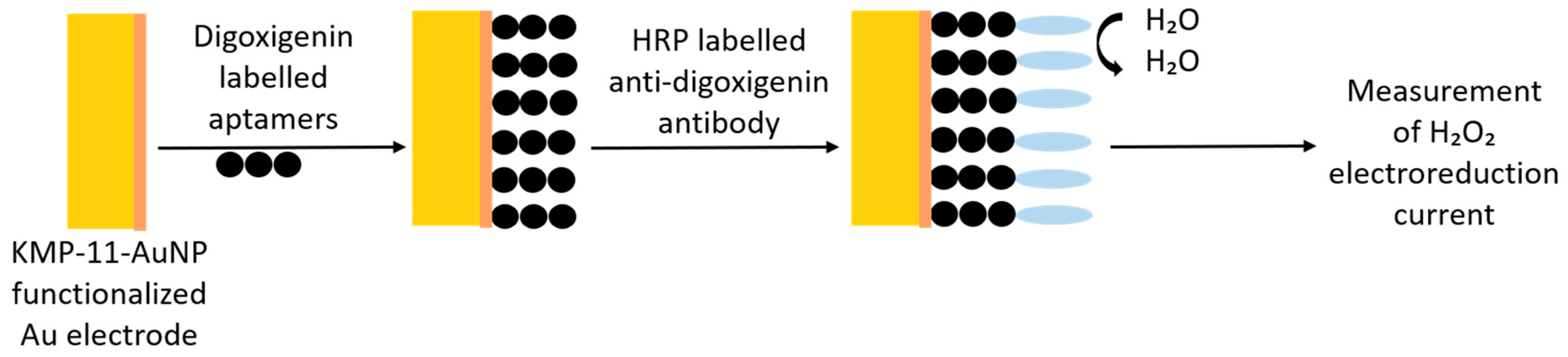
3.6. Aptamers over Antibodies
3.6.1. L. infantum Histone-Specific Aptamers
3.6.2. Aptamer Targeting Poly-A Binding Protein
3.6.3. Aptamer against Kinetoplast Surface Protein
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- De las Heras, F.G. Overview of Neglected Tropical Diseases. In Third World Diseases, Elliott, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–46. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Herrera, G.; Barragán, N.; Luna, N.; Martínez, D.; De Martino, F.; Medina, J.; Niño, S.; Páez, L.; Ramírez, A.; Vega, L.; et al. An interactive database of Leishmania species distribution in the Americas. Sci. Data 2020, 7, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e035671. [Google Scholar] [CrossRef] [PubMed]
- Quinnell, R.J.; Courtenay, O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology 2009, 136, 1915–1934. [Google Scholar] [CrossRef] [PubMed]
- Aronson, N.; Herwaldt, B.L.; Libman, M.; Pearson, R.; Lopez-Velez, R.; Weina, P.; Carvalho, E.; Ephros, M.; Jeronimo, S.; Magill, A. Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am. J. Trop. Med. Hyg. 2017, 96, 24–45. [Google Scholar] [CrossRef]
- McGwire, B.S.; Satoskar, A.R. Leishmaniasis: Clinical syndromes and treatment. QJM Int. J. Med. 2013, 107, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Varma, N.; Naseem, S. Hematologic changes in visceral leishmaniasis/kala azar. Indian J. Hematol. Blood Transfus. 2010, 26, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Akhoundi, M.; Downing, T.; Votýpka, J.; Kuhls, K.; Lukeš, J.; Cannet, A.; Ravel, C.; Marty, P.; Delaunay, P.; Kasbari, M.; et al. Leishmania infections: Molecular targets and diagnosis. Mol. Asp. Med. 2017, 57, 1–29. [Google Scholar] [CrossRef]
- Roatt, B.M.; De Oliveira Cardoso, J.M.; De Brito, R.C.F.; Coura-Vital, W.; De Oliveira Aguiar-Soares, R.D.; Reis, A.B. Recent advances and new strategies on leishmaniasis treatment. Appl. Microbiol. Biotechnol. 2020, 104, 8965–8977. [Google Scholar] [CrossRef]
- Goto, H.; Lindoso, J.A. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev. Anti-Infect. Ther. 2010, 8, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, S.P.; Makaritsis, K.P.; Dalekos, G.N. Leishmaniasis revisited: Current aspects on epidemiology, diagnosis and treatment. J. Transl. Int. Med. 2015, 3, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Kotb, E.E.; Antonio Sampedro, M.; Javier, R.-G.; Yannick, H.-M.; Ahamd, A.; Jose Mari Navarro, M.; Jose Gutierrez, F. Diagnosis of leishmaniasis. J. Infect. Dev. Ctries. 2014, 8. [Google Scholar] [CrossRef]
- Liu, J.; Cao, Z.; Lu, Y. Functional Nucleic Acid Sensors. Chem. Rev. 2009, 109, 1948–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, N.; Memar, M.Y.; Moaddab, S.R.; Kafil, H.S. Aptamer-assisted novel technologies for detecting bacterial pathogens. Biomed. Pharmacother. 2017, 93, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, M.; Hayat, A.; Marty, J.L. Aptamer-based assays and aptasensors for detection of pathogenic bacteria in food samples. TrAC Trends Anal. Chem. 2018, 107, 60–77. [Google Scholar] [CrossRef]
- Blum, J.; Desjeux, P.; Schwartz, E.; Beck, B.; Hatz, C. Treatment of cutaneous leishmaniasis among travellers. J. Antimicrob. Chemother. 2004, 53, 158–166. [Google Scholar] [CrossRef]
