Dexamethasone-Loaded Lipomers: Development, Characterization, and Skin Biodistribution Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of DEX-Loaded LPNCs, Fluorescent-Loaded LPNCs, and Non-Vesiculated Control Solutions
2.3. Screening of Experimental Variables on Nanoparticle Properties
2.4. LPNCs Physic-Chemical Characterization
2.5. DEX Quantification
2.6. In Vitro Release Tests of the DEX-Loaded LPNCs
2.7. Pig Skin Permeation
2.7.1. Confocal Microscopy Biodistribution of Fluorescent Probes
2.7.2. Immunohistofluorescence Biodistribution
2.8. In Vitro Cytotoxicity/Anti-TNFα Efficacy
3. Results and Discussion
3.1. Screening of Experimental Variables on Nanoparticle Properties
3.2. Physicochemical Characterization of the LPNCs
3.3. In Vitro Release Test of DEX-Loaded LPNCs and Free DEX
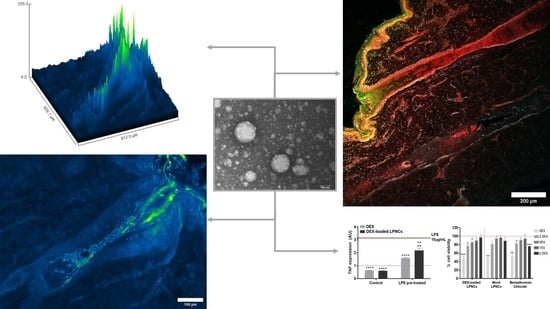
3.4. Confocal Microscopy Biodistribution of Fluorescent Probes
3.5. Immunohistofluorescence Biodistribution
3.6. Cytotoxicity and Anti-TNFα Efficacy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elias, P.M. Stratum Corneum Defensive Functions: An Integrated View. J. Investig. Dermatol. 2005, 125, 183–200. [Google Scholar] [CrossRef]
- Gupta, M.; Agrawal, U.; Vyas, S.P. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin. Drug Deliv. 2012, 9, 783–804. [Google Scholar] [CrossRef]
- Pena-Rodríguez, E.; Moreno, M.C.; Blanco-Fernandez, B.; González, J.; Fernández-Campos, F.; Fernandez, B.-. Epidermal Delivery of Retinyl Palmitate Loaded Transfersomes: Penetration and Biodistribution Studies. Pharmaceutics 2020, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Weiss, B.; Schaefer, U.F.; Lehr, C.-M.; Wepf, R. Nanoparticles—An efficient carrier for drug delivery into the hair follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venturini, C.G.; Jäger, E.; Oliveira, C.P.; Bernardi, A.; Battastini, A.M.; Guterres, S.S.; Pohlmann, A.R. Formulation of lipid core nanocapsules. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 200–208. [Google Scholar] [CrossRef]
- Bazylińska, U.; Lewińska, A.; Lamch, Ł.; Wilk, K.A. Polymeric nanocapsules and nanospheres for encapsulation and long sustained release of hydrophobic cyanine-type photosensitizer. Colloids Surf. A Physicochem. Eng. Asp. 2014, 442, 42–49. [Google Scholar] [CrossRef]
- Saraiya, N.V.; A Goldstein, D. Dexamethasone for ocular inflammation. Expert Opin. Pharmacother. 2011, 12, 1127–1131. [Google Scholar] [CrossRef]
- Yamamoto, K.A.; Klossek, R.; Flesch, F.; Rancan, M.; Weigand, I.; Bykova, M.; Bechtel, S.; Ahlberg, A.; Vogt, U.; Blume-Peytavi, P.; et al. Influence of the skin barrier on the penetration of topical-ly-applied dexamethasone probed by soft X-ray spectromicroscopy. Eur. J. Pharm. Biopharm. 2017, 118, 30–37. [Google Scholar] [CrossRef]
- Leyden, J.L.; Kligman, A.M. Treatment of alopecia areata with steroid solution. Arch Dermatol. 1972, 106, 924. [Google Scholar] [CrossRef]
- Shapiro, J. Current Treatment of Alopecia Areata. J. Investig. Dermatol. Symp. Proc. 2013, 16, S42–S44. [Google Scholar] [CrossRef] [Green Version]
