Complying with the Guideline for Quality and Equivalence for Topical Semisolid Products: The Case of Clotrimazole Cream
Abstract
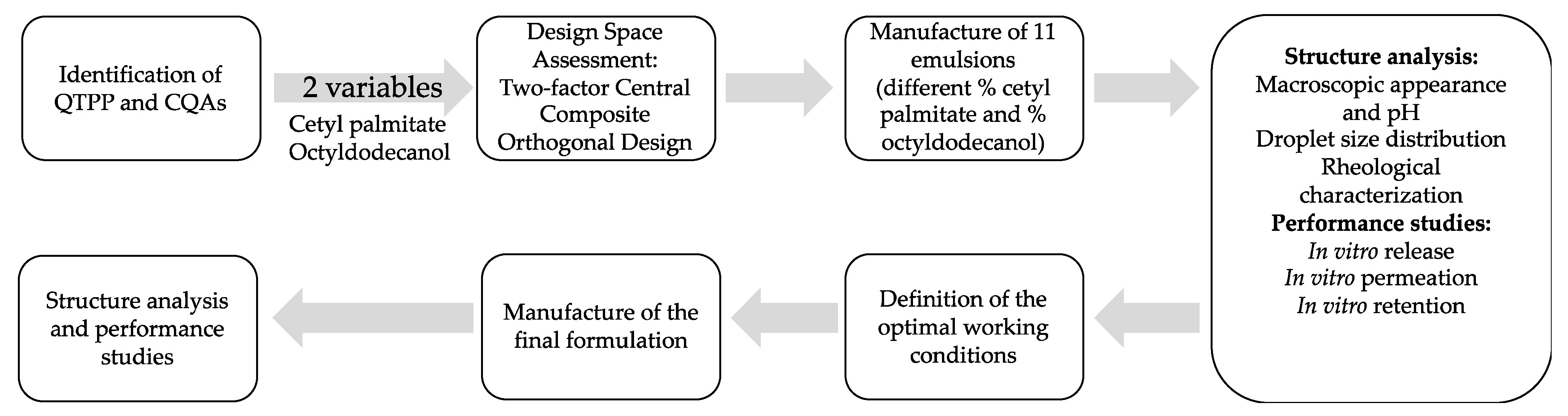
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. QbD Approach-Identification of Quality Target Product Profile (QTPP) and Critical Quality Attributes (CQAs)
2.2.2. QbD Approach—Design Space (DS) Assessment
2.2.3. Manufacturing Process
2.2.4. Structure Analysis of Emulsions
Macroscopic Appearance and pH Determination
Droplet Size Distribution
Rheological Characterization
Rotational Measurements
Oscillatory Measurements
2.2.5. In Vitro Release Testing (IVRT)
2.2.6. In Vitro Permeation Testing
2.2.7. In Vitro Retention Testing
2.2.8. Drug Quantification
2.2.9. Statistical Analysis for Optimal Working Conditions
3. Results and Discussion
3.1. Description and Composition of the Drug Formulation
3.2. Identification of QTPP and CQAs
3.2.1. Definition of Variables
3.2.2. Definition and Application of the Experimental Design
3.3. Product Characterization
3.3.1. Macroscopic Appearance and pH Determination
3.3.2. Droplet Size Distribution
3.3.3. Rheological Characterization—Structure Analysis
Rotational Measurements
3.4. Product Performance
3.4.1. IVRT
3.4.2. IVPT
3.5. Optimal Working Conditions
3.6. Final Formulation
3.6.1. Droplet Size Distribution
3.6.2. Rheological Characterization
Rotational Measurements
Oscillatory Measurements
3.6.3. Product Performance (IVRT and IVPT)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Council of Europe. European Pharmacopoeia 8; European Directorate for the Quality of Medicines and Healthcare; Council of Europe: Strasbourg, France, 2014. [Google Scholar]
- Simões, A.; Veiga, F.; Vitorino, C. Developing Cream Formulations: Renewed Interest in an Old Problem. J. Pharm. Sci. 2019, 108, 3240–3251. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Sousa, J.J.; Veiga, F.; Cardoso, C.; Vitorino, C. Bioequivalence of Topical Generic Products. Part 1: Where Are We Now? Eur. J. Pharm. Sci. 2018, 123, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Hornecker, J.R. Generic Drugs: History, Approval Process, and Current Challenges. US Pharm. Generic Drug Rev. Suppl. 2009, 34, 26–30. [Google Scholar]
- European Medicines Agency. Draft Guideline on Quality and Equivalence of Topical Products; EMA: Amsterdam, The Netherlands, 2018. [Google Scholar]
- U.S. Food & Drug Administration. Concept Paper on the Development of a Guideline on Quality and Equivalence of Topical Products; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2014.
