Sesame Oil-Based Nanostructured Lipid Carriers of Nicergoline, Intranasal Delivery System for Brain Targeting of Synergistic Cerebrovascular Protection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Experimental Design
2.2.2. Validation of the Experimental Method
2.3. Preparation of NIC–NLCs
2.4. Characterization of the Prepared NIC–NLCs
2.4.1. Particle Size (PS) and Size Distribution
2.4.2. Entrapment Efficiency (EE%) and Loading Capacity (LC)
2.4.3. Zeta Potential (ZP)
2.4.4. Determination of the NIC Release Rate from the Optimized NLC Formulations
Kinetic Modeling of Release Data
2.4.5. Solid State Characterizations and Compatibility Studies
Differential Scanning Calorimetry (DSC)
Infrared Spectroscopy (IR)
X-ray Diffraction (XRD)
2.4.6. pH Measurement
2.5. Ex Vivo Nasal Permeation Studies
2.6. In Vivo Bioavailability and Brain Distribution Studies
2.6.1. Study Design
2.6.2. Analysis of Samples and Drug Determination
Chromatographic Conditions:
Sample Preparation:
Pharmacokinetics Calculations:
Statistical Analysis of Pharmacokinetic Data
2.7. Stability Study
3. Results and Discussion
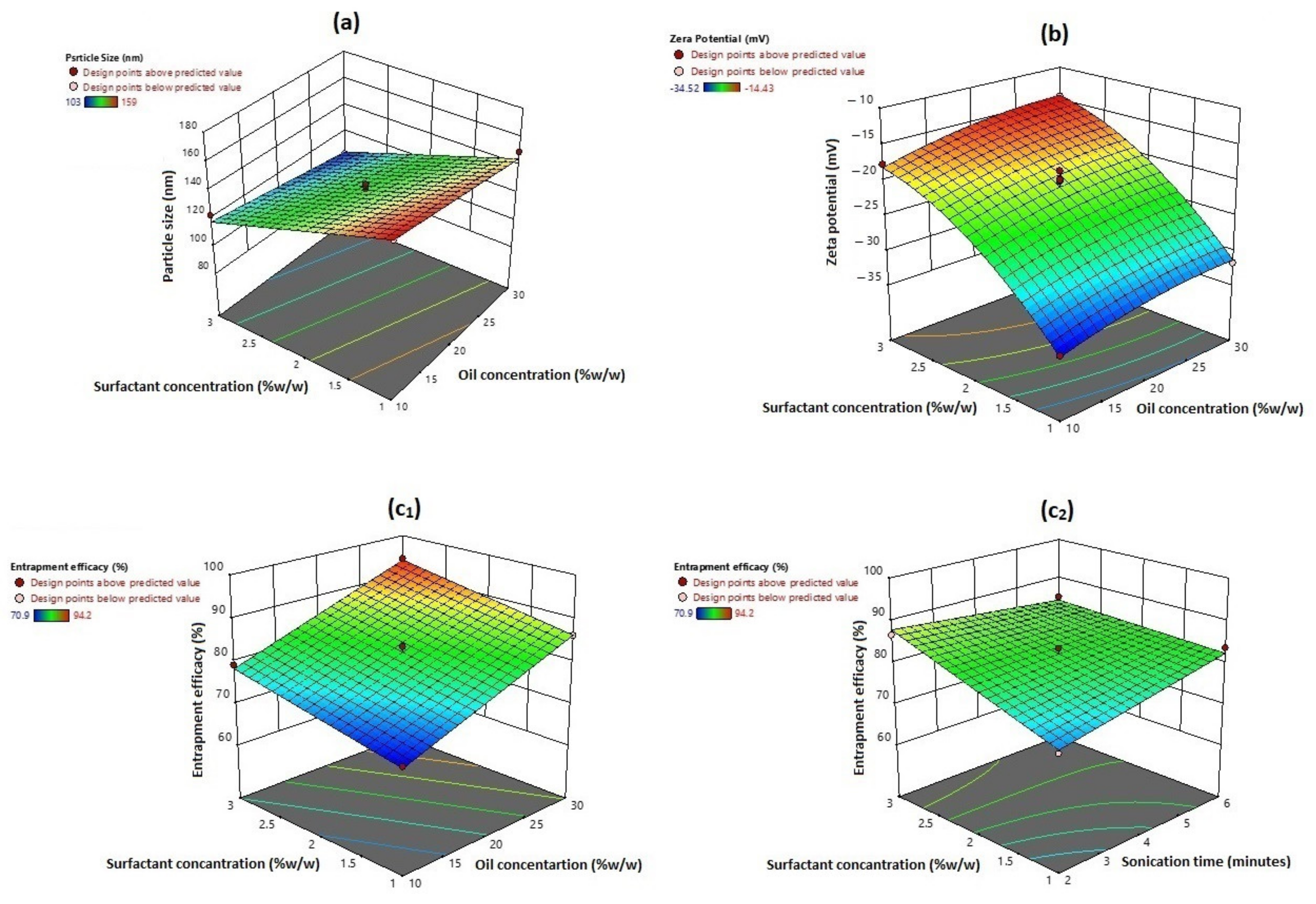
3.1. Effect on Particle Size
3.2. Effect on Zeta Potential
3.3. Effects on Entrapment Efficiency
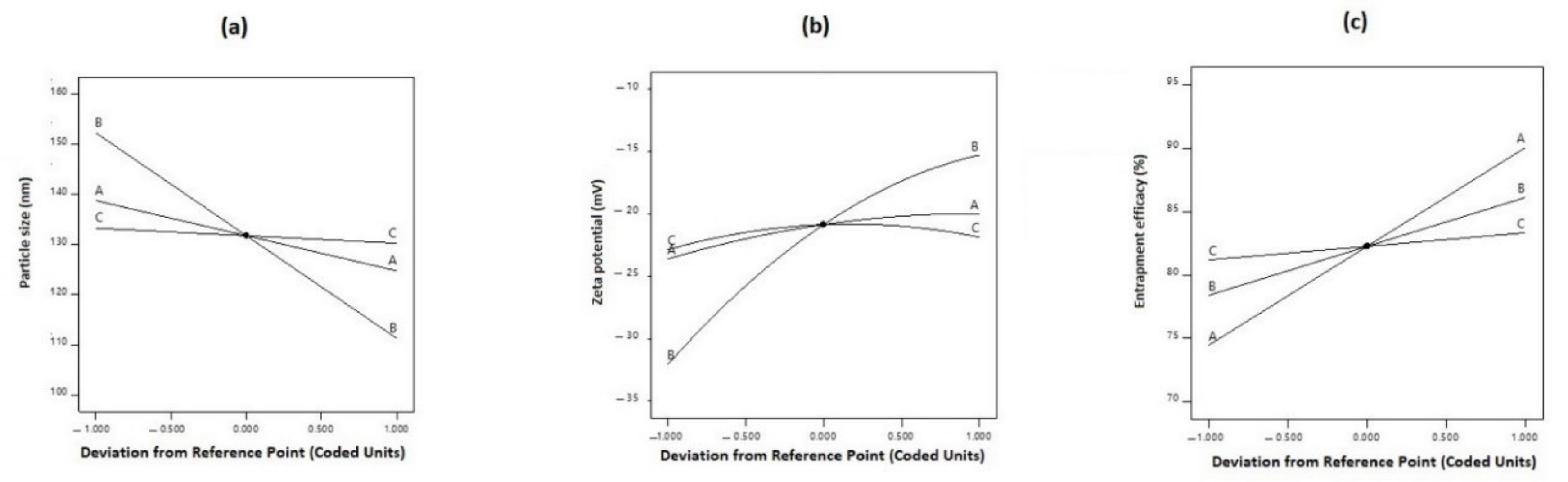
3.4. Optimization and Validation of the Collected Data
3.5. NIC Release Studies
3.6. Thermal (DSC) Analysis
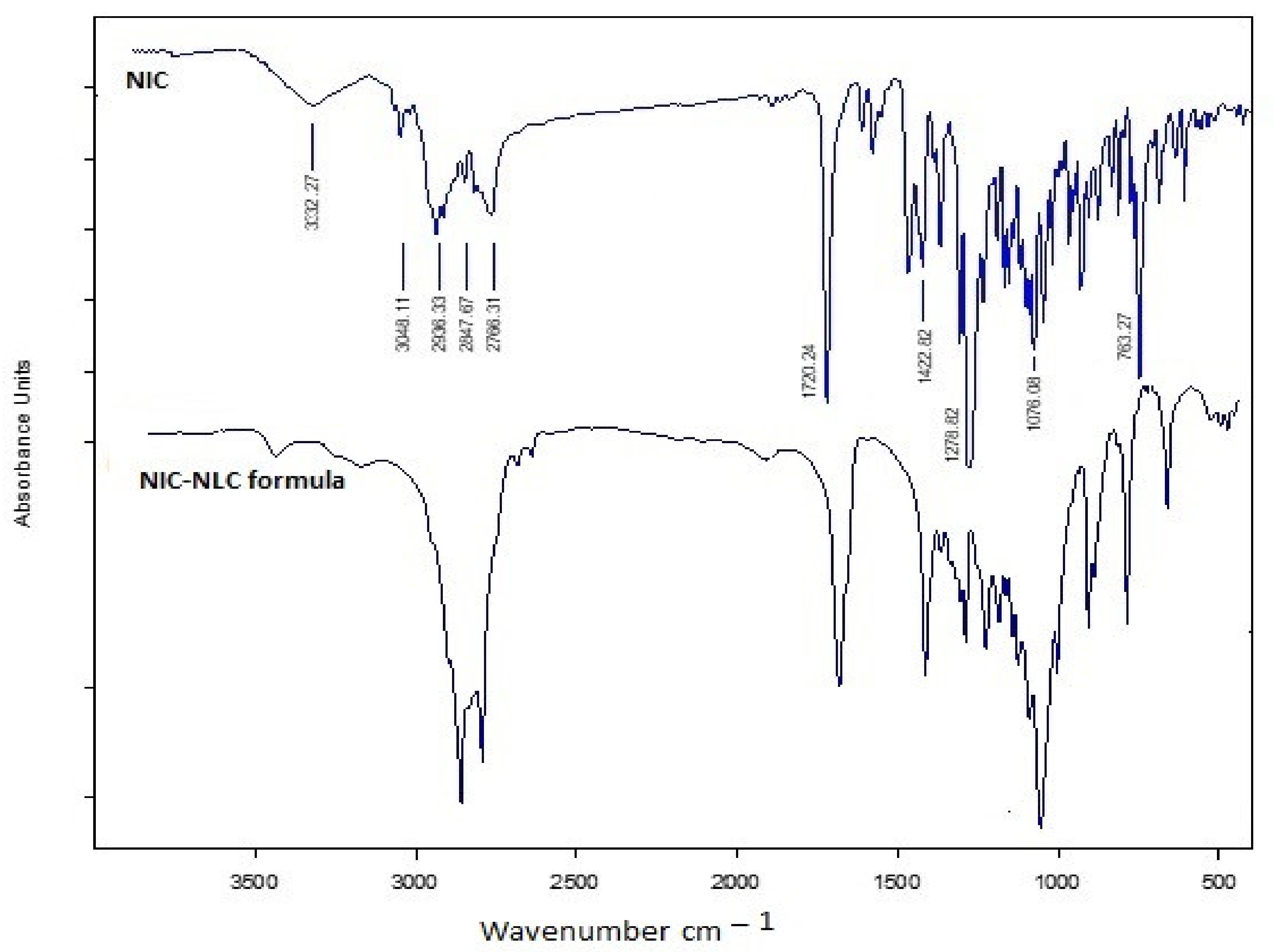
3.7. Infrared Spectroapy Stuies
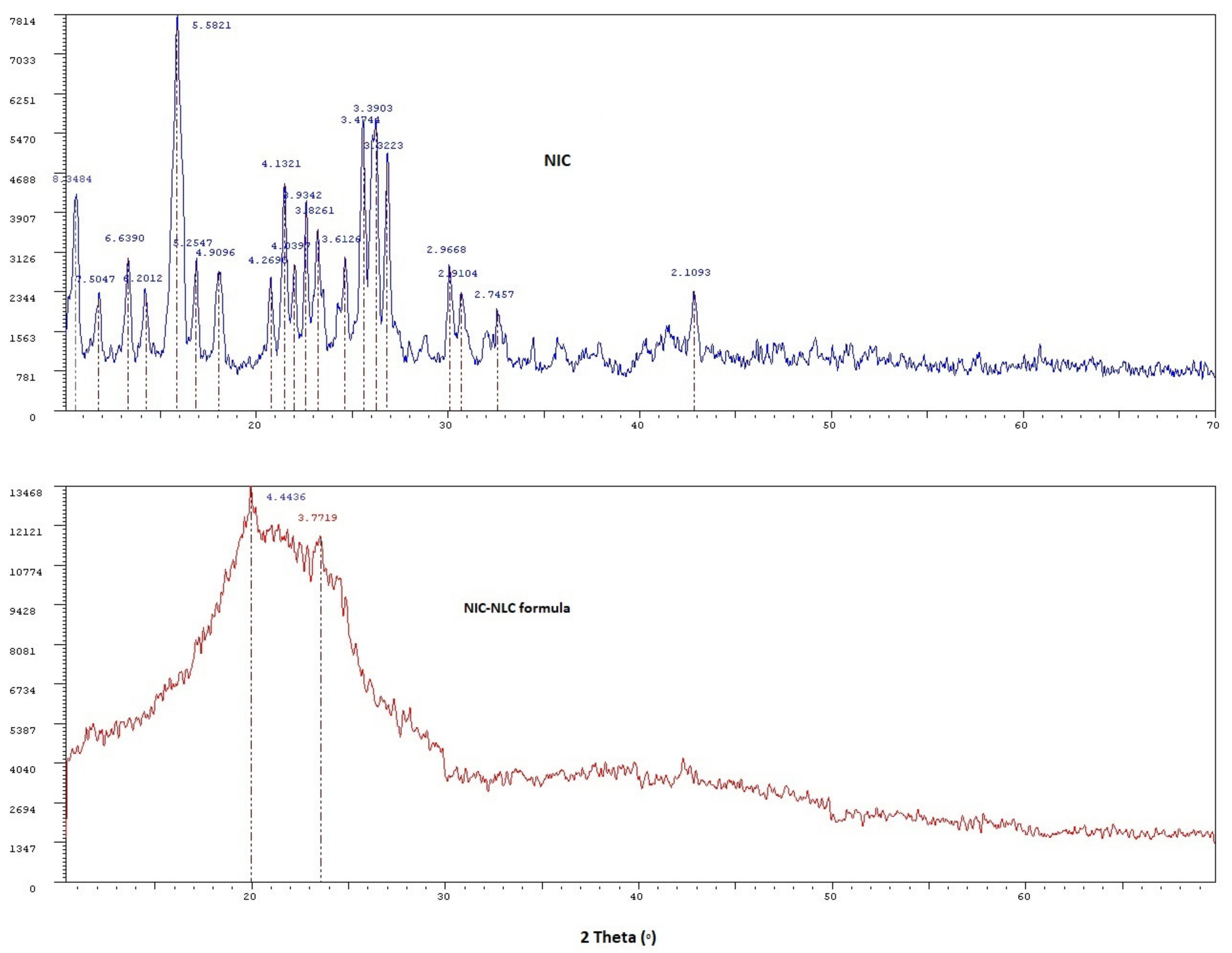
3.8. XRD Analysis
3.9. pH Measurement
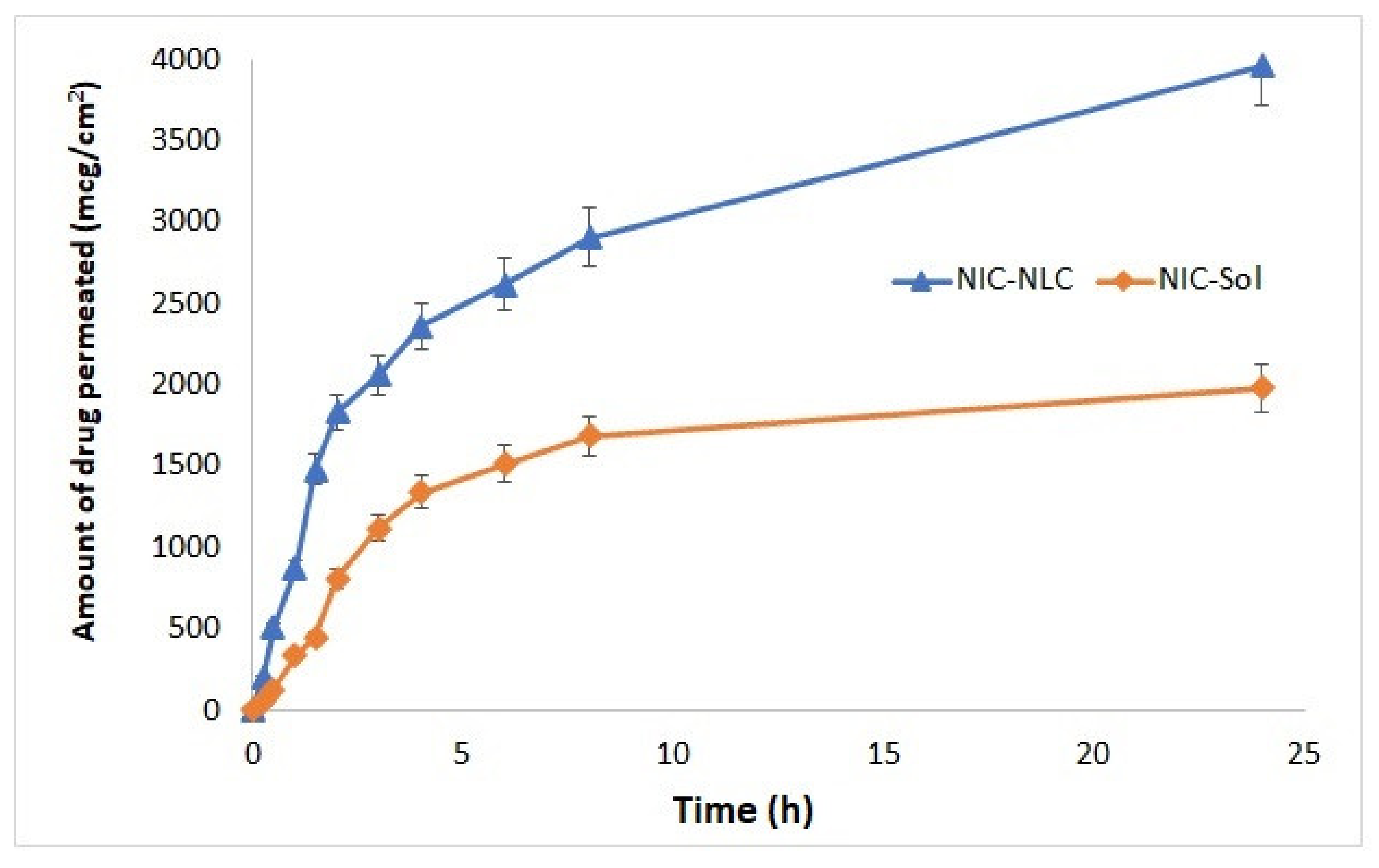
3.10. Ex Vivo Permeation Studies
3.11. In Vivo Bioavailability and Brain Distribution Studies
3.12. Stability Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloom, D.E.; Canning, D.; Fink, G. Population aging and economic growth. Glob. Growth 2010, 297–328. [Google Scholar] [CrossRef]
- Buffington, A.L.; Lipski, D.M.; Westfall, E. Dementia: An evidence-based review of common presentations and family-based interventions. J. Am. Osteopath. Assoc. 2013, 113, 768–775. [Google Scholar] [CrossRef] [Green Version]
