Development of a Nanostructured Lipid Carrier (NLC) by a Low-Energy Method, Comparison of Release Kinetics and Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Nanostructured Lipid Carrier (NLC) and Loading of Rhodamine 123
2.3. Incorporation Efficiency and Drug Loading
2.4. Particle Size, Polydispersity Index, Zeta Potential Analysis, and NLC Particle Concentration
2.5. Morphology of NLC
2.6. Calorimetric Evaluation of NLC
2.7. Freeze-Drying and Reconstitution Studies
2.8. Colloidal Stability
2.9. pH Stability of NLC-Rho
2.10. Release Studies
2.11. Molecular Dynamics Simulations
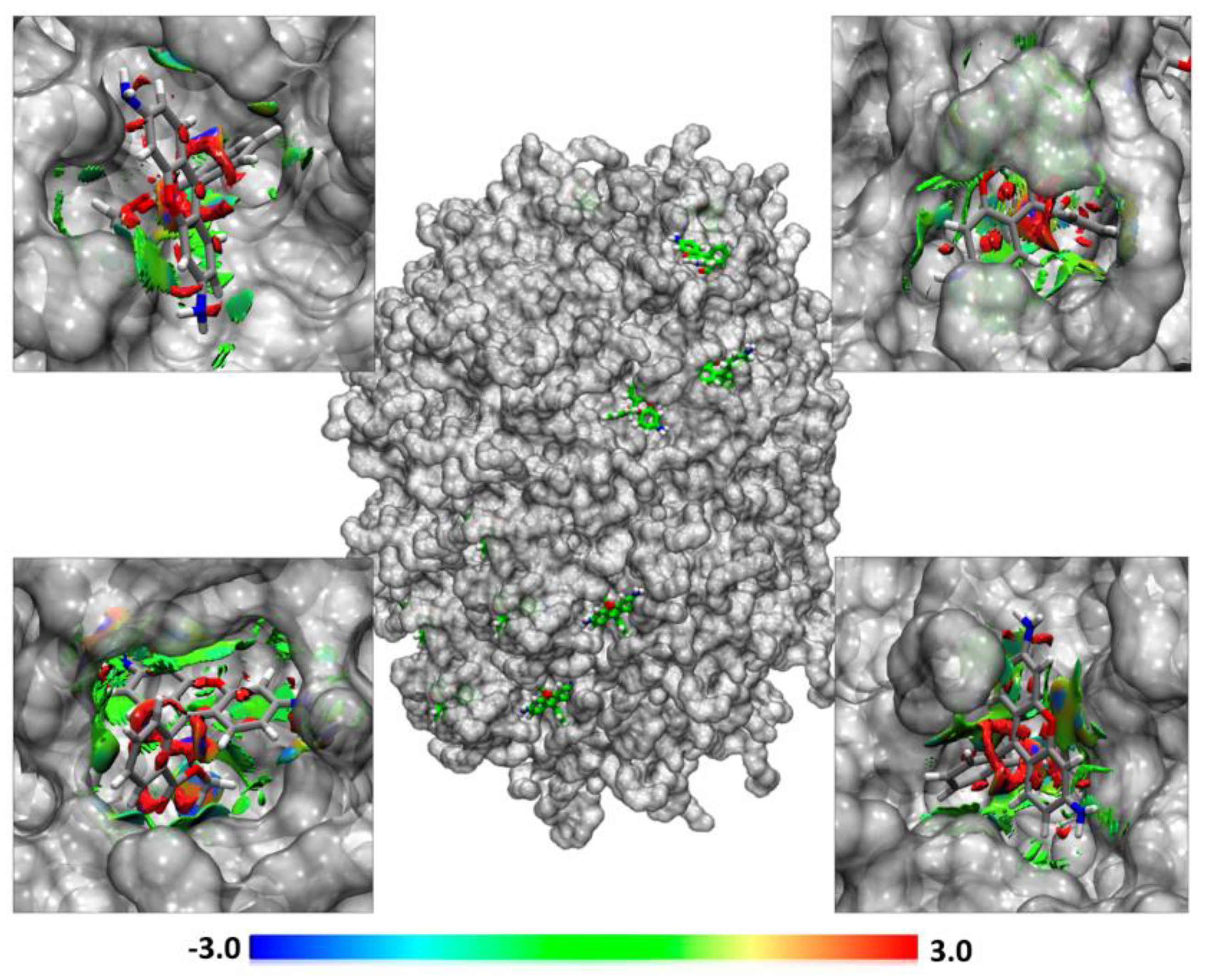
2.12. Non-Covalent Interaction Index
2.13. Data Analysis and Statistics
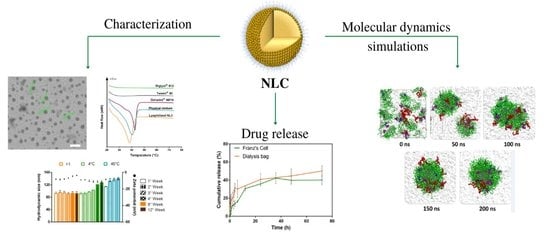
3. Results and Discussion
3.1. Low-Energy Synthesis and Physicochemical Properties of Novel NLC
3.2. Morphology
3.3. Physical State Evaluation of NLC
3.4. Freeze-Drying and Reconstitution Studies
3.5. Incorporation Efficiency and Drug Loading
3.6. Colloidal Stability
3.7. pH Stability of NLC-Rho
3.8. Release Studies
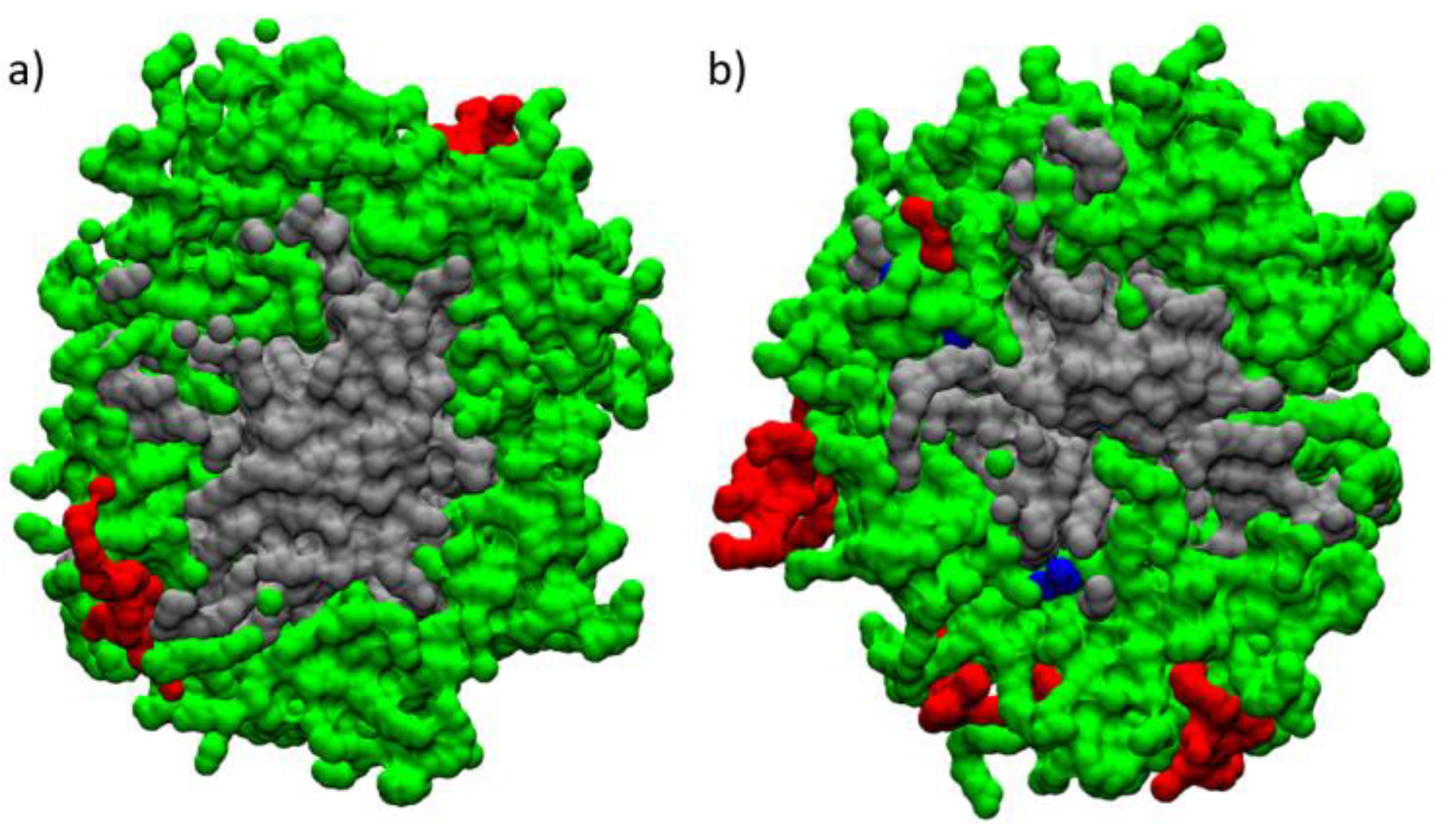
3.9. Molecular Dynamics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hu, Y.; Niemeyer, C.M. From DNA Nanotechnology to Material Systems Engineering. Adv. Mater. 2019, 31, 1806294. [Google Scholar] [CrossRef] [Green Version]
- Cheon, J.; Chan, W.; Zuhorn, I. The Future of Nanotechnology: Cross-Disciplined Progress to Improve Health and Medicine. Acc. Chem. Res. 2019, 52, 2405. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Xiao, L.; Zhang, J.; Fu, H. The Role of Nanotechnology in Subgrade and Pavement Engineering: A Review. J. Nanosci. Nanotechnol. 2020, 20, 4607–4618. [Google Scholar] [CrossRef]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramaniam, B.; Siddik, Z.H.; Nagoor, N.H. Optimization of Nanostructured Lipid Carriers: Understanding the Types, Designs, and Parameters in the Process of Formulations. J. Nanopart. Res. 2020, 22, 141. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured Lipid Carriers and Their Current Application in Targeted Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured Lipid Carriers for Site-Specific Drug Delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, L. Nanoscale Delivery System for Nutraceuticals: Preparation, Application, Characterization, Safety, and Future Trends. Food Eng. Rev. 2020, 12, 14–31. [Google Scholar] [CrossRef]
- Nothnagel, L.; Wacker, M.G. How to Measure Release from Nanosized Carriers? Eur. J. Pharm. Sci. 2018, 120, 199–211. [Google Scholar] [CrossRef]