- Molina, I.; Fisa, R.; Riera, C.; Falcó, V.; Elizalde, A.; Salvador, F.; Crespo, M.; Curran, A.; López-Chejade, P.; Tebar, S.; et al. Ultrasensitive real-time PCR for the clinical management of visceral leishmaniasis in HIV-Infected patients. Am. J. Trop. Med. Hyg. 2013, 89, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Hira, P.R.; Saroj, G.; Philip, R.; Al-Ali, F.; Madda, P.J.; Sher, A. Imported visceral leishmaniasis: Diagnostic dilemmas and comparative analysis of three assays. J. Clin. Microbiol. 2002, 40, 475–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boelaert, M.; Rijal, S.; Regmi, S.; Singh, R.; Karki, B.; Jacquet, D.; Chappuis, F.; Campino, L.; Desjeux, P.; Le Ray, D.; et al. A comparative study of the effectiveness of diagnostic tests for visceral leishmaniasis. Am. J. Trop. Med. Hyg. 2004, 70, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Rai, M. Laboratory Diagnosis of Visceral Leishmaniasis. Clin. Diagn. Lab. Immunol. 2002, 9, 951–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedras, M.J.; De Gouvêa Viana, L.; De Oliveira, E.J.; Rabello, A. Comparative evaluation of direct agglutination test, rK39 and soluble antigen ELISA and IFAT for the diagnosis of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 172–178. [Google Scholar] [CrossRef] [PubMed]
- De Assis, T.S.M.; Caligiorne, R.B.; Romero, G.A.S.; Rabello, A. Detection of Leishmania kDNA in human serum samples for the diagnosis of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, A.; Schubach, A.; Paula, C.C.d.; Bogio, A.; Antonio, L.d.F.; Schiavoni, P.B.; Monteiro, V.d.S.; Madeira, M.d.F.; Quintella, L.P.; Valete-Rosalino, C.M.; et al. Evaluation of polymerase chain reaction in the routine diagnosis for tegumentary leishmaniasis in a referral centre. J. Memórias Inst. Oswaldo Cruz. 2010, 105, 109–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Castro Zacche-Tonini, A.; Fonseca, G.S.F.; De Jesus, L.; Barros, G.B.; Coelho-Dos-Reis, J.G.A.; Béla, S.R.; Machado, A.S.; Carneiro, A.; Andrade, G.M.Q.; Vasconcelos-Santos, D.V.; et al. Establishing tools for early diagnosis of congenital toxoplasmosis: Flow cytometric IgG avidity assay as a confirmatory test for neonatal screening. J. Immunol. Methods 2017, 451, 37–47. [Google Scholar] [CrossRef]
- Alessio, G.D.; De Araújo, F.F.; Sales Júnior, P.A.; Gomes, M.S.; Amaral, L.R.D.; Pascoal Xavier, M.A.; Teixeira-Carvalho, A.; De Lana, M.; Martins-Filho, O.A. Accomplishing the genotype-specific serodiagnosis of single and dual Trypanosoma cruzi infections by flow cytometry Chagas-Flow ATE-IgG2a. PLoS Negl. Trop. Dis. 2018, 12, e0006140. [Google Scholar] [CrossRef]
- Mary, C.; Faraut, F.; Lascombe, L.; Dumon, H. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J. Clin. Microbiol. 2004, 42, 5249–5255. [Google Scholar] [CrossRef] [Green Version]
- Schönian, G.; Kuhls, K.; Mauricio, I.L. Molecular approaches for a better understanding of the epidemiology and population genetics of Leishmania. Parasitology 2011, 138, 405–425. [Google Scholar] [CrossRef] [Green Version]