- Kalaria, R.N.; Maestre, G.E.; Arizaga, R.; Friedland, R.P.; Galasko, D.; Hall, K.; Luchsinger, J.A.; Ogunniyi, A.; Perry, E.K.; Potocnik, F. Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008, 7, 812–826. [Google Scholar] [CrossRef] [Green Version]
- Winblad, B.; Fioravanti, M.; Dolezal, T.; Logina, I.; Milanov, I.G.; Popescu, D.C.; Solomon, A. Therapeutic use of nicergoline. Clin. Drug Investig. 2008, 28, 533–552. [Google Scholar] [CrossRef]
- Simpson, J.R. DSM-5 and neurocognitive disorders. J. Am. Acad. Psychiatry Law Online 2014, 42, 159–164. [Google Scholar]
- Blazer, D. Neurocognitive disorders in DSM-5. Am. J. Psychiatry 2013, 170, 585–587. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and management of dementia. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef]
- Raz, L.; Knoefel, J.; Bhaskar, K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 2016, 36, 172–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fioravanti, M.; Flicker, L. Nicergoline for dementia and other age associated forms of cognitive impairment. Cochrane Database Syst. Rev. 2001, 4. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.A.; Firbank, M.J.; Widdrington, M.; Blamire, A.M.; Kalaria, R.N.; O’Brien, J.T. Post-stroke dementia: The contribution of thalamus and basal ganglia changes. Int. Psychogeriatr. 2012, 24, 568. [Google Scholar] [CrossRef] [PubMed]
- Lezak, M.D.; Howieson, D.B.; Loring, D.W.; Fischer, J.S. Neuropsychological Assessment; Oxford University Press, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Salmon, D.P.; Filoteo, J.V. Neuropsychology of cortical versus subcortical dementia syndromes. Semin. Neurol. 2007, 27, 007–021. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-T.; Chow, P.K.; Song, Y.-Q.; Edwin, C.; Chan, A.C.; Lee, T.M.; Lam, J.H. Mental and physical activities delay cognitive decline in older persons with dementia. Am. J. Geriatr. Psychiatry 2014, 22, 63–74. [Google Scholar] [CrossRef]