- Gupta, P.; Garg, S. Recent Advances in Semisolid Dosage Forms for Dermatological Application. Pharm. Technol. 2002, 5, 144–162. [Google Scholar]
- Dhoot, A.S.; Fernandes, G.J.; Naha, A.; Rathnanand, M.; Kumar, L. Design of Experiments in Pharmaceutical Development. Pharm. Chem. J. 2019, 53, 730–735. [Google Scholar] [CrossRef]
- ICHQ8(R2). ICH Harmonised Tripartite Guideline on Pharmaceutical Development Q8(R2). 2017. Available online: www.ich.Org (accessed on 30 September 2020).
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding Pharmaceutical Quality by Design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Fowler, M. Quality by Design (QbD) Approach to Generic Transdermal or Topical Product Development. Available online: https://www.americanpharmaceuticalreview.Com/Featured-Articles/172883-Quality-by-Design-QbDApproach-to-Generic-Transdermal-or-Topical-Product-Development/ (accessed on 30 September 2020).
- Fukuda, I.M.; Pinto, C.F.F.; Moreira, C.D.S.; Saviano, A.M.; Lourenço, F.R. Design of Experiments (DoE) Applied to Pharmaceutical and Analytical Quality by Design (QbD). Braz. J. Pharm. Sci. 2018, 54. [Google Scholar] [CrossRef]
- Hami, A.E.; Pugnet, P. Embedded Mechatronics Systems: Analysis of Failures, Modeling, Simulation and Optimization; ISTE Press Ltd.: London, UK; Elsevier: Amsterdam, The Netherlands, 2015; p. 2. [Google Scholar]
- OECD. Test No. 428: Skin Absorption: In Vitro Method; OECD Publishing: Paris, France, 2004. [Google Scholar]
- Banker, G.S.; Rhodes, C.T. Modern Pharmaceutics, 4th ed.; Marcel Dekker: New York, NY, USA, 2002; p. 121. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Chang, R.-K.; Raw, A.; Lionberger, R.; Yu, L. Generic Development of Topical Dermatologic Products, Part II: Quality by Design for Topical Semisolid Products. AAPS J. 2013, 15, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Welage, L.S.; Kirking, D.M.; Ascione, F.J.; Gaither, C.A. Understanding the Scientific Issues Embedded in the Generic Drug Approval Process. J. Am. Pharm. Assoc. 2001, 41, 856–867. [Google Scholar] [CrossRef]
- Korhonen, M.; Niskanen, H.; Kiesvaara, J.; Yliruusi, J. Determination of Optimal Combination of Surfactants in Creams Using Rheology Measurements. Int. J. Pharm. 2000, 197, 143–151. [Google Scholar] [CrossRef]
- Korhonen, M.; Lehtonen, J.; Hellen, L.; Hirvonen, J.; Yliruusi, J. Rheological Properties of Three Component Creams Containing Sorbitan Monoesters as Surfactants. Int. J. Pharm. 2002, 247, 103–114. [Google Scholar] [CrossRef]
- Lukic, M.; Jaksic, I.; Krstonosic, V.; Cekic, N.; Savic, S. A Combined Approach in Characterization of an Effective w/o Hand Cream: The Influence of Emollient on Textural, Sensorial and in Vivo Skin Performance. Int. J. Cosmet. Sci. 2011, 34, 140–149. [Google Scholar] [CrossRef]
- Miranda, M.; Cardoso, C.; Vitorino, C. Fast Screening Methods for the Analysis of Topical Drug Products. Processes 2020, 8, 397. [Google Scholar] [CrossRef] [Green Version]
- Blokhina, S.; Sharapova, A.; Ol’Khovich, M.; Perlovich, G. Experimental Solubility of Clotrimazole and Some Thermodynamic Aspects of Dissolution in Different Solvents. Thermochim. Acta 2019, 682, 178431. [Google Scholar] [CrossRef]
