Antidiabetic Flavonoids from Fruits of Morus alba Promoting Insulin-Stimulated Glucose Uptake via Akt and AMP-Activated Protein Kinase Activation in 3T3-L1 Adipocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Extraction, and Isolation
2.2. Chemical Reagents
2.3. 3T3-L1 Preadipocyte Differentiation
2.4. Cell Viability Assay
2.5. Glucose Uptake Assay
2.6. Western Blot Analysis
2.7. Oil-Red-O Staining
2.8. Statistical Analysis
3. Results
3.1. Isolation of Compounds
3.2. Effect of Rutin and Q3G on 3T3-L1 Cell Viability
3.3. Effect of Rutin and Q3G on Glucose Uptake in Differentiated 3T3-L1 Adipocytes
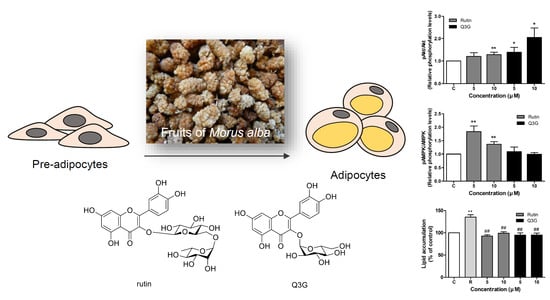
3.4. Effect of Rutin and Q3G on Akt or AMPK Phosphorylation
3.5. Effect of Rutin and Q3G on Lipid Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Joost, H.-G.; Thorens, B. The extended GLUT-family of sugar/polyol transport facilitators: Nomenclature, sequence characteristics, and potential function of its novel members. Mol. Membr. Biol. 2001, 18, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.T.; Kanzaki, M.; Pessin, J.E. Regulated Membrane Trafficking of the Insulin-Responsive Glucose Transporter 4 in Adipocytes. Endocr. Rev. 2004, 25, 177–204. [Google Scholar] [CrossRef]
- Herman, M.A.; Kahn, B.B. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J. Clin. Investig. 2006, 116, 1767–1775. [Google Scholar] [CrossRef]
- Cong, L.-N.; Chen, H.; Li, Y.; Zhou, L.; McGibbon, M.A.; Taylor, S.I.; Quon, M.J. Physiological Role of Akt in Insulin-Stimulated Translocation of GLUT4 in Transfected Rat Adipose Cells. Mol. Endocrinol. 1997, 11, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Cushman, S.W.; Wardzala, L.J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J. Biol. Chem. 1980, 255, 4758–4762. [Google Scholar] [CrossRef]
- Wiernsperger, N. Is non-insulin dependent glucose uptake a therapeutic alternative? Part 1: Physiology, mechanisms and role of non insulin-dependent glucose uptake in type 2 diabetes. Diabetes Metab. 2005, 31, 415–426. [Google Scholar] [CrossRef]
- Sesti, G. Pathophysiology of insulin resistance. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 665–679. [Google Scholar] [CrossRef]
- Newgard, C.B.; Hirsch, L.J.; Foster, D.W.; McGarry, J.D. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J. Biol. Chem. 1983, 258, 8046–8052. [Google Scholar] [CrossRef]
- De Meyts, P.; Whittaker, J. Structural biology of insulin and IGF1 receptors: Implications for drug design. Nat. Rev. Drug Discov. 2002, 1, 769–783. [Google Scholar] [CrossRef]
- White, M.F. IRS proteins and the common path to diabetes. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E413–E422. [Google Scholar] [CrossRef]
- Alessi, D.R.; Downes, C.P. The role of PI 3-kinase in insulin action. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 1998, 1436, 151–164. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Alessi, D.R. The PI3K–PDK1 connection: More than just a road to PKB. Biochem. J. 2000, 346, 561–576. [Google Scholar]
- Beeson, M.; Sajan, M.P.; Dizon, M.; Grebenev, D.; Gomez-Daspet, J.; Miura, A.; Kanoh, Y.; Powe, J.; Bandyopadhyay, G.; Standaert, M.L.; et al. Activation of Protein Kinase C-by Insulin and Phosphatidylinositol-3,4,5-(PO4)3 Is Defective in Muscle in Type 2 Diabetes and Impaired Glucose Tolerance: Amelioration by Rosiglitazone and Exercise. Diabetes 2003, 52, 1926–1934. [Google Scholar] [CrossRef]