- Rebok, G.W.; Ball, K.; Guey, L.T.; Jones, R.N.; Kim, H.Y.; King, J.W.; Marsiske, M.; Morris, J.N.; Tennstedt, S.L.; Unverzagt, F.W. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J. Am. Geriatr. Soc. 2014, 62, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Adelman, R.D.; Tmanova, L.L.; Delgado, D.; Dion, S.; Lachs, M.S. Caregiver burden: A clinical review. JAMA 2014, 311, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.L. Harrison’s Principles of Internal Medicine; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Schneider, L.S.; Dagerman, K.S.; Higgins, J.P.; McShane, R. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch. Neurol. 2011, 68, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Fowler, N.R.; Chen, Y.-F.; Thurton, C.A.; Men, A.; Rodriguez, E.G.; Donohue, J.M. The impact of Medicare prescription drug coverage on the use of antidementia drugs. BMC Geriatr. 2013, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Malaj, L.; Censi, R.; Capsoni, D.; Pellegrino, L.; Bini, M.; Ferrari, S.; Gobetto, R.; Massarotti, V.; Di Martino, P. Characterization of nicergoline polymorphs crystallized in several organic solvents. J. Pharm. Sci. 2011, 100, 2610–2622. [Google Scholar] [CrossRef]
- Kurosu, S.; Mori, Y.; Kobayashi, N. Relationship of ERP to intellectual function and cerebral blood flow following Sermion (nicergoline) treatment in patients with cerebrovascular dementia. Pharma Med. 2002, 20, 231–236. [Google Scholar]
- Im, J.J.; Jeong, H.S.; Park, J.-S.; Yang, Y.; Na, S.-H.; Oh, J.K.; Chung, Y.-A.; Song, I.-U. Changes in Regional Cerebral Perfusion after Nicergoline Treatment in Early Alzheimer’s Disease: A Pilot Study. Dement. Neurocogn. Disord. 2017, 16, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Saletu, B.; Garg, A.; Shoeb, A. Safety of nicergoline as an agent for management of cognitive function disorders. BioMed Res. Int. 2014, 2014, 610103. [Google Scholar] [CrossRef] [Green Version]
