UVA-Degradable Collagenase Nanocapsules as a Potential Treatment for Fibrotic Diseases
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Bisacrylamide-Photolinker (PL) Synthesis and Photosensitivity Evaluation
2.3. Synthesis of UVA-Degradable Collagenase Nanocapsules (nCol-PL)
2.4. Nanocapsules Photocleavage Evaluation
2.5. Nanocapsules Enzymatic Activity Measurements
2.6. Nanocapsules Collagen Gel Digestion Capacity
2.7. In Vitro Cytotoxicity Evaluation of nCol-PL
3. Results and Discussion
3.1. Synthesis of UVA Sensitive Photolinker
3.2. Photo-Lability Evaluation of PL
3.3. Synthesis of UVA-Sensitive Collagenase Nanocapsules (nCol-PL)
3.4. In Vitro Cleavage Evaluation of nCol-PL and Enzymatic Activity Measurement
3.5. UVA-Degradation and Enzymatic Activity Evaluation Of Nanocapsules
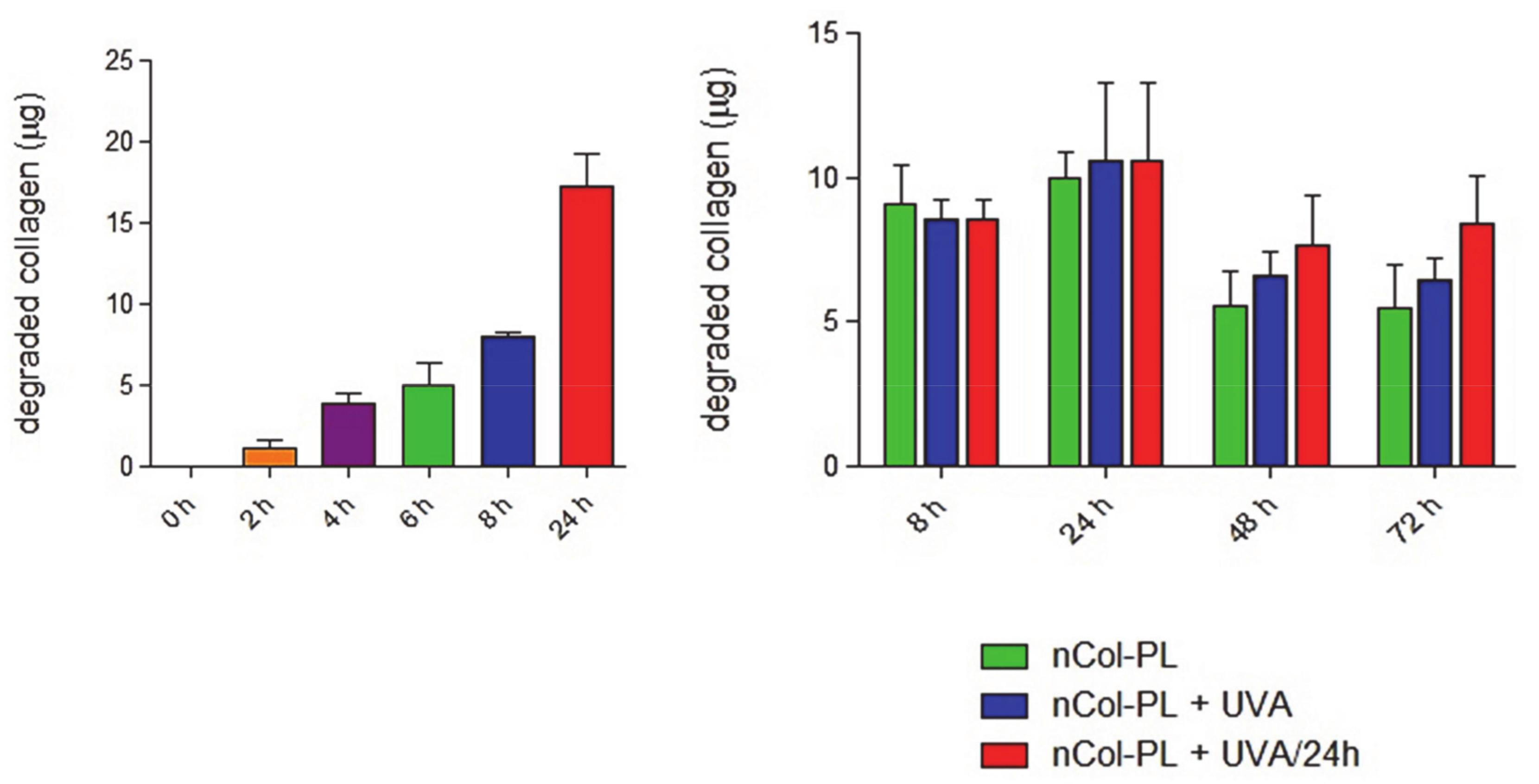
3.6. Nanocapsules Kinetics for Collagen Degradation Within Collagen Gels
3.7. Evaluation of nCol-PL Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Distler, J.H.W.; Györfi, A.H.; Ramanujam, M.; Whitfield, M.L.; Königshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef]
- Trojanowska, M.; LeRoy, E.C.; Eckes, B.; Krieg, T. Pathogenesis of fibrosis: Type 1 collagen and the skin. J. Mol. Med. 1998, 76, 266–274. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. DMM Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.Y.; Lagares, D.; Tager, A.M.; Kapoor, M. Fibrosis-A lethal component of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Šmíd, V. Liver fibrosis. Vnitr. Lek. 2020, 66, e36–e41. [Google Scholar] [CrossRef]
- Arias, D.; Borbujo-Martínez, J. Localized scleroderma (Morphea). FMC Form. Med. Contin. Aten. Primaria 2005, 12, 680. [Google Scholar] [CrossRef]
- Levine, L.A.; Larsen, S.M. Surgical correction of persistent peyronie’s disease following collagenase clostridium histolyticum treatment. J. Sex. Med. 2015, 12, 259–264. [Google Scholar] [CrossRef]
- Patel, D.P.; Christensen, M.B.; Hotaling, J.M.; Pastuszak, A.W. A review of inflammation and fibrosis: Implications for the pathogenesis of Peyronie’s disease. World J. Urol. 2020, 38, 253–261. [Google Scholar] [CrossRef]
- Soreide, E.; Murad, M.H.; Denbeigh, J.M.; Dudakovic, A.; Kakar, S.; Lewallen, E.A.; Nordsletten, L.; Van Wijnen, A.J. Treatment of Dupuytren’s contracture: A systematic review. Bone Jt. J. 2018, 100B, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Al-Qattan, M.M. Factors in the pathogenesis of Dupuytren’s contracture. J. Hand Surg. Am. 2006, 31, 1527–1534. [Google Scholar] [CrossRef]
- Nellas, C.L.; Crawford, N.; Scherbel, A.L. Pancreatic collagenase therapy for severe, progressive systemic sclerosis: Effect on skin and on hydroxyproline content in urine. Clin. Pharmacol. Ther. 1965, 6, 367–371. [Google Scholar] [CrossRef]
- Mills, S.A.; Gelbard, M.K. Sixty years in the making: Collagenase Clostridium histolyticum, from benchtop to FDA approval and beyond. World J. Urol. 2020, 38, 269–277. [Google Scholar] [CrossRef]
- Gilpin, D.; Coleman, S.; Hall, S.; Houston, A.; Karrasch, J.; Jones, N. Injectable collagenase clostridium histolyticum: A new nonsurgical treatment for Dupuytren’s disease. J. Hand Surg. Am. 2010, 35, 2027–2038.e1. [Google Scholar] [CrossRef]