- Gonzalez, E.; McGraw, T.E. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 7004–7009. [Google Scholar] [CrossRef]
- Gr, S.; Be, K. amPK in health and disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Fryer, L.G.D.; Parbu-Patel, A.; Carling, D. The Anti-diabetic Drugs Rosiglitazone and Metformin Stimulate AMP-activated Protein Kinase through Distinct Signaling Pathways. J. Biol. Chem. 2002, 277, 25226–25232. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Katahira, H.; Ozawa, S.; Nakamichi, Y.; Tanaka, T.; Shimoyama, T.; Takahashi, K.; Yoshimoto, K.; Imaizumi, M.O.; Nagamatsu, S.; et al. Activators of AMP-activated protein kinase enhance GLUT4 translocation and its glucose transport activity in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E643–E649. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. What We Talk About When We Talk About Fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, J.; Stephen, R.D. Obesity, Insulin Resistance, Diabetes, and Cardiovascular Risk in Children. Circulation 2003, 107, 1448–1453. [Google Scholar] [CrossRef]
- Qatanani, M.; Lazar, M.A. Mechanisms of obesity-associated insulin resistance: Many choices on the menu. Genes Dev. 2007, 21, 1443–1455. [Google Scholar] [CrossRef]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef]
- Vázquez-Vela, M.E.F.; Torres, N.; Tovar, A.R. White Adipose Tissue as Endocrine Organ and Its Role in Obesity. Arch. Med. Res. 2008, 39, 715–728. [Google Scholar] [CrossRef]
- Sethi, J.K.; Vidal-Puig, A. Visfatin: The missing link between intra-abdominal obesity and diabetes? Trends Mol. Med. 2005, 11, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Parulkar, A.A.; Pendergrass, M.L.; Granda-Ayala, R.; Lee, T.R.; Fonseca, V.A. Nonhypoglycemic effects of thiazolidinediones. Ann. Intern. Med. 2001, 134, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, D.; Ryoo, R.; Kim, J.C.; Park, H.B.; Kang, K.S.; Kim, K.H. Calvatianone, a Sterol Possessing a 6/5/6/5-Fused Ring System with a Contracted Tetrahydrofuran B-Ring, from the Fruiting Bodies of Calvatia nipponica. J. Nat. Prod. 2020, 83, 2737–2742. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Kang, H.S.; Yoo, M.J.; Yi, S.A.; Beemelmanns, C.; Lee, J.C.; Kim, K.H. Anti-adipogenic Pregnane Steroid from a Hydractinia-associated Fungus, Cladosporium sphaerospermum SW67. Nat. Prod. Sci. 2020, 26, 230–235. [Google Scholar]
- Lee, S.; Ryoo, R.; Choi, J.H.; Kim, J.H.; Kim, S.H.; Kim, K.H. Trichothecene and tremulane sesquiterpenes from a hallucinogenic mushroom Gymnopilus junonius and their cytotoxicity. Arch. Pharm. Res. 2020, 43, 214–223. [Google Scholar] [CrossRef]
- Trinh, T.A.; Park, E.J.; Lee, D.; Song, J.H.; Lee, H.L.; Kim, K.H.; Kim, Y.; Jung, K.; Kang, K.S.; Yoo, J.E. Estrogenic Activity of Sanguiin H-6 through Activation of Estrogen Receptor α Coactivator-binding Site. Nat. Prod. Sci. 2019, 25, 28–33. [Google Scholar] [CrossRef][Green Version]
- Ha, J.W.; Kim, J.; Kim, H.; Jang, W.; Kim, K.H. Mushrooms: An Important Source of Natural Bioactive Compounds. Nat. Prod. Sci. 2020, 26, 118–131. [Google Scholar]