- Borhade, V.; Pathak, S.; Sharma, S.; Patravale, V. Clotrimazole Nanoemulsion for Malaria Chemotherapy. Part I: Preformulation Studies, Formulation Design and Physicochemical Evaluation. Int. J. Pharm. 2012, 431, 138–148. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin PH: From Basic SciencE to Basic Skin Care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Simões, A.; Veiga, F.; Vitorino, C. Progressing Towards the Sustainable Development of Cream Formulations. Pharmaceutics 2020, 12, 647. [Google Scholar] [CrossRef]
- Langley, N.; Michniak-Kohn, B.; Osborne, D.W. (Eds.) The Role of Microstructure in Topical Drug Product Development; Springer: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Isaac, V.L.B.; Chiari-Andréo, B.G.; Marto, J.M.; Moraes, J.D.D.; Leone, B.A.; Corrêa, M.A.; Ribeiro, H.M. Rheology as a Tool to Predict the Release of Alpha-Lipoic Acid from Emulsions Used for the Prevention of Skin Aging. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Park, E.-K.; Song, K.-W. Rheological Evaluation of Petroleum Jelly as a Base Material in Ointment and Cream Formulations: Steady Shear Flow Behavior. Arch. Pharmacal Res. 2010, 33, 141–150. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.; Xu, X.; Rahman, Z.; Yang, Y.; Katragadda, U.; Lionberger, R.; Peters, J.R.; Uhl, K.; Khan, M.A. Development of Performance Matrix for Generic Product Equivalence of Acyclovir Topical Creams. Int. J. Pharm. 2014, 475, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Lobo, J.M.S. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Contreras, S.; Castillo, C.; Olivera-Nappa, Á.; Townley, B.; Ihle, C.F. A New Statistically-Based Methodology for Variability Assessment of Rheological Parameters in Mineral Processing. Miner. Eng. 2020, 156, 106494. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook for Users of Rotational and Oscillatory Rheometers, 2nd ed.; Vincentz: Hannover, Germany, 2006. [Google Scholar]
- Davis, S.S. Viscoelastic Properties of Pharmaceutical Semisolids III: Nondestructive Oscillatory Testing. J. Pharm. Sci. 1971, 60, 1351–1356. [Google Scholar] [CrossRef]
- Gore, E.; Picard, C.; Savary, G. Spreading Behavior of Cosmetic Emulsions: Impact of the Oil Phase. Biotribology 2018, 16, 17–24. [Google Scholar] [CrossRef]
- Pereira, J.; Gonçalves, R.; Barreto, M.; Dias, C.; Carvalho, F.; Almeida, A.J.; Ribeiro, H.M.; Marto, J. Development of Gel-in-Oil Emulsions for Khellin Topical Delivery. Pharmaceutics 2020, 12, 398. [Google Scholar] [CrossRef]
- Tamarkin, D.; Friedman, D.; Shemer, A. Emollient Foam in Topical Drug Delivery. Expert Opin. Drug Deliv. 2006, 3, 799–807. [Google Scholar] [CrossRef]
- Gore, E.; Picard, C.; Savary, G. Complementary Approaches to Understand the Spreading Behavior on Skin of O/W Emulsions Containing Different Emollientss. Colloids Surf. B Biointerfaces 2020, 193, 111132. [Google Scholar] [CrossRef]
- Miranda, M.; Cruz, M.T.; Vitorino, C.; Cabral, C. Nanostructuring Lipid Carriers Using Ridolfia segetum (L.) Moris Essential Oil. Mater. Sci. Eng. C 2019, 103, 109804. [Google Scholar] [CrossRef]
- Singhvi, G.; Singh, M. Review: In-Vitro Drug Release Characterization Models. Int. J. Pharm. Stud. Res. 2011, 2, 77–84. [Google Scholar]
- U.S. Food & Drug Administration. FDA Fact Sheet—What’s Involved in Reviewing and Approving Generic Drug Applications? U.S. Food & Drug Administration: Silver Spring, MD, USA. Available online: https://www.fda.gov/media/99163/download (accessed on 30 September 2020).