- Sheshala, R.; Anuar, N.K.; Abu Samah, N.H.; Wong, T.W. In Vitro Drug Dissolution/Permeation Testing of Nanocarriers for Skin Application: A Comprehensive Review. AAPS PharmSciTech 2019, 20, 164. [Google Scholar] [CrossRef]
- Yu, M.; Yuan, W.; Li, D.; Schwendeman, A.; Schwendeman, S.P. Predicting Drug Release Kinetics from Nanocarriers inside Dialysis Bags. J. Control. Release 2019, 315, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Karplus, M.; McCammon, J.A. Molecular Dynamics Simulations of Biomolecules. Nat. Struct. Biol. 2002, 9, 646–652. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Ye, T.; Chen, F.; Sun, X.; Kong, J.; Yang, X.; Pan, W.; Li, S. Design, Characterization, and in Vitro Cellular Inhibition and Uptake of Optimized Genistein-Loaded NLC for the Prevention of Posterior Capsular Opacification Using Response Surface Methodology. Int. J. Pharm. 2013, 454, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Ditchfield, R. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Dodda, L.S.; Vilseck, J.Z.; Tirado-Rives, J.; Jorgensen, W.L. 1.14*CM1A-LBCC: Localized Bond-Charge Corrected CM1A Charges for Condensed-Phase Simulations. J. Phys. Chem. B 2017, 121, 3864–3870. [Google Scholar] [CrossRef] [Green Version]
- Dodda, L.S.; Cabeza de Vaca, I.; Tirado-Rives, J.; Jorgensen, W.L. LigParGen Web Server: An Automatic OPLS-AA Parameter Generator for Organic Ligands. Nucleic Acids Res. 2017, 45, W331–W336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, W.L.; Tirado-Rives, J. Potential Energy Functions for Atomic-Level Simulations of Water and Organic and Biomolecular Systems. Proc. Natl. Acad. Sci. USA 2005, 102, 6665–6670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eastman, P.; Swails, J.; Chodera, J.D.; McGibbon, R.T.; Zhao, Y.; Beauchamp, K.A.; Wang, L.P.; Simmonett, A.C.; Harrigan, M.P.; Stern, C.D.; et al. OpenMM 7: Rapid Development of High Performance Algorithms for Molecular Dynamics. PLoS Comput. Biol. 2017, 13, 1–17. [Google Scholar] [CrossRef]
- Eastman, P.; Pande, V.S. Constant Constraint Matrix Approximation: A Robust, Parallelizable Constraint Method for Molecular Simulations. J. Chem. Theory Comput. 2010, 6, 434–437. [Google Scholar] [CrossRef]