- De Padua, L.S.; Bunyapraphatsara, N.; Lemmens, R.H.M.J. Plant Resources of South-East Asia 12:(1) Medicinal and Poisonous Plants 1; Backhuys Publishers: Leiden, The Netherlands, 1999. [Google Scholar]
- Suttie, J.M.; Reynolds, S.G.; Batello, C. Grasslands of the World; Food and Agriculture Organization: Rome, Italy, 2005; Volume 34. [Google Scholar]
- Bae, S.-H.; Suh, H.-J. Antioxidant activities of five different mulberry cultivars in Korea. LWT Food Sci. Technol. 2007, 40, 955–962. [Google Scholar] [CrossRef]
- Katsube, T.; Imawaka, N.; Kawano, Y.; Yamazaki, Y.; Shiwaku, K.; Yamane, Y. Antioxidant flavonol glycosides in mulberry (Morus alba L.) leaves isolated based on LDL antioxidant activity. Food Chem. 2006, 97, 25–31. [Google Scholar] [CrossRef]
- Machii, H.; Koyama, A.; Yamanouchi, H. Mulberry for Animal Production. Mulberry breeding, cultivation and utilization in Japan. FAO Anim. Prod. Health Pap. 2002, 147, 63. [Google Scholar]
- Chan, E.W.; Lye, P.-Y.; Wong, S.-K. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar]
- Ramappa, V.K.; Srivastava, D.; Singh, P.; Kumar, U.; Kumar, D.; Gosipatala, S.B.; Saha, S.; Kumar, D.; Raj, R. Mulberries: A Promising Fruit for Phytochemicals, Nutraceuticals, and Biological Activities. Int. J. Fruit Sci. 2020, 1–26. [Google Scholar] [CrossRef]
- Lee, S.R.; Park, J.Y.; Yu, J.S.; Lee, S.O.; Ryu, J.Y.; Choi, S.Z.; Kang, K.S.; Yamabe, N.; Kim, K.H. Odisolane, a Novel Oxolane Derivative, and Antiangiogenic Constituents from the Fruits of Mulberry (Morus alba L.). J. Agric. Food Chem. 2016, 64, 3804–3809. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Lee, D.; Lee, S.R.; Lee, J.W.; Choi, C.I.; Jang, T.S.; Kang, K.S.; Kim, K.H. Chemical characterization of cytotoxic indole acetic acid derivative from mulberry fruit (Morus alba L.) against human cervical cancer. Bioorg. Chem. 2018, 76, 28–36. [Google Scholar] [CrossRef]
- Lee, D.; Yu, J.S.; Lee, S.R.; Hwang, G.S.; Kang, K.S.; Park, J.G.; Kim, H.Y.; Kim, K.H.; Yamabe, N. Beneficial effects of bioactive compounds in mulberry fruits against cisplatin-induced nephrotoxicity. Int. J. Mol. Sci. 2018, 19, 1117. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, D.; Lee, S.R.; Yu, J.S.; Jang, T.S.; Nam, J.W.; Kim, K.H.; Kang, K.S. Identification of bioactive heterocyclic compounds from mulberry and their protective effect against streptozotocin-induced apoptosis in INS-1 cells. Mol. Med. Rep. 2018, 17, 5982–5987. [Google Scholar] [CrossRef]
- Xin, C.; Quan, H.; Kim, J.M.; Hur, Y.H.; Shin, J.Y.; Bae, H.B.; Choi, J.I. Ginsenoside Rb1 increases macrophage phagocytosis through p38 mitogen-activated protein kinase/Akt pathway. J. Ginseng Res. 2019, 43, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, R.F.; Zhou, Y.; Hu, H.J.; Yang, Y.B.; Yang, L.; Wang, Z.T. Dammarane-type triterpene oligoglycosides from the leaves and stems of Panax notoginseng and their antiinflammatory activities. J. Ginseng Res. 2019, 43, 377–384. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Miranda-Torres, A.C.; González-Chávez, M.M.; Salazar-Olivo, L.A. Cecropia obtusifolia Bertol and its active compound, chlorogenic acid, stimulate 2-NBDglucose uptake in both insulin-sensitive and insulin-resistant 3T3 adipocytes. J. Ethnopharmacol. 2008, 120, 458–464. [Google Scholar] [CrossRef]