- Caraci, F.; Chisari, M.; Frasca, G.; Canonico, P.L.; Battaglia, A.; Calafiore, M.; Battaglia, G.; Bosco, P.; Nicoletti, F.; Copani, A. Nicergoline, a drug used for age-dependent cognitive impairment, protects cultured neurons against β-amyloid toxicity. Brain Res. 2005, 1047, 30–37. [Google Scholar] [CrossRef]
- Xiaofeng, L.; Changlin, H. Effect of nicergoline on cognition function of patients with cerebral infarction. Chin. J. Tissue Eng. Res. 2005, 9, 186–187. [Google Scholar]
- Mizuno, T.; Kuno, R.; Nitta, A.; Nabeshima, T.; Zhang, G.; Kawanokuchi, J.; Wang, J.; Jin, S.; Takeuchi, H.; Suzumura, A. Protective effects of nicergoline against neuronal cell death induced by activated microglia and astrocytes. Brain Res. 2005, 1066, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Miccheli, A.; Puccetti, C.; Capuani, G.; Di Cocco, M.E.; Giardino, L.; Calzà, L.; Battaglia, A.; Battistin, L.; Conti, F. [1-13C] Glucose entry in neuronal and astrocytic intermediary metabolism of aged rats: A study of the effects of nicergoline treatment by 13C NMR spectroscopy. Brain Res. 2003, 966, 116–125. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Abe, A.; Ueda, M.; Katsura, K.-i.; Katayama, Y. Nicergoline increases serum substance P levels in patients with an ischaemic stroke. Cerebrovasc. Dis. 2010, 29, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Hattori, N.; Okimoto, M.; Yanagida, J.; Kohno, N. Nicergoline improves dysphagia by upregulating substance P in the elderly. Medicine 2011, 90, 279–283. [Google Scholar] [CrossRef]
- Winkler, F.; Koedel, U.; Kastenbauer, S.; Pfister, H.W. Differential expression of nitric oxide synthases in bacterial meningitis: Role of the inducible isoform for blood-brain barrier breakdown. J. Infect. Dis. 2001, 183, 1749–1759. [Google Scholar] [CrossRef]
- Ezan, E.; Delestre, L.; Legendre, S.; Riviere, R.; Doignon, J.-L.; Grognet, J.-M. Immunoassays for the detection of nicergoline and its metabolites in human plasma. J. Pharm. Biomed. Anal. 2001, 25, 123–130. [Google Scholar] [CrossRef]