- Wang, L.-P.; Martinez, T.J.; Pande, V.S. Building Force Fields: An Automatic, Systematic, and Reproducible Approach. J. Phys. Chem. Lett. 2014, 5, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. Packmol: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the Design of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Targeting Brain Diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Joos, A.; Weiss, J.; McClements, D.J. Fabrication of Lipophilic Nanoparticles by Spontaneous Emulsification: Stabilization by Cosurfactants. Food Biophys 2015, 10, 83–93. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: A Review Emphasizing on Particle Structure and Drug Release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, H.; Cheng, S.; Zhai, G.; Shen, C. Development of Curcumin Loaded Nanostructured Lipid Carrier Based Thermosensitive in Situ Gel for Dermal Delivery. Colloids Surf. Physicochem. Eng. Asp. 2016, 506, 356–362. [Google Scholar] [CrossRef]
- Carbone, C.; Campisi, A.; Musumeci, T.; Raciti, G.; Bonfanti, R.; Puglisi, G. FA-Loaded Lipid Drug Delivery Systems: Preparation, Characterization and Biological Studies. Eur. J. Pharm. Sci. 2014, 52, 12–20. [Google Scholar] [CrossRef]
- Sun, M.; Zhu, Z.; Wang, H.; Han, C.; Liu, D.; Tian, L.; Yang, X.; Pan, W. Surface Density of Polyarginine Influence the Size, Zeta Potential, Cellular Uptake and Tissue Distribution of the Nanostructured Lipid Carrier. Drug Deliv. 2017, 24, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of Food Grade Nanostructured Lipid Carrier (NLC) for Potential Applications in Medicinal-Functional Foods. J. Drug Deliv. Sci. Technol. 2017, 39, 50–58. [Google Scholar] [CrossRef]
- Alvarez-Trabado, J.; Diebold, Y.; Sanchez, A. Designing Lipid Nanoparticles for Topical Ocular Drug Delivery. Int. J. Pharm. 2017, 532, 204–217. [Google Scholar] [CrossRef]
- Marengo, E.; Cavalli, R.; Caputo, O.; Rodriguez, L.; Gasco, M.R. Scale-up of the Preparation Process of Solid Lipid Nanospheres. Part I. Int. J. Pharm. 2000, 205, 3–13. [Google Scholar] [CrossRef]
- Gao, S.; McClements, D.J. Formation and Stability of Solid Lipid Nanoparticles Fabricated Using Phase Inversion Temperature Method. Colloids Surf. Physicochem. Eng. Asp. 2016, 499, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Mehnert, W.; Mäder, K. Solid Lipid Nanoparticles: Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Fernandez, S.; Rodier, J.-D.; Ritter, N.; Mahler, B.; Demarne, F.; Carrière, F.; Jannin, V. Lipolysis of the Semi-Solid Self-Emulsifying Excipient Gelucire® 44/14 by Digestive Lipases. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2008, 1781, 367–375. [Google Scholar] [CrossRef]