- Hossain, F.; Ghosh, P.; Khan, M.A.A.; Duthie, M.S.; Vallur, A.C.; Picone, A.; Howard, R.F.; Reed, S.G.; Mondal, D. Real-time PCR in detection and quantitation of Leishmania donovani for the diagnosis of Visceral Leishmaniasis patients and the monitoring of their response to treatment. PLoS ONE 2017, 12, e0185606. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Cong, Y.; Huang, Y.; Du, X. Nanomaterials-Based Electrochemical Immunosensors. Micromachines 2019, 10, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, M.; Khurana, S.; Mehra, K.; Yadav, N.; Mishra, S.; Kukreti, S. Emerging Trends in Advanced Nanomaterials Based Electrochemical Genosensors. Curr. Pharm. Des. 2018, 24, 3697–3709. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, Y.-P.; Dikici, E.; Deo, S.K.; Daunert, S. Beyond Antibodies as Binding Partners: The Role of Antibody Mimetics in Bioanalysis. Annu. Rev. Anal. Chem. 2017, 10, 293–320. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Ahlawat, W.; Kumar, R.; Dilbaghi, N. Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare. Biosens. Bioelectron. 2015, 70, 498–503. [Google Scholar] [CrossRef]
- Shavanova, K.; Bakakina, Y.; Burkova, I.; Shtepliuk, I.; Viter, R.; Ubelis, A.; Beni, V.; Starodub, N.; Yakimova, R.; Khranovskyy, V. Application of 2D Non-Graphene Materials and 2D Oxide Nanostructures for Biosensing Technology. Sensors 2016, 16, 223. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cheng, H.; Zhao, X.; Wu, J.; Muhammad, F.; Lin, S.; He, J.; Zhou, L.; Zhang, C.; Deng, Y.; et al. Surface-Enhanced Raman Scattering Active Gold Nanoparticles with Enzyme-Mimicking Activities for Measuring Glucose and Lactate in Living Tissues. ACS Nano 2017, 11, 5558–5566. [Google Scholar] [CrossRef]
- Jain, S.; Huang, Z.; Dixon, B.R.; Sattar, S.; Liu, J. Cryptosporidium parvum oocyst directed assembly of gold nanoparticles and graphene oxide. Front. Chem. Sci. Eng. 2019, 13, 608–615. [Google Scholar] [CrossRef]
- Jain, S.; Costa Melo, T.G.; Dolabella, S.S.; Liu, J. Current and emerging tools for detecting protozoan cysts and oocysts in water. TrAC Trends Anal. Chem. 2019, 121, 115695. [Google Scholar] [CrossRef]
- Andreadou, M.; Liandris, E.; Gazouli, M.; Mataragka, A.; Tachtsidis, I.; Goutas, N.; Vlachodimitropoulos, D.; Ikonomopoulos, J. Detection of Leishmania-specific DNA and surface antigens using a combination of functionalized magnetic beads and cadmium selenite quantum dots. J. Microbiol. Methods 2016, 123, 62–67. [Google Scholar] [CrossRef]
- Toubanaki, D.K.; Athanasiou, E.; Karagouni, E. Gold nanoparticle-based lateral flow biosensor for rapid visual detection of Leishmania-specific DNA amplification products. J. Microbiol. Methods 2016, 127, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Sattarahmady, N.; Movahedpour, A.; Heli, H.; Hatam, G.R. Gold nanoparticles-based biosensing of Leishmania major kDNA genome: Visual and spectrophotometric detections. Sens. Actuators B Chem. 2016, 235, 723–731. [Google Scholar] [CrossRef]
- Andreadou, M.; Liandris, E.; Gazouli, M.; Taka, S.; Antoniou, M.; Theodoropoulos, G.; Tachtsidis, I.; Goutas, N.; Vlachodimitropoulos, D.; Kasampalidis, I.; et al. A novel non-amplification assay for the detection of Leishmania spp. in clinical samples using gold nanoparticles. J. Microbiol. Methods 2014, 96, 56–61. [Google Scholar] [CrossRef]
- Anfossi, L.; Di Nardo, F.; Profiti, M.; Nogarol, C.; Cavalera, S.; Baggiani, C.; Giovannoli, C.; Spano, G.; Ferroglio, E.; Mignone, W.; et al. A versatile and sensitive lateral flow immunoassay for the rapid diagnosis of visceral leishmaniasis. Anal. Bioanal. Chem. 2018, 410, 4123–4134. [Google Scholar] [CrossRef] [PubMed]