- Martena, V.; Censi, R.; Hoti, E.; Malaj, L.; Di Martino, P. A new nanospray drying method for the preparation of nicergoline pure nanoparticles. J. Nanoparticle Res. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Hassan, A.H.; Haleem, A.M.A.; El-Nabarawy, M.A.; Abd-Elbary, A. Formulation and Evaluation of Taste-Masked Orally Disintegrating Tablets of Nicergoline based on [beta]-cyclodextrin Inclusion Complexation. Int. J. Drug Deliv. 2013, 5, 110. [Google Scholar]
- Schneider, J.A. High blood pressure and microinfarcts: A link between vascular risk factors, dementia, and clinical Alzheimer’s disease. J. Am. Geriatr. Soc. 2009, 57, 2146–2147. [Google Scholar] [CrossRef]
- Farrall, A.J.; Wardlaw, J.M. Blood–brain barrier: Ageing and microvascular disease–systematic review and meta-analysis. Neurobiol. Aging 2009, 30, 337–352. [Google Scholar] [CrossRef]
- Rosenberg, G. Blood-brain barrier permeability in aging and Alzheimer’s disease. J. Prev. Alzheimer’s Dis. 2014, 1, 138. [Google Scholar]
- Bell, R.D.; Winkler, E.A.; Singh, I.; Sagare, A.P.; Deane, R.; Wu, Z.; Holtzman, D.M.; Betsholtz, C.; Armulik, A.; Sallstrom, J. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012, 485, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Sagare, A.P.; Bell, R.D.; Zhao, Z.; Ma, Q.; Winkler, E.A.; Ramanathan, A.; Zlokovic, B.V. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat. Commun. 2013, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Starr, J.M.; Farrall, A.J.; Armitage, P.; McGurn, B.; Wardlaw, J. Blood–brain barrier permeability in Alzheimer’s disease: A case–control MRI study. Psychiatry Res. Neuroimaging 2009, 171, 232–241. [Google Scholar] [CrossRef]
- Hsieh, P.F.; Hou, C.-W.; Yao, P.-W.; Wu, S.-P.; Peng, Y.-F.; Shen, M.-L.; Lin, C.-H.; Chao, Y.-Y.; Chang, M.-H.; Jeng, K.-C. Sesamin ameliorates oxidative stress and mortality in kainic acid-induced status epilepticus by inhibition of MAPK and COX-2 activation. J. Neuroinflamm. 