- Panigrahi, K.C.; Patra, C.N.; Jena, G.K.; Ghose, D.; Jena, J.; Panda, S.K.; Sahu, M. Gelucire: A Versatile Polymer for Modified Release Drug Delivery System. Future J. Pharm. Sci. 2018, 4, 102–108. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukovac, S.; Gooijert, K.E.G.; Gregory, P.C.; Shlieout, G.; Stellaard, F.; Rings, E.H.H.M.; Verkade, H.J. Gelucire®44/14 Improves Fat Absorption in Rats with Impaired Lipolysis. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2010, 1801, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Borrás-Linares, I.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A.; Souto, E.B. Polyphenols-Enriched Hibiscus Sabdariffa Extract-Loaded Nanostructured Lipid Carriers (NLC): Optimization by Multi-Response Surface Methodology. J. Drug Deliv. Sci. Technol. 2019, 49, 660–667. [Google Scholar] [CrossRef]
- Baig, M.S.; Owida, H.; Njoroge, W.; Siddiqui, A.-R.; Yang, Y. Development and Evaluation of Cationic Nanostructured Lipid Carriers for Ophthalmic Drug Delivery of Besifloxacin. J. Drug Deliv. Sci. Technol. 2020, 55, 101496. [Google Scholar] [CrossRef]
- Levit, S.L.; Stwodah, R.M.; Tang, C. Rapid, Room Temperature Nanoparticle Drying and Low-Energy Reconstitution via Electrospinning. J. Pharm. Sci. 2018, 107, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-J.; Wu, P.-C.; Huang, Y.-B.; Chang, J.-S.; Lin, C.-L.; Tsai, Y.-H.; Fang, J.-Y. Baicalein Loaded in Tocol Nanostructured Lipid Carriers (Tocol NLCs) for Enhanced Stability and Brain Targeting. Int. J. Pharm. 2012, 423, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Design and Characterization of Astaxanthin-Loaded Nanostructured Lipid Carriers. Innov. Food Sci. Emerg. Technol. 2014, 26, 366–374. [Google Scholar] [CrossRef]
- Adabi, M.; Naghibzadeh, M.; Adabi, M.; Zarrinfard, M.A.; Esnaashari, S.S.; Seifalian, A.M.; Faridi-Majidi, R.; Aiyelabegan, H.T.; Ghanbari, H. Biocompatibility and Nanostructured Materials: Applications in Nanomedicine. Artif. Cells Nanomed. Biotechnol. 2017, 45, 833–842. [Google Scholar] [CrossRef]
- Yue, Z.-G.; Wei, W.; Lv, P.-P.; Yue, H.; Wang, L.-Y.; Su, Z.-G.; Ma, G.-H. Surface Charge Affects Cellular Uptake and Intracellular Trafficking of Chitosan-Based Nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Kedmi, R.; Ben-Arie, N.; Peer, D. The Systemic Toxicity of Positively Charged Lipid Nanoparticles and the Role of Toll-like Receptor 4 in Immune Activation. Biomaterials 2010, 31, 6867–6875. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [Green Version]