- Li, Y.L.; Li, J.; Wang, N.L.; Yao, X.S. Flavonoids and a new polyacetylene from Bidens parviflora Willd. Molecules 2008, 13, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Han, J.T.; Bang, M.H.; Chun, O.K.; Kim, D.O.; Lee, C.Y.; Baek, N.I. Flavonol glycosides from the aerial parts of Aceriphyllum rossii and their antioxidant activities. Arch. Pharm. Res. 2004, 27, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R.; Baird, K.L.; Flier, J.S.; Grunfeld, C.; Harmon, J.T.; Harrison, L.C.; Karlsson, F.A.; Kasuga, M.; King, G.L.; Lang, U.C.; et al. Insulin Receptors, Receptor Antibodies, and the Mechanism of Insulin Action. In Proceedings of the 1980 Laurentian Hormone Conference; Academic Press: Cambridge, MA, USA, 1981; pp. 477–538. [Google Scholar]
- Szendroedi, J.; Phielix, E.; Roden, M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Hollander, P. Anti-Diabetes and Anti-Obesity Medications: Effects on Weight in People with Diabetes. Diabetes Spectr. 2007, 20, 159–165. [Google Scholar] [CrossRef]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Selloum, L.; Bouriche, H.; Tigrine, C.; Boudoukha, C. Anti-inflammatory effect of rutin on rat paw oedema, and on neutrophils chemotaxis and degranulation. Exp. Toxicol. Pathol. 2003, 54, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Mellou, F.; Loutrari, H.; Stamatis, H.; Roussos, C.; Kolisis, F.N. Enzymatic esterification of flavonoids with unsaturated fatty acids: Effect of the novel esters on vascular endothelial growth factor release from K562 cells. Process Biochem. 2006, 41, 2029–2034. [Google Scholar] [CrossRef]
- La Casa, C.; Villegas, I.; Alarcón de la Lastra, C.; Motilva, V.; Martín Calero, M.J. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J. Ethnopharmacol. 2000, 71, 45–53. [Google Scholar] [CrossRef]
- Janbaz, K.H.; Saeed, S.A.; Gilani, A.H. Protective effect of rutin on paracetamol- and CCl4-induced hepatotoxicity in rodents. Fitoterapia 2002, 73, 557–563. [Google Scholar] [CrossRef]
- Richetti, S.K.; Blank, M.; Capiotti, K.M.; Piato, A.L.; Bogo, M.R.; Vianna, M.R.; Bonan, C.D. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav. Brain Res. 2011, 217, 10–15. [Google Scholar] [CrossRef]
- Javed, H.; Khan, M.M.; Ahmad, A.; Vaibhav, K.; Ahmad, M.E.; Khan, A.; Ashafaq, M.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience 2012, 210, 340–352. [Google Scholar] [CrossRef]
- Sadeghnia, H.R.; Yousefsani, B.S.; Rashidfar, M.; Boroushaki, M.T.; Asadpour, E.; Ghorbani, A. Protective effect of rutin on hexachlorobutadiene-induced nephrotoxicity. Renal Fail. 2013, 35, 1151–1155. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, Y.; Morrison, R.F.; Bucher, N.L.; Farmer, S.R. PPARgamma induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPalpha during the conversion of 3T3 fibroblasts into adipocytes. J. Clin. Investig. 1998, 101, 22–32. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Pessin, J. Insulin regulation of glucose uptake: A complex interplay of intracellular signalling pathways. Diabetologia 2002, 45, 1475–1483. [Google Scholar]