- Baeza, A.; Guisasola, E.; Torres-Pardo, A.; González-Calbet, J.M.; Melen, G.J.; Ramirez, M.; Vallet-Regí, M. Hybrid enzyme-polymeric capsules/mesoporous silica nanodevice for in situ cytotoxic agent generation. Adv. Funct. Mater. 2014, 24, 4625–4633. [Google Scholar] [CrossRef] [Green Version]
- Simmchen, J.; Baeza, A.; Ruiz-Molina, D.; Vallet-Regí, M. Improving catalase-based propelled motor endurance by enzyme encapsulation. Nanoscale 2014, 6, 8907–8913. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.R.; Baeza, A.; Vallet-Regí, M. Hybrid collagenase nanocapsules for enhanced nanocarrier penetration in tumoral tissues. ACS Appl. Mater. Interfaces 2015, 7, 24075–24081. [Google Scholar] [CrossRef] [Green Version]
- Villegas, M.R.; Baeza, A.; Noureddine, A.; Durfee, P.N.; Butler, K.S.; Agola, J.O.; Brinker, C.J.; Vallet-Regí, M. Multifunctional protocells for enhanced penetration in 3D extracellular tumoral matrices. Chem. Mater. 2018, 30, 112–120. [Google Scholar] [CrossRef]
- Villegas, M.R.; Baeza, A.; Usategui, A.; Ortiz-Romero, P.L.; Pablos, J.L.; Vallet-Regí, M. Collagenase nanocapsules: An approach to fibrosis treatment. Acta Biomater. 2018, 74, 430–438. [Google Scholar] [CrossRef]
- Moreno, V.M.; Baeza, A.; Vallet-regí, M. Evaluation of the penetration process of fluorescent collagenase nanocapsules in a 3D collagen gel. Acta Biomater. 2021, 121, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Cal, R.; Janett, E.; Hoffmann, V.; Bochet, C.G.; Constable, E.; Beaufils, F.; Wymann, M.P. Cell-permeant and photocleavable chemical inducer of dimerization. Angew. Chem. Int. Ed. 2014, 53, 4717–4720. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Lorenzo, C.; Bromberg, L.; Concheiro, A. Light-sensitive intelligent drug delivery systems. Photochem. Photobiol. 2009, 85, 848–860. [Google Scholar] [CrossRef]
- Fomina, N.; McFearin, C.; Sermsakdi, M.; Edigin, O.; Almutairi, A. UV and near-IR triggered release from polymeric nanoparticles. J. Am. Chem. Soc. 2010, 132, 9540–9542. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Zheng, J.; Zhou, Z.; Zhang, D. Approaches for the synthesis of o-nitrobenzyl and coumarin linkers for use in photocleavable biomaterials and bioconjugates and their biomedical applications. Acta Biomater. 2020, 115, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.; Guardado-Alvarez, T.M.; Ayaz-Gunner, S.; Ge, Y.; Jin, S. A family of photolabile nitroveratryl-based surfactants that self-assemble into photodegradable supramolecular structures. Langmuir 2016, 32, 3963–3969. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Miguel, V.S.; Del Campo, A. Light-triggered multifunctionality at surfaces mediated by photolabile protecting groups. Macromol. Rapid Commun. 2013, 34, 310–329. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Baeza, A.; Rodriguez-Milla, M.A.; García-Castro, J.; Vallet-Regí, M. Mesoporous silica nanoparticles grafted with a light-responsive protein shell for highly cytotoxic antitumoral therapy. J. Mater. Chem. B 2015, 3, 5746–5752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegner, S.V.; Sentürk, O.I.; Spatz, J.P. Photocleavable linker for the patterning of bioactive molecules. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Feeney, M.J.; McIntosh, E.; Mullahoo, J.; Jia, F.; Xu, Q.; Thomas, S.W. Triggered release of encapsulated cargo from photoresponsive polyelectrolyte nanocomplexes. ACS Appl. Mater. Interfaces 2016, 8, 23517–23522. [Google Scholar] [CrossRef]