2011, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.H.; Wang, S.H.; Kuan, I.I.; Kao, Y.S.; Wu, P.J.; Liang, C.J.; Chien, H.F.; Kao, C.H.; Huang, C.J.; Chen, Y.L. Sesamin attenuates intercellular cell adhesion molecule-1 expression in vitro in TNF-α-treated human aortic endothelial cells and in vivo in apolipoprotein-E-deficient mice. Mol. Nutr. Food Res. 2010, 54, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, A.; Chopra, K. Effect of sesamol on diabetes-associated cognitive decline in rats. Exp. Brain Res. 2008, 185, 411–420. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Arbabi, E.; Rostami, F. The ameliorative effects of sesamol against seizures, cognitive impairment and oxidative stress in the experimental model of epilepsy. Iran. J. Basic Med. Sci. 2014, 17, 100. [Google Scholar] [PubMed]
- Angeline, M.S.; Sarkar, A.; Anand, K.; Ambasta, R.; Kumar, P. Sesamol and naringenin reverse the effect of rotenone-induced PD rat model. Neuroscience 2013, 254, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Xu, Z.-M.; Yang, G.-Y.; Yang, D.-X.; Ding, J.; Chen, H.; Yuan, F.; Tian, H.-L. Sesamin alleviates blood-brain barrier disruption in mice with experimental traumatic brain injury. Acta Pharmacol. Sin. 2017, 38, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- VanGilder, R.L.; Huber, J.D. Sesamol: A treatment for diabetes-associated blood-brain barrier dysfunction. Postdoc J. J. Postdr. Res. Postdr. Aff. 2014, 2, 13. [Google Scholar] [CrossRef]
- Chen, P.R.; Tsai, C.E.; Chang, H.; Liu, T.L.; Lee, C.C. Sesamol induces nitric oxide release from human umbilical vein endothelial cells. Lipids 2005, 40, 955–961. [Google Scholar] [CrossRef] [PubMed]
- VanGilder, R.L.; Rosen, C.L.; Barr, T.L.; Huber, J.D. Targeting the neurovascular unit for treatment of neurological disorders. Pharmacol. Ther. 2011, 130, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Bellavance, M.-A.; Blanchette, M.; Fortin, D. Recent advances in blood–brain barrier disruption as a CNS delivery strategy. AAPS J. 2008, 10, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Pardridge, W.M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Kaya, M.; Gulturk, S.; Elmas, I.; Kalayci, R.; Arican, N.; Kocyildiz, Z.C.; Kucuk, M.; Yorulmaz, H.; Sıvas, A. The effects of magnesium sulfate on blood-brain barrier disruption caused by intracarotid injection of hyperosmolar mannitol in rats. Life Sci. 2004, 76, 201–212. [Google Scholar] [CrossRef]