- Leite-Silva, V.R.; Grice, J.E.; Mohammed, Y.; Moghimi, H.R.; Roberts, M.S. The Influence of Emollients on Dermal and Transdermal Drug Delivery. Percutaneous Penetration Enhancers Drug Penetration Into/Through Skin 2017, 37, 77–93. [Google Scholar] [CrossRef]
- Maru, S.M.; Gathu, L.W.; Mathenge, A.W.; Okaru, A.O.; Kamau, F.N.; Chepkwony, H.K. In Vitro Drug Release Studies of Metronidazole Topical Formulations through Cellulose Membrane. East Cent. Afr. J. Pharm. Sci. 2012, 15, 57–62. [Google Scholar]
- Cogdill, R.; Drennen, J.; Anderson, C. Risk Analysis for Near Infrared Method Development. NIR News 2004, 15, 12. [Google Scholar] [CrossRef]
- Preece, D.A.; Montgomery, D.C. Design and Analysis of Experiments. Int. Stat. Rev. 1978, 46, 120. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Wikström, C.; Wold, S. Design of Experiments, Principles and Applications. J. Chemom. 2001, 15, 495–496. [Google Scholar]
- Pal, R. Effect of Droplet Size on the Rheology of Emulsions. AIChE J. 1996, 42, 3181–3190. [Google Scholar] [CrossRef]
- Capdevila, M.; Maestro, A.; Porras, M.; Gutierrez, J.M. Preparation of Span 80/oil/Water Highly Concentrated Emulsions: Influence of Composition and Formation Variables and Scale-up. J. Colloid Interface Sci. 2010, 345, 27–33. [Google Scholar] [CrossRef]
- Silva, A.C.; Santos, D.; Ferreira, D.C.; Souto, E.B. Minoxidil-Loaded Nanostructured Lipid Carriers (NLC): Characterization and Rhe-Ological Behaviour of Topical Formulations. Pharmazie 2009, 64, 177–182. [Google Scholar] [CrossRef]
- Ng, S.P.; Khor, Y.P.; Lim, H.K.; Lai, O.M.; Wang, Y.; Wang, Y.; Cheong, L.Z.; Nehdi, I.A.; Mansour, L.; Tan, C.P. Fabrication of Concentrated Palm Olein-Based Diacylglycerol Oil–Soybean Oil Blend Oil-In-Water Emulsion: In-Depth Study of the Rheological Properties and Storage Stability. Foods 2020, 9, 877. [Google Scholar] [CrossRef]
- Bom, S.; Santos, C.; Barros, R.; Martins, A.M.; Paradiso, P.; Cláudio, R.; Pinto, P.C.; Ribeiro, H.M.; Marto, J. Effects of Starch Incorporation on the Physicochemical Properties and Release Kinetics of Alginate-Based 3D Hydrogel Patches for Topical Delivery. Pharmaceutics 2020, 12, 719. [Google Scholar] [CrossRef]







| Samples | Jss (μg/cm2/h) | ER (Jss) | Kp (cm/h) | Q12 h (μg/cm2) | Q24 h (μg/cm2) | Lag Time (h) |
|---|---|---|---|---|---|---|
| F1 | 0.20 ± 0.03 | 1.16 | 9.9 × 10−6 ± 1.7 × 10−6 | 2.2 ± 0.1 | 4.7 ± 0.2 | 2.6 ± 0.6 |
| F2 | 0.17 ± 0.02 | 0.97 | 8.4 × 10−6 ± 1.1 × 10−6 | 2.1 ± 0.5 | 4.2 ± 0.3 | 3.1 ± 1.8 |
| F3 | 0.18 ± 0.05 | 1.06 | 9.2 × 10−6 ± 2.3 × 10−6 | 3.1 ± 0.1 | 5.5 ± 0.7 | 6.4 ± 5.0 |