- Chaowattanapanit, S.; Choonhakarn, C.; Foocharoen, C.; Julanon, N. Phototherapy in systemic sclerosis: Review. Photodermatol. Photoimmunol. Photomed. 2017, 33, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Sunderkötter, C.; Kuhn, A.; Hunzelmann, N.; Beissert, S. Phototherapy: A promising treatment option for skin sclerosis in scleroderma? Rheumatology 2006, 45, 52–54. [Google Scholar] [CrossRef] [Green Version]
- Gambichler, T.; Terras, S.; Kreuter, A. Treatment regimens, protocols, dosage, and indications for UVA1 phototherapy: Facts and controversies. Clin. Dermatol. 2013, 31, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Stoscheck, C.M. Quantitation of protein. Methods Enzymol. 1990, 182, 50–68. [Google Scholar] [CrossRef]
- Šolomek, T.; Mercier, S.; Bally, T.; Bochet, C.G. Photolysis of ortho-nitrobenzylic derivatives: The importance of the leaving group. Photochem. Photobiol. Sci. 2012, 11, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Diamond, S.L. Photocleavage of o-nitrobenzyl ether derivatives for rapid biomedical release applications. Bioorg. Med. Chem. Lett. 2006, 16, 4007–4010. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.R.; Baeza, A.; Vallet-Regí, M. Nanotechnological strategies for protein delivery. Molecules 2018, 23, 1008. [Google Scholar] [CrossRef] [Green Version]
- Schlaak, M.; Schwind, S.; Wetzig, T.; Maschke, J.; Treudler, R.; Basara, N.; Lange, T.; Simon, J.C.; Niederwieser, D.; Al-Ali, H.K. UVA (UVA-1) therapy for the treatment of acute GVHD of the skin. Bone Marrow Transplant. 2010, 45, 1741–1748. [Google Scholar] [CrossRef] [Green Version]
- Khaskhely, N.M.; Maruno, M.; Takamiyagi, A.; Uezato, H.; Kasem, K.M.A.; Hosokawa, A.; Kariya, K.I.; Hashiguchi, Y.; Landires, E.A.G.; Nonaka, S. Pre-exposure with low-dose UVA suppresses lesion development and enhances Th1 response in BALB/c mice infected with Leishmania (Leishmania) amazonensis. J. Dermatol. Sci. 2001, 26, 217–232. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, V.M.; Meroño, C.; Baeza, A.; Usategui, A.; Ortiz-Romero, P.L.; Pablos, J.L.; Vallet-Regí, M. UVA-Degradable Collagenase Nanocapsules as a Potential Treatment for Fibrotic Diseases. Pharmaceutics 2021, 13, 499. https://doi.org/10.3390/pharmaceutics13040499
Moreno VM, Meroño C, Baeza A, Usategui A, Ortiz-Romero PL, Pablos JL, Vallet-Regí M. UVA-Degradable Collagenase Nanocapsules as a Potential Treatment for Fibrotic Diseases. Pharmaceutics. 2021; 13(4):499. https://doi.org/10.3390/pharmaceutics13040499
Chicago/Turabian StyleMoreno, Víctor M., Carolina Meroño, Alejandro Baeza, Alicia Usategui, Pablo L. Ortiz-Romero, José L. Pablos, and María Vallet-Regí. 2021. "UVA-Degradable Collagenase Nanocapsules as a Potential Treatment for Fibrotic Diseases" Pharmaceutics 13, no. 4: 499. https://doi.org/10.3390/pharmaceutics13040499