- Lachenmeier, D.W. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, S.; Brief, E.; Langlais, D.; Kitson, N.; Lafleur, M.; Thewalt, J. Ethanol perturbs lipid organization in models of stratum corneum membranes: An investigation combining differential scanning calorimetry, infrared and 2H NMR spectroscopy. Biochim. Biophys. Acta Biomembr. 2012, 1818, 1410–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179, Erratum in 2001, 14, 227. [Google Scholar] [CrossRef] [Green Version]
- Balzus, B.; Sahle, F.F.; Hönzke, S.; Gerecke, C.; Schumacher, F.; Hedtrich, S.; Kleuser, B.; Bodmeier, R. Formulation and ex vivo evaluation of polymeric nanoparticles for controlled delivery of cortico-steroids to the skin and the corneal epithelium. Eur. J. Pharm. Biopharm. 2012, 115, 122–130. [Google Scholar] [CrossRef]
- Al-Amin, M.D.; Bellato, F.; Mastrotto, F.; Garofalo, M.; Malfanti, A.; Salmaso, S.; Caliceti, P. Dexamethasone Loaded Liposomes by Thin-Film Hydration and Microfluidic Procedures: Formulation Challenges. Int. J. Mol. Sci. 2020, 21, 1611. [Google Scholar] [CrossRef] [Green Version]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Lymberopoulos, A.; Demopoulou, C.; Kyriazi, M.; Katsarou, M.; Demertzis, N.; Hatziandoniou, S.; Maswadeh, H.; Papaioanou, G.; Demetzos, C.; Maibach, H.; et al. Liposome percutaneous penetration in vivo. Toxicol. Res. Appl. 2017. [Google Scholar] [CrossRef] [Green Version]
- European Directorate for the Quality of Medicines & HealthCare, European Pharmacopoeia 7.0., Chapter 2.4.24. Identification and Control of Residual Solvents, 01/2008:20424. Available online: https://www.drugfuture.com/Pharmacopoeia/EP7/DATA/20424E.PDF (accessed on 10 February 2021).
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Monica, L.-L.; Jordi, G.; Francisco, F.-C. In situ bioadhesive film-forming system for topical delivery of mometasone furoate: Characterization and biopharmaceutical properties. J. Drug Deliv. Sci. Technol. 2020, 59, 101852. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Supramaniam, J.; Adnan, R.; Kaus, N.H.M.; Bushra, R. Magnetic nanocellulose alginate hydrogel beads as potential drug delivery system. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Rekhi, G.S.; Jambhekar, S.S. Ethylcellulose—A Polymer Review. Drug Dev. Ind. Pharm. 1995, 21, 61–77. [Google Scholar] [CrossRef]
- Gurtler, F.; Kaltsatos, V.; Boisramé, B.; Gurny, R. Long-acting soluble bioadhesive ophthalmic drug insert (BODI) containing gentamicin for veterinary use: Optimization and clinical investigation. J. Control. Release 1995, 33, 231–236. [Google Scholar] [CrossRef]
- Wasilewska, K.; Winnicka, K. Ethylcellulose—A Pharmaceutical Excipient with Multidirectional Application in Drug Dosage Forms Development. Materials 2019, 12, 3386. [Google Scholar] [CrossRef] [Green Version]
- Kala, S.; Gurudiwan, P.; Juyal, D. Formulation and Evaluation of Besifloxacin Loaded in Situ Gel for Ophthalmic Delivery. Pharm. Biosci. J. 2018, 6, 36. [Google Scholar] [CrossRef]
- Committee for Human Medicinal Products, ICH guideline Q3C (R6) on impurities: Guideline for residual solvents 9 August 2019, EMA/CHMP/ICH/82260/2006. Available online: https://www.ema.europa.eu/en/ich-q3c-r6-residual-solvents (accessed on 18 March 2021).