- Kumar, T.P.; Sirisha, B.; Raju, P.N.; Reddy, G.N. Nasal drug delivery: A potential route for brain targeting. Pharma Innov. 2013, 2, 77. [Google Scholar]
- Djupesland, P.G.; Mahmoud, R.A.; Messina, J.C. Accessing the brain: The nose may know the way. J. Cereb. Blood Flow Metab. 2013, 33, 793–794. [Google Scholar] [CrossRef] [Green Version]
- Minn, A.; Leclerc, S.; Heydel, J.-M.; Minn, A.-L.; Denizot, C.; Cattarelli, M.; Netter, P.; Gradinaru, D. Drug transport into the mammalian brain: The nasal pathway and its specific metabolic barrier. J. Drug Target. 2002, 10, 285–296. [Google Scholar] [CrossRef]
- Jain, K.; Sood, S.; Gowthamarajan, K. Optimization of artemether-loaded NLC for intranasal delivery using central composite design. Drug Deliv. 2015, 22, 940–954. [Google Scholar] [CrossRef] [Green Version]
- Omar, M.M.; Eleraky, N.E.; El Sisi, A.M.; Hasan, O.A. Development and evaluation of in-situ nasal gel formulations of nanosized transferosomal sumatriptan: Design, optimization, in vitro and in vivo evaluation. Drug Des. Dev. Ther. 2019, 13, 4413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, D.; Ali, A.; Md, S.; Baboota, S.; Sahni, J.K.; Ali, J. Insights into direct nose to brain delivery: Current status and future perspective. Drug Deliv. 2014, 21, 75–86. [Google Scholar] [CrossRef]
- Sonvico, F.; Clementino, A.; Buttini, F.; Colombo, G.; Pescina, S.; Stanisçuaski Guterres, S.; Raffin Pohlmann, A.; Nicoli, S. Surface-modified nanocarriers for nose-to-brain delivery: From bioadhesion to targeting. Pharmaceutics 2018, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Elsenosy, F.M.; Abdelbary, G.A.; Elshafeey, A.H.; Elsayed, I.; Fares, A.R. Brain Targeting of Duloxetine HCL via Intranasal Delivery of Loaded Cubosomal Gel: In vitro Characterization, ex vivo Permeation, and in vivo Biodistribution Studies. Int. J. Nanomed. 2020, 15, 9517. [Google Scholar] [CrossRef]