- Perinoto, Â.C.; Maki, R.M.; Colhone, M.C.; Santos, F.R.; Migliaccio, V.; Daghastanli, K.R.; Stabeli, R.G.; Ciancaglini, P.; Paulovich, F.V.; De Oliveira, M.C.; et al. Biosensors for efficient diagnosis of leishmaniasis: Innovations in bioanalytics for a neglected disease. Anal. Chem. 2010, 82, 9763–9768. [Google Scholar] [CrossRef] [PubMed]
- Souto, D.E.; Fonseca, A.M.; Barragan, J.T.; Luz Rde, C.; Andrade, H.M.; Damos, F.S.; Kubota, L.T. SPR analysis of the interaction between a recombinant protein of unknown function in Leishmania infantum immobilised on dendrimers and antibodies of the visceral leishmaniasis: A potential use in immunodiagnosis. Biosens. Bioelectron. 2015, 70, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Heli, H.; Sattarahmady, N.; Hatam, G.R.; Reisi, F.; Vais, R.D. An electrochemical genosensor for Leishmania major detection based on dual effect of immobilization and electrocatalysis of cobalt-zinc ferrite quantum dots. Talanta 2016, 156–157, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, M.; Sattarahmady, N.; Hatam, G.; Heli, H. Electrochemical Genosensing of Leishmania major using Gold Hierarchical Nanoleaflets. J. Biol. Today’s World 2016, 5, 128–136. [Google Scholar] [CrossRef]
- Nazari-Vanani, R.; Sattarahmady, N.; Yadegari, H.; Delshadi, N.; Hatam, G.R.; Heli, H. Electrochemical quantitation of Leishmania infantum based on detection of its kDNA genome and transduction of non-spherical gold nanoparticles. Anal. Chim. Acta 2018, 1041, 40–49. [Google Scholar] [CrossRef]
- Mobed, A.; Mehri, P.; Hasanzadeh, M.; Mokhtarzadeh, A. Binding of Leishmania spp with gold nanoparticles supported polyethylene glycol and its application for the sensitive detection of infectious photogenes in human plasma samples: A novel biosensor. J. Mol. Recognit. 2020, 33, e2839. [Google Scholar] [CrossRef]
- Diouani, M.F.; Ouerghi, O.; Belgacem, K.; Sayhi, M.; Ionescu, R.; Laouini, D. Casein-Conjugated Gold Nanoparticles for Amperometric Detection of Leishmania infantum. Biosensors 2019, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- De la Escosura-Muñiz, A.; Baptista-Pires, L.; Serrano, L.; Altet, L.; Francino, O.; Sánchez, A.; Merkoçi, A. Magnetic Bead/Gold Nanoparticle Double-Labeled Primers for Electrochemical Detection of Isothermal Amplified Leishmania DNA. Small 2016, 12, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, M.; González, V.M.; Rincón, E.; Domingo, A.; Domínguez, E. Aptasensor based on the selective electrodeposition of protein-linked gold nanoparticles on screen-printed electrodes. Analyst 2011, 136, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Song, B.; Li, D.; Zhu, C.; Qi, W.; Wen, Y.; Wang, L.; Song, S.; Fang, H.; Fan, C. A Graphene Nanoprobe for Rapid, Sensitive, and Multicolor Fluorescent DNA Analysis. Adv. Funct. Mater. 2010, 20, 453–459. [Google Scholar] [CrossRef]
- Liu, Z.; Su, X. A novel fluorescent DNA sensor for ultrasensitive detection of Helicobacter pylori. Biosens. Bioelectron. 2017, 87, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Dubertret, B.; Calame, M.; Libchaber, A.J. Single-mismatch detection using gold-quenched fluorescent oligonucleotides. Nat. Biotechnol. 2001, 19, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Wang, L.; Tian, J.; Sun, X. Nucleic acid detection using carbon nanoparticles as a fluorescent sensing platform. Chem. Commun. 2011, 47, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-K.; Ma, H.-L.; Cai, P.-F.; Wu, J.-Y. Highly selective and sensitive nucleic acid detection based on polysaccharide-functionalized silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 17–21. [Google Scholar] [CrossRef]