- Nnamani, P.O.; Hansen, S.; Windbergs, M.; Lehr, C.-M. Development of Artemether-Loaded Nanostructured Lipid Carrier (NLC) Formulation for Topical Application. Int. J. Pharm. 2014, 477, 208–217. [Google Scholar] [CrossRef]
- Varma, L.T.; Singh, N.; Gorain, B.; Choudhury, H.; Tambuwala, M.M.; Kesharwani, P.; Shukla, R. Recent Advances in Self-Assembled Nanoparticles for Drug Delivery. Curr. Drug Deliv. 2020, 17, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Damian, F.; Blaton, N.; Naesens, L.; Balzarini, J.; Kinget, R.; Augustijns, P.; Van den Mooter, G. Physicochemical Characterization of Solid Dispersions of the Antiviral Agent UC-781 with Polyethylene Glycol 6000 and Gelucire 44/14. Eur. J. Pharm. Sci. 2000, 10, 311–322. [Google Scholar] [CrossRef]
- Caon, T.; Konig, R.A.; da Cruz, A.C.C.; Cardoso, S.G.; Campos, C.E.M.; Cuffini, S.L.; Koester, L.S.; Simões, C.M.O. Development and Physicochemical Characterization of Saquinavir Mesylate Solid Dispersions Using Gelucire 44/14 or PEG 4000 as Carrier. Arch. Pharm. Res. 2013, 36, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Čerpnjak, K.; Zvonar, A.; Vrečer, F.; Gašperlin, M. Development of a Solid Self-Microemulsifying Drug Delivery System (SMEDDS) for Solubility Enhancement of Naproxen. Drug Dev. Ind. Pharm. 2015, 41, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, M.Y.; Kim, S.; Lee, J. Cryoprotectants for Freeze Drying of Drug Nano-Suspensions: Effect of Freezing Rate. J. Pharm. Sci. 2009, 98, 4808–4817. [Google Scholar] [CrossRef]
- Varshosaz, J.; Eskandari, S.; Tabbakhian, M. Freeze-Drying of Nanostructure Lipid Carriers by Different Carbohydrate Polymers Used as Cryoprotectants. Carbohydr. Polym. 2012, 88, 1157–1163. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Fessi, H. Investigation of Nanocapsules Stabilization by Amorphous Excipients during Freeze-Drying and Storage. Eur. J. Pharm. Biopharm. 2006, 63, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.C.; Casas, I.; Mella, P.; Naranjo, O.; Pizarro, N.; Vega, A.; Cerda-Opazo, P.; García, L.; Morales, J.O.; Cepeda-Plaza, M. Aptamer-Functionalized Lipid-Core Micelles Loaded with Rhenium Tricarbonyl Complex. Polyhedron 2021, 195, 114963. [Google Scholar] [CrossRef]
- Salvi, V.R.; Pawar, P. Nanostructured Lipid Carriers (NLC) System: A Novel Drug Targeting Carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]
- Safwat, S.; Ishak, R.A.H.; Hathout, R.M.; Mortada, N.D. Nanostructured Lipid Carriers Loaded with Simvastatin: Effect of PEG/Glycerides on Characterization, Stability, Cellular Uptake Efficiency and in Vitro Cytotoxicity. Drug Dev. Ind. Pharm. 2017, 43, 1112–1125. [Google Scholar] [CrossRef]