- Sharma, N.; Madan, P.; Lin, S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study. Asian J. Pharm. Sci. 2016, 11, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.B.; Chu, W.C.; Yussof, N.S.; Abas, F.; Mirhosseini, H.; Cheah, Y.K.; Nehdi, I.A.; Tan, C.P. Physicochemical, morphological and cellular uptake properties of lutein nanodispersions prepared by using surfactants with different stabilizing mechanisms. Food Funct. 2016, 7, 2043–2051. [Google Scholar] [CrossRef]
- Jäger, E.; Venturini, C.G.; Poletto, F.S.; Colomé, L.M.; Pohlmann, J.P.U.; Bernardi, A.; Battastini, A.M.O.; Guterres, S.S.; Pohlmann, A.R. Sustained Release from Lipid-Core Nanocapsules by Varying the Core Viscosity and the Particle Surface Area. J. Biomed. Nanotechnol. 2009, 5, 130–140. [Google Scholar] [CrossRef]
- Singhvi, G.; Singh, M. Review: In vitro drug release characterization models. Int. J. Pharm. Stud. Res. II 2011, 77–84. [Google Scholar]
- Morgen, M.; Lu, G.W.; Du, D.; Stehle, R.; Lembke, F.; Cervantes, J.; Ciotti, S.; Haskell, R.; Smithey, D.; Haley, K.; et al. Targeted delivery of a poorly water-soluble compound to hair follicles using polymeric nanoparticle suspensions. Int. J. Pharm. 2011, 416, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Döge, N.; Hönzke, S.; Schumacher, F.; Balzus, B.; Colombo, M.; Hadam, S.; Rancan, F.; Blume-Peytavi, U.; Schäfer-Korting, M.; Schindler, A.; et al. Ethyl cellulose nanocarriers and nanocrystals differentially deliver dexamethasone into intact, tape-stripped or sodium lauryl sulfate-exposed ex vivo human skin—Assessment by intradermal microdialysis and extraction from the different skin layers. J. Control Release. 2016, 242, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, A.; Jacobi, U.; Ossadnik, M.; Richter, H.; Koch, S.; Sterry, W.; Lademann, J. Differential Stripping: Determination of the Amount of Topically Applied Substances Penetrated into the Hair Follicles. J. Investig. Dermatol. 2005, 125, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicoli, S.; Bunge, A.L.; Delgado-Charro, M.B.; Guy, R.H. Dermatopharmacokinetics: Factors Influencing Drug Clearance from the Stratum Corneum. Pharm. Res. 2008, 26, 865–871. [Google Scholar] [CrossRef] [Green Version]
- FDA Inactive Ingredient Database. Available online: http://www.accessdata.fda.gov/scripts/cder/iig/index.cfm (accessed on 18 March 2021).