- de Goikoetxea, O.G.L. Nanostructured Lipid Carriers for Nose-to-Brain Delivery in Neurodegenerative Diseases Therapy; Universidad del País Vasco-Euskal Herriko Unibertsitatea: Biscay, Spain, 2016. [Google Scholar]
- Eleraky, N.E.; Omar, M.M.; Mahmoud, H.A.; Abou-Taleb, H.A. Nanostructured lipid carriers to mediate brain delivery of temazepam: Design and in vivo study. Pharmaceutics 2020, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Gidwani, B.; Kaur, C.D. Development of mebendazole loaded nanostructured lipid carriers for lymphatic targeting: Optimization, characterization, in-vitro and in-vivo evaluation. Part. Sci. Technol. 2020, 39, 380–390. [Google Scholar] [CrossRef]
- Arregui, J.R.; Kovvasu, S.P.; Betageri, G.V. Daptomycin proliposomes for oral delivery: Formulation, characterization, and in vivo pharmacokinetics. Aaps Pharmscitech 2018, 19, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Varsha, B.; Sowjanya, G.N.; Ajitha, A.; Uma Maheswara Rao, V.; Sura, R.S. Stability indicating rp-hplc method development andvalidation for estimation of nicergoline in bulk and tablet dosage form. World J. Pharm. Res. 2015, 4, 803–813. [Google Scholar]
- Khames, A.; Khaleel, M.A.; El-Badawy, M.F.; El-Nezhawy, A.O. Natamycin solid lipid nanoparticles–sustained ocular delivery system of higher corneal penetration against deep fungal keratitis: Preparation and optimization. Int. J. Nanomed. 2019, 14, 2515. [Google Scholar] [CrossRef] [Green Version]
- Diksha Sharma, S.A. Formulation and evaluation of in situ mucoadhesive thermoreversible nasal gel of sertraline hydrochloridedi. Asian J. Pharm. Clin. Res. 2019, 12, 195–202. [Google Scholar] [CrossRef]
- Abdelrahman, F.E.; Elsayed, I.; Gad, M.K.; Badr, A.; Mohamed, M.I. Investigating the cubosomal ability for transnasal brain targeting: In vitro optimization, ex vivo permeation and in vivo biodistribution. Int. J. Pharm. 2015, 490, 281–291. [Google Scholar] [CrossRef]