- Bashiri, S.; Ghanbarzadeh, B.; Ayaseh, A.; Dehghannya, J.; Ehsani, A. Preparation and Characterization of Chitosan-Coated Nanostructured Lipid Carriers (CH-NLC) Containing Cinnamon Essential Oil for Enriching Milk and Anti-Oxidant Activity. LWT 2020, 119, 108836. [Google Scholar] [CrossRef]
- Nakach, M.; Authelin, J.-R.; Tadros, T.; Galet, L.; Chamayou, A. Engineering of Nano-Crystalline Drug Suspensions: Employing a Physico-Chemistry Based Stabilizer Selection Methodology or Approach. Int. J. Pharm. 2014, 476, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.-O.; Aditya, N.P.; Ko, S. Effect of Aqueous PH and Electrolyte Concentration on Structure, Stability and Flow Behavior of Non-Ionic Surfactant Based Solid Lipid Nanoparticles. Food Chem. 2014, 147, 239–244. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Development of Nanostructured Lipid Carriers for the Encapsulation and Controlled Release of Vitamin D3. Food Chem. 2017, 225, 213–219. [Google Scholar] [CrossRef]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Nanoemulsion Delivery Systems for Oil-Soluble Vitamins: Influence of Carrier Oil Type on Lipid Digestion and Vitamin D3 Bioaccessibility. Food Chem. 2015, 187, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Soleimanian, Y.; Goli, S.A.H.; Varshosaz, J.; Di Cesare Mannelli, L.; Ghelardini, C.; Cirri, M.; Maestrelli, F. β-Sitosterol Loaded Nanostructured Lipid Carrier: Physical and Oxidative Stability, In Vitro Simulated Digestion and Hypocholesterolemic Activity. Pharmaceutics 2020, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured Lipid Carriers (NLC): A Potential Delivery System for Bioactive Food Molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- Garg, N.K.; Tyagi, R.K.; Singh, B.; Sharma, G.; Nirbhavane, P.; Kushwah, V.; Jain, S.; Katare, O.P. Nanostructured Lipid Carrier Mediates Effective Delivery of Methotrexate to Induce Apoptosis of Rheumatoid Arthritis via NF-ΚB and FOXO1. Int. J. Pharm. 2016, 499, 301–320. [Google Scholar] [CrossRef]
- Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L. (Ed.) Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. ISBN 978-0-08-100092-2. [Google Scholar]
- Alam, M.; Nikita, S.A.; Moon, G.; Aqil, M.; Sultana, Y. Chemical Engineering of a Lipid Nano-Scaffold for the Solubility Enhancement of an Antihyperlipidaemic Drug, Simvastatin; Preparation, Optimization, Physicochemical Characterization and Pharmacodynamic Study. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1908–1919. [Google Scholar] [CrossRef] [Green Version]