- Huang, S.; Czech, M.P. The GLUT4 Glucose Transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-H.; Lin, C.-H.; Shih, C.-C. Caffeamide 36-13 Regulates the Antidiabetic and Hypolipidemic Signs of High-Fat-Fed Mice on Glucose Transporter 4, AMPK Phosphorylation, and Regulated Hepatic Glucose Production. Evid. Based Complement. Alternat. Med. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Grisouard, J.; Timper, K.; Radimerski, T.M.; Frey, D.M.; Peterli, R.; Kola, B.; Korbonits, M.; Herrmann, P.; Krähenbühl, S.; Zulewski, H.; et al. Mechanisms of metformin action on glucose transport and metabolism in human adipocytes. Biochem. Pharmacol. 2010, 80, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ye, C.; Liu, W.; Zhao, W.; Zhang, Y.-J.; Zhang, H.; Ying, H. AICAR enhances insulin signaling via downregulation of miR-29. Can. J. Physiol. Pharmacol. 2016, 94, 199–205. [Google Scholar] [CrossRef]
- Gaidhu, M.P.; Ceddia, R.B. The Role of Adenosine Monophosphate Kinase in Remodeling White Adipose Tissue Metabolism. Exerc. Sport Sci. Rev. 2011, 39, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.P.; Lin, J.K. Epigallocatechin gallate (EGCG) and rutin suppress the glucotoxicity through activating IRS2 and AMPK signaling in rat pancreatic beta cells. J. Agric. Food Chem. 2009, 57, 9817–9827. [Google Scholar] [CrossRef]
- Yeo, C.-R.; Yang, C.; Wong, T.-Y.; Popovich, D.G. A Quantified Ginseng (Panax ginseng C.A. Meyer) Extract Influences Lipid Acquisition and Increases Adiponectin Expression in 3T3-L1 Cells. Molecules 2011, 16, 477–492. [Google Scholar] [CrossRef]
- Avram, M.M.; Avram, A.S.; James, W.D. Subcutaneous fat in normal and diseased states: 3. Adipogenesis: From stem cell to fat cell. J. Am. Acad. Dermatol. 2007, 56, 472–492. [Google Scholar] [CrossRef]
- Camp, H.S.; Ren, D.; Leff, T. Adipogenesis and fat-cell function in obesity and diabetes. Trends. Mol. Med. 2002, 8, 442–447. [Google Scholar] [CrossRef]
- White, U.A.; Stephens, J.M. Transcriptional factors that promote formation of white adipose tissue. Mol. Cell. Endocrinol. 2010, 318, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, T.-F.; Chang, C.J.; Liu, I.-M. 6-Gingerol Inhibits Rosiglitazone-Induced Adipogenesis in 3T3-L1 Adipocytes. Phytother. Res. 2014, 28, 187–192. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.H.; Yu, J.S.; Lee, H.S.; Choi, C.-I.; Kim, K.H. Antidiabetic Flavonoids from Fruits of Morus alba Promoting Insulin-Stimulated Glucose Uptake via Akt and AMP-Activated Protein Kinase Activation in 3T3-L1 Adipocytes. Pharmaceutics 2021, 13, 526. https://doi.org/10.3390/pharmaceutics13040526
Lim SH, Yu JS, Lee HS, Choi C-I, Kim KH. Antidiabetic Flavonoids from Fruits of Morus alba Promoting Insulin-Stimulated Glucose Uptake via Akt and AMP-Activated Protein Kinase Activation in 3T3-L1 Adipocytes. Pharmaceutics. 2021; 13(4):526. https://doi.org/10.3390/pharmaceutics13040526
Chicago/Turabian StyleLim, Sung Ho, Jae Sik Yu, Ho Seon Lee, Chang-Ik Choi, and Ki Hyun Kim. 2021. "Antidiabetic Flavonoids from Fruits of Morus alba Promoting Insulin-Stimulated Glucose Uptake via Akt and AMP-Activated Protein Kinase Activation in 3T3-L1 Adipocytes" Pharmaceutics 13, no. 4: 526. https://doi.org/10.3390/pharmaceutics13040526
APA StyleLim, S. H., Yu, J. S., Lee, H. S., Choi, C.-I., & Kim, K. H. (2021). Antidiabetic Flavonoids from Fruits of Morus alba Promoting Insulin-Stimulated Glucose Uptake via Akt and AMP-Activated Protein Kinase Activation in 3T3-L1 Adipocytes. Pharmaceutics, 13(4), 526. https://doi.org/10.3390/pharmaceutics13040526