- Bülbül, G.; Hayat, A.; Mustafa, F.; Andreescu, S. DNA assay based on Nanoceria as Fluorescence Quenchers (NanoCeracQ DNA assay). Sci. Rep. 2018, 8, 2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loo, A.H.; Sofer, Z.; Bouša, D.; Ulbrich, P.; Bonanni, A.; Pumera, M. Carboxylic Carbon Quantum Dots as a Fluorescent Sensing Platform for DNA Detection. ACS Appl. Mater. Interfaces 2016, 8, 1951–1957. [Google Scholar] [CrossRef]
- Zhao, H.-Q.; Qiu, G.-H.; Liang, Z.; Li, M.-M.; Sun, B.; Qin, L.; Yang, S.-P.; Chen, W.-H.; Chen, J.-X. A zinc(II)-based two-dimensional MOF for sensitive and selective sensing of HIV-1 ds-DNA sequences. Anal. Chim. Acta 2016, 922, 55–63. [Google Scholar] [CrossRef]
- Tang, G.; Gao, J.; Wang, C.; Tan, H. Luminescent lanthanide coordination polymer as a platform for DNA colorimetric detection. Sens. Actuators B Chem. 2017, 244, 571–576. [Google Scholar] [CrossRef]
- Zhu, C.; Zeng, Z.; Li, H.; Li, F.; Fan, C.; Zhang, H. Single-Layer MoS2-Based Nanoprobes for Homogeneous Detection of Biomolecules. J. Am. Chem. Soc. 2013, 135, 5998–6001. [Google Scholar] [CrossRef]
- Mo, L.; Li, J.; Liu, Q.; Qiu, L.; Tan, W. Nucleic acid-functionalized transition metal nanosheets for biosensing applications. Biosens. Bioelectron. 2017, 89, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, S.; Wang, L.; Luo, Y.; Tian, J.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Novel Use of Poly(3,4-ethylenedioxythiophene) Nanoparticles for Fluorescent Nucleic Acid Detection. ACS Comb. Sci. 2012, 14, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Qin, X.; Chang, G.; Sun, X. 2,4,6-Tris (2-pyridyl)-1,3,5-triazine nanobelts as an effective fluorescent sensing platform for DNA detection. J. Nanosci. Nanotechnol. 2012, 12, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Pedro, G.C.; Gorza, F.D.S.; Da Silva, R.J.; Do Nascimento, K.T.O.; Medina-Llamas, J.C.; Chávez-Guajardo, A.E.; Alcaraz-Espinoza, J.J.; De Melo, C.P. A novel nucleic acid fluorescent sensing platform based on nanostructured films of intrinsically conducting polymers. Anal. Chim. Acta 2019, 1047, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, M.; Liandris, E.; Kasampalidis, I.N.; Taka, S.; Antoniou, M.; Ntais, P.; Vaiopoulou, A.; Theodoropoulos, G.; Gazouli, M.; Ikonomopoulos, J. Evaluation of the performance of selected in-house and commercially available PCR and real-time PCR assays for the detection of Leishmania DNA in canine clinical samples. Exp. Parasitol. 2012, 131, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Souto, D.E.P.; Silva, J.V.; Martins, H.R.; Reis, A.B.; Luz, R.C.S.; Kubota, L.T.; Damos, F.S. Development of a label-free immunosensor based on surface plasmon resonance technique for the detection of anti-Leishmania infantum antibodies in canine serum. Biosens. Bioelectron. 2013, 46, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Jimmy Huang, P.-J.; Ding, J.; Liu, J. Aptamer-based biosensors for biomedical diagnostics. Analyst 2014, 139, 2627–2640. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yan, W.; Fu, S.; Hu, S.; Wang, Y.; Xu, J.; Ye, C. Multiple Cross Displacement Amplification Coupled With Nanoparticles-Based Lateral Flow Biosensor for Detection of Staphylococcus aureus and Identification of Methicillin-Resistant S. aureus. Front. Microbiol. 2018, 9, 907. [Google Scholar] [CrossRef] [Green Version]