- Suk, V.R.E.; Latif, F.M.; Teo, Y.Y.; Misran, M. Development of Nanostructured Lipid Carrier (NLC) Assisted with Polysorbate Nonionic Surfactants as a Carrier for l-Ascorbic Acid and Gold Tri.E 30. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Jelvehgari, M.; Zakeri-Milani, P.; Siahi-Shadbad, M.R.; Loveymi, B.D.; Nokhodchi, A.; Azari, Z.; Valizadeh, H. Development of PH-Sensitive Insulin Nanoparticles Using Eudragit L100-55 and Chitosan with Different Molecular Weights. AAPS PharmSciTech 2010, 11, 1237–1242. [Google Scholar] [CrossRef] [Green Version]
- Balzus, B.; Colombo, M.; Sahle, F.F.; Zoubari, G.; Staufenbiel, S.; Bodmeier, R. Comparison of Different in Vitro Release Methods Used to Investigate Nanocarriers Intended for Dermal Application. Int. J. Pharm. 2016, 513, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Rowe, E.L. Effect of Emulsifier Concentration and Type on the Particle Size Distribution of Emulsions. J. Pharm. Sci. 1965, 54, 260–264. [Google Scholar] [CrossRef]
- Schreier, S.; Malheiros, S.V.P.; de Paula, E. Surface Active Drugs: Self-Association and Interaction with Membranes and Surfactants. Physicochemical and Biological Aspects. Biochim. Biophys. Acta BBA Biomembr. 2000, 1508, 210–234. [Google Scholar] [CrossRef] [Green Version]
- Chandler, D. Interfaces and the Driving Force of Hydrophobic Assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef]
- Stephenson, B.C.; Goldsipe, A.; Blankschtein, D. Molecular Dynamics Simulation and Thermodynamic Modeling of the Self-Assembly of the Triterpenoids Asiatic Acid and Madecassic Acid in Aqueous Solution. J. Phys. Chem. B 2008, 112, 2357–2371. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zhang, L.; Wei, X.-H.; Wang, Q. Molecular Dynamics of Paclitaxel Encapsulated by Salicylic Acid-Grafted Chitosan Oligosaccharide Aggregates. Biomaterials 2013, 34, 1843–1851. [Google Scholar] [CrossRef] [PubMed]









| Raw Materials | % p/p Components | Role |
|---|---|---|
| Solid lipid (Gelucire® 44/14) Liquid lipid (Miglyol® 812) | 4 1 | Lipid component |
| Tween® 80 | 2 | Surfactant |
| Water | 93 | Vehicle |
| Cryoprotectant | Concentration (%) |
|---|---|
| Trehalose | 2.5, 10, 15 |
| Mannitol | 2.5, 10, 15 |
| Dextrose | 2.5, 10, 15 |
| Lactose | 2.5, 10, 15 |
| Kinetic Models | Dialysis Bag | Franz’s Cell | |||
|---|---|---|---|---|---|
| 25 °C | 37 °C | 25 °C | 37 °C | ||