| F4 | 0.19 ± 0.01 | 1.14 | 9.9 × 10−6 ± 5.5 × 107 | 2.6 ± 0.3 | 5.0 ± 0.5 | 2.9 ± 1.1 |
| F5 | 0.20 ± 0.03 | 1.16 | 1.0 × 10−5 ± 1.4 × 10−6 | 1.9 ± 0.5 | 4.9 ± 0.6 | 1.9 ± 0.6 |
| F6 | 0.16 ± 0.02 | 1.13 | 9.7 × 10−6 ± 7.3 × 10−7 | 2.1 ± 0.4 | 4.4 ± 0.2 | 1.4 ± 0.6 |
| F7 | 0.13 ± 0.03 | 0.73 | 6.4 × 10−6 ± 1.7±10−6 | 1.6 ± 0.3 | 3.1 ± 0.9 | 0.4 ± 0.0 |
| F8 | 0.26 ± 0.00 | 1.50 | 1.3 × 10−5 ± 2.2 × 107 | 3.4 ± 0.4 | 6.2 ± 0.4 | 1.1 ± 0.4 |
| F9 | 0.18 ± 0.01 | 1.03 | 8.9 × 10−6 ± 6.5 × 10−7 | 2.3 ± 0.2 | 4.4 ± 0.3 | 0.9 ± 0.2 |
| F10 | 0.17 ± 0.01 | 1.01 | 8.7 × 10−6 ± 4.8 × 10−7 | 2.4 ± 0.2 | 4.5 ± 0.3 | 1.9 ± 0.5 |
| F11 | 0.18 ± 0.00 | 1.02 | 8.9 × 10−6 ± 1.4 × 10−6 | 2.5 ± 0.2 | 4.6 ± 0.2 | 1.9 ± 0.8 |
| RF | 0.17 ± 0.03 | 1.00 | 8.7 × 10−6 ± 1.2 × 10−6 | 2.3 ± 0.2 | 4.2 ± 0.3 | 1.7 ± 0.8 |
| Samples | Droplet Size Distribution (μm) | |||
|---|---|---|---|---|
| Span | d(10) | d(50) | d(90) | |
| FF | 2.9 ± 0.0 | 1.5 ± 0.1 | 9.2 ± 0.8 | 29.1 ± 2.7 |
| RF | 4.7 ± 1.1 | 1.2 ± 0.0 | 5.3 ± 0.6 | 20.9 ± 0.3 |
| Samples | Release 12h (%) | DE 12h (%) | AUC | Jss (μg/cm2/h) | ER (Jss) | Kp (cm/h) | Q12 h (μg/cm2) | Q24 h (μg/cm2) | tlag (h) | Retention (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| FF | 19.6 ± 5.6 | 9.8 ± 2.8 | 156.2 ± 25.7 | 0.22 ± 0.03 | 1.13 | 1.1 × 10−5 ± 1.7 × 10−6 | 1.9 ± 0.8 | 4.9 ± 0.7 | 1.7 ± 0.9 | 3.5 ± 0.9 |
| RF | 19.9 ± 5.5 | 9.9 ± 2.7 | 158.3 ± 44.8 | 0.20 ± 0.06 | 1.00 | 9.7 × 10−6 ± 2.8 × 10−6 | 2.2 ± 0.3 | 4.5 ± 0.9 | 1.7 ± 0.0 | 3.3 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, T.; Arranca, D.; Martins, A.; Ribeiro, H.; Raposo, S.; Marto, J. Complying with the Guideline for Quality and Equivalence for Topical Semisolid Products: The Case of Clotrimazole Cream. Pharmaceutics 2021, 13, 555. https://doi.org/10.3390/pharmaceutics13040555
Alves T, Arranca D, Martins A, Ribeiro H, Raposo S, Marto J. Complying with the Guideline for Quality and Equivalence for Topical Semisolid Products: The Case of Clotrimazole Cream. Pharmaceutics. 2021; 13(4):555. https://doi.org/10.3390/pharmaceutics13040555
Chicago/Turabian StyleAlves, Teresa, Daniel Arranca, Ana Martins, Helena Ribeiro, Sara Raposo, and Joana Marto. 2021. "Complying with the Guideline for Quality and Equivalence for Topical Semisolid Products: The Case of Clotrimazole Cream" Pharmaceutics 13, no. 4: 555. https://doi.org/10.3390/pharmaceutics13040555
APA StyleAlves, T., Arranca, D., Martins, A., Ribeiro, H., Raposo, S., & Marto, J. (2021). Complying with the Guideline for Quality and Equivalence for Topical Semisolid Products: The Case of Clotrimazole Cream. Pharmaceutics, 13(4), 555. https://doi.org/10.3390/pharmaceutics13040555