- Posthuma-Trumpie, G.A.; Korf, J.; Van Amerongen, A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef] [Green Version]
- Rivas, L.; Reuterswärd, P.; Rasti, R.; Herrmann, B.; Mårtensson, A.; Alfvén, T.; Gantelius, J.; Andersson-Svahn, H. A vertical flow paper-microarray assay with isothermal DNA amplification for detection of Neisseria meningitidis. Talanta 2018, 183, 192–200. [Google Scholar] [CrossRef]
- Moradi, M.; Sattarahmady, N.; Rahi, A.; Hatam, G.R.; Sorkhabadi, S.M.R.; Heli, H. A label-free, PCR-free and signal-on electrochemical DNA biosensor for Leishmania major based on gold nanoleaves. Talanta 2016, 161, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, J.; Schneider, P.; Etges, R.; Bordier, C. Peptide substrate specificity of the membrane-bound metalloprotease of Leishmania. Biochemistry 1990, 29, 10113–10119. [Google Scholar] [CrossRef]
- Yao, C.; Donelson, J.E.; Wilson, M.E. The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression, and function. Mol. Biochem. Parasitol. 2003, 132, 1–16. [Google Scholar] [CrossRef]
- Bayat, P.; Nosrati, R.; Alibolandi, M.; Rafatpanah, H.; Abnous, K.; Khedri, M.; Ramezani, M. SELEX methods on the road to protein targeting with nucleic acid aptamers. Biochimie 2018, 154, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Villa, J.D.; Zamorano-Carrillo, A.; Castañón-Sánchez, C.A.; Ramírez-Moreno, E.; Marchat, L.A. Aptamers as a promising approach for the control of parasitic diseases. Braz. J. Infect. Dis. 2016, 20, 610–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolter, O.; Mayer, G. Aptamers as Valuable Molecular Tools in Neurosciences. J. Neurosci. 2017, 37, 2517–2523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Q.; Luo, F.; Liu, M.; Zhang, X.-L. Oligonucleotide aptamers: Promising and powerful diagnostic and therapeutic tools for infectious diseases. J. Infect. 2018, 77, 83–98. [Google Scholar] [CrossRef]
- Ruiz Ciancio, D.; Vargas, M.R.; Thiel, W.H.; Bruno, M.A.; Giangrande, P.H.; Mestre, M.B. Aptamers as Diagnostic Tools in Cancer. Pharmaceuticals 2018, 11, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, M.; Rincón, E.; Piñeiro, D.; Fernández, G.; Domingo, A.; Jiménez-Ruíz, A.; Salinas, M.; González, V.C.M. Selection of aptamers against KMP-11 using colloidal gold during the SELEX process. Biochem. Biophys. Res. Commun. 2003, 308, 214–218. [Google Scholar] [CrossRef]
- Ramos, E.; Piñeiro, D.; Soto, M.; Abanades, D.R.; Martín, M.E.; Salinas, M.; González, V.M. A DNA aptamer population specifically detects Leishmania infantum H2A antigen. Lab. Investig. J. Tech. Methods Pathol. 2007, 87, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.E.; García-Hernández, M.; García-Recio, E.M.; Gómez-Chacón, G.F.; Sánchez-López, M.; González, V.M. DNA aptamers selectively target Leishmania infantum H2A protein. PLoS ONE 2013, 8, e078886. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Pérez, N.; Ramos, E.; García-Hernández, M.; Pinto, C.; Soto, M.; Martín, M.E.; González, V.M. Molecular and Functional Characterization of ssDNA Aptamers that Specifically Bind Leishmania infantum PABP. PLoS ONE 2015, 10, e0140048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frezza, V.; Pinto-Díez, C.; Fernández, G.; Soto, M.; Martín, M.E.; García-Sacristán, A.; González, V.M. DNA aptamers targeting Leishmania infantum H3 protein as potential diagnostic tools. Anal. Chim. Acta 2020, 1107, 155–163. [Google Scholar] [CrossRef] [PubMed]