| Zero order | K | 1.92 | 2.20 | 2.48 | 1.83 |
| R2 | −1.16 | 0.78 | −1.93 | 0.77 | |
| First order | K | 0.03 | 0.03 | 0.03 | 0.02 |
| R2 | −0.45 | −0.05 | −1.50 | 0.86 | |
| Higuchi | K | 10.08 | 10.75 | 10.43 | 7.43 |
| R2 | 0.46 | 0.73 | −0.19 | 0.96 | |
| Korsmeyer–Peppas | n | 0.25 | 0.59 | 0.15 | 0.56 |
| kKP | 17.53 | 16.83 | 22.08 | 6.43 | |
| R2 | 0.87 | 0.94 | 0.93 | 0.97 | |
| Methodology | Hydrodynamic Size (nm ± SD) | PdI | Zeta Potential (mV ± SD) | |
|---|---|---|---|---|
| NLC-Rho | t = 0 | 106.7 ± 1.6 | 0.1 ± 0.01 | −9.3 ± 0.6 |
| Dialysis bag | 25 °C | 118.5 ± 1.7 | 0.1 ± 0.01 | −18.4 ± 1.6 |
| 37 °C | 115.1 ± 2.8 | 0.3 ± 0.01 | −19.1 ± 1.3 | |
| Franz’s Cells | 25 °C | 117.2 ± 1.4 | 0.1 ± 0.01 | −19.0 ± 1.0 |
| 37 °C | 114.3 ± 3.1 | 0.2 ± 0.01 | −16.0 ± 0.5 | |
| Molecules | MD-1 (300 K) | MD-2 (358 K) | MD-3 (358 K) | MD-4 (358 K) | |
|---|---|---|---|---|---|
| Rhodamine | Rhodamine 123 | - | - | - | 12 |
| Miglyol® 812 | Capric Triglyceride | 3 | 3 | - | 3 |
| Caprylic Triglyceride | 3 | 3 | - | 3 | |
| Tween® 80 | Polysorbate 80 | 8 | 8 | 8 | 8 |
| Gelucire® 44/14 | Lauric acid PEG | 200 | 200 | 200 | 200 |
| Mono-glyceride Lauric acid | 17 | 17 | 17 | 17 | |
| Di-glyceride Lauric acid | 17 | 17 | 17 | 17 | |
| Tri-glyceride Lauric acid | 17 | 17 | 17 | 17 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, A.C.; Yañez, O.; Salas-Huenuleo, E.; Morales, J.O. Development of a Nanostructured Lipid Carrier (NLC) by a Low-Energy Method, Comparison of Release Kinetics and Molecular Dynamics Simulation. Pharmaceutics 2021, 13, 531. https://doi.org/10.3390/pharmaceutics13040531
Ortiz AC, Yañez O, Salas-Huenuleo E, Morales JO. Development of a Nanostructured Lipid Carrier (NLC) by a Low-Energy Method, Comparison of Release Kinetics and Molecular Dynamics Simulation. Pharmaceutics. 2021; 13(4):531. https://doi.org/10.3390/pharmaceutics13040531
Chicago/Turabian StyleOrtiz, Andrea C., Osvaldo Yañez, Edison Salas-Huenuleo, and Javier O. Morales. 2021. "Development of a Nanostructured Lipid Carrier (NLC) by a Low-Energy Method, Comparison of Release Kinetics and Molecular Dynamics Simulation" Pharmaceutics 13, no. 4: 531. https://doi.org/10.3390/pharmaceutics13040531
APA StyleOrtiz, A. C., Yañez, O., Salas-Huenuleo, E., & Morales, J. O. (2021). Development of a Nanostructured Lipid Carrier (NLC) by a Low-Energy Method, Comparison of Release Kinetics and Molecular Dynamics Simulation. Pharmaceutics, 13(4), 531. https://doi.org/10.3390/pharmaceutics13040531