- Kasumagic-Halilovic, E.; Prohic, A.; Cavaljuga, S. Tumor necrosis factor-alpha in patients with alopecia areata. Indian J. Dermatol. 2011, 56, 494–496. [Google Scholar] [CrossRef]
- Tauber, M.; Buche, S.; Reygagne, P.; Berthelot, J.-M.; Aubin, F.; Ghislain, P.-D.; Cohen, J.-D.; Coquerelle, P.; Goujon, E.; Jullien, D.; et al. Alopecia areata occurring during anti-TNF therapy: A national multicenter prospective study. J. Am. Acad. Dermatol. 2014, 70, 1146–1149. [Google Scholar] [CrossRef]







| Factor | Lower Level | Higher Level |
|---|---|---|
| % Tween 80 (w/w) | 1.5 | 2.5 |
| % Span 60 (w/w) | 0.16 | 0.32 |
| Kinetic Model | Equation |
|---|---|
| First Order | |
| Higuchi | |
| Korsmeyer–Peppas | |
| Weibull |
| EA:ET Ratio | Viscosity (cP) |
|---|---|
| 1:1 | 16 |
| 5:1 | 20.5 |
| 1:5 | 24 |
| 1:0 | 70.5 |
| 0:1 | 25 |
| Batch | % Tween 80 | % Span 60 | Z-Average (nm) | PDI | Z-Potential (mV) | % EE |
|---|---|---|---|---|---|---|
| LP01 | 2.5 | 0.32 | 117.6 ± 1.2 | 0.263 ± 0.005 | 21.7 | 96.76 |
| LP02 | 1.5 | 0.32 | 130.5 ± 1.1 | 0.215 ± 0.011 | 27.2 | 96.68 |
| LP03 | 1.5 | 0.16 | 114.1 ± 1.1 | 0.239 ± 0.002 | 29.1 | 96.93 |
| LP04 | 2.5 | 0.16 | 125.9 ± 0.5 | 0.256 ± 0.006 | 23.5 | 98.32 |
| Evaporation Time (min) | Residual EA (ppm) | Residual ET (ppm) |
|---|---|---|
| 5 | <LOQ | 153.0 ± 10.0 |
| 7 | <LOQ | 34.4 ± 4.5 |
| 10 | <LOQ | 12.5 ± 2.3 |
| 15 | <LOQ | 1.5 ± 0.0 |
| Formulation | Model | AIC | Parameters | Value |
|---|---|---|---|---|
| LPNCs-DEX | First order | 31.84 | k (h−1) | 0.128 |
| Higuchi | 39.98 | kH (%h−1/2) | 16.263 | |
| Korsmeyer–Peppas | 39.13 | kKP (%h−n) n | 7.91 0.573 | |
| Weibull | 28.81 | Td (h) β | 13.49 0.79 | |
| FREE-DEX | First-order | 68.51 | k (h−1) Fmax (%) | 0.192 104.35 |
| Higuchi | 71.60 | kH (%h−1/2) | 22.475 | |
| Korsmeyer–Peppas | 44.86 | kKP (%h−n) n | 42.78 0.271 | |
| Weibull | 47.43 | β Td (h) | 0.698 3.21 |
| Formulation | Model | Parameters | Value |
|---|---|---|---|
| LPNCs-DEX | Weibull | β Td (h) | 0.82 ± 0.17 14.16 ± 4.11 |
| FREE-DEX | Korsmeyer–Peppas | kKP (%h−n) n | 42.39 ± 5.96 0.278 ± 0.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pena-Rodríguez, E.; Lajarin-Reinares, M.; Mata-Ventosa, A.; Pérez-Torras, S.; Fernández-Campos, F. Dexamethasone-Loaded Lipomers: Development, Characterization, and Skin Biodistribution Studies. Pharmaceutics 2021, 13, 533. https://doi.org/10.3390/pharmaceutics13040533
Pena-Rodríguez E, Lajarin-Reinares M, Mata-Ventosa A, Pérez-Torras S, Fernández-Campos F. Dexamethasone-Loaded Lipomers: Development, Characterization, and Skin Biodistribution Studies. Pharmaceutics. 2021; 13(4):533. https://doi.org/10.3390/pharmaceutics13040533
Chicago/Turabian StylePena-Rodríguez, Eloy, Maria Lajarin-Reinares, Aida Mata-Ventosa, Sandra Pérez-Torras, and Francisco Fernández-Campos. 2021. "Dexamethasone-Loaded Lipomers: Development, Characterization, and Skin Biodistribution Studies" Pharmaceutics 13, no. 4: 533. https://doi.org/10.3390/pharmaceutics13040533
APA StylePena-Rodríguez, E., Lajarin-Reinares, M., Mata-Ventosa, A., Pérez-Torras, S., & Fernández-Campos, F. (2021). Dexamethasone-Loaded Lipomers: Development, Characterization, and Skin Biodistribution Studies. Pharmaceutics, 13(4), 533. https://doi.org/10.3390/pharmaceutics13040533