| Type of Biosensor | Nanomaterial Used | Type of Pathogen | Target Biomolecule | Limit of Detection | References |
|---|---|---|---|---|---|
| Genosensor/Optical | PPY PANI | L. infantum | FAM-ssDNA | 1.1 nM † 1.3 nM | [23] |
| Genosensor/Optical | cadmium selenite QD | Leishmania spp. | Conserved specific genomic DNA LPG and gp36 antigens | 3.125 ng/μL ‡ | [40] |
| Immunosensor/Optical | 103 cells/mL ¥ | ||||
| Genosensor/Optical | AuNP | L. infantum | KinetoplastDNA | 100 fmol | [41] |
| Genosensor/Optical | AuNP | L. major | KinetoplastDNA | 7.0 pg/μL | [42] |
| Genosensor/Optical | AuNP | Leishmania spp. | KinetoplastDNA | 11.5 ng/μL | [43] |
| Immunosensor/Optical | AuNP | L. infantum | Chimeric recombinant antigens (K9, K39, and K26) | - | [44] |
| Immunosensor/Impedimetric | PAMAM | L. amazonensis | membrane proteins | 10−5 mg/mL | [45] |
| Immunosensor/SPR | PAMAM | L. infantum | hypothetical C1protein | 7.83 nmol/L | [46] |
| Genosensor/Electrochemical | cobalt-zinc ferrite QD | L. major | KinetoplastDNA | 1.8 × 10−14 ng/µL | [47] |
| Genosensor/Electrochemical | AuNP | L. major | L. major specific DNA | 1.8 × 10−20 mol/L 0.7 ng/µL | [48] |
| Genosensor/Electrochemical | AuNP | L. infantum | KinetoplastDNA | 2 × 10−19 mol/L | [49] |
| Genosensor/Electrochemical | AuNP | Leishmania spp. | Leishmania specific DNA | 1ZM | [50] |
| Casein-gp63 interaction/Electrochemical | AuNP | L. infantum | gp63 surface protein | 0.55 parasite/mL | [51] |
| Genosensor/Electrochemical | AuNP | Leishmania spp. | KinetoplastDNA | 0.8 parasite/mL | [52] |
| Aptasensor/Electrochemical | AuNP | L. infantum | Kinetoplastid membrane protein-11 | 2.27 mM | [53] |
| Aptamer | Target Protein | Organism | Affinity (Kd) | Minimum Number of Detectable Promastigotes | References | |
|---|---|---|---|---|---|---|
| AptLiH2A#2, AptLiH2A#1 | H2A | L. infantum | 0.96 ± 0.17 nM | 7500 | [85] | |
| 1.16 ± 0.28 nM | 7500 | |||||
| AptLiH3#4 AptLiH3#10 | H3 | L. infantum | 0.52 ± 0.05 nM | 6000 | [87] | |
| 0.37 ± 0.05 nM | 9000 | |||||
| ApPABP#3 | PAPB | L. infantum | 5.4 ± 1.1 nM | 2500 | [86] | |
| ApPABP#7 | 6.0 ± 2.6 nM | |||||
| ApPABP#11 | 10.8 ± 2.7 nM | |||||
| Aptamer population | KMP-11 | L. infantum | - | - | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, S.; Santana, W.; Dolabella, S.S.; Santos, A.L.S.; Souto, E.B.; Severino, P. Are Nanobiosensors an Improved Solution for Diagnosis of Leishmania? Pharmaceutics 2021, 13, 491. https://doi.org/10.3390/pharmaceutics13040491
Jain S, Santana W, Dolabella SS, Santos ALS, Souto EB, Severino P. Are Nanobiosensors an Improved Solution for Diagnosis of Leishmania? Pharmaceutics. 2021; 13(4):491. https://doi.org/10.3390/pharmaceutics13040491
Chicago/Turabian StyleJain, Sona, Wanessa Santana, Silvio S. Dolabella, André L. S. Santos, Eliana B. Souto, and Patrícia Severino. 2021. "Are Nanobiosensors an Improved Solution for Diagnosis of Leishmania?" Pharmaceutics 13, no. 4: 491. https://doi.org/10.3390/pharmaceutics13040491
APA StyleJain, S., Santana, W., Dolabella, S. S., Santos, A. L. S., Souto, E. B., & Severino, P. (2021). Are Nanobiosensors an Improved Solution for Diagnosis of Leishmania? Pharmaceutics, 13(4), 491. https://doi.org/10.3390/pharmaceutics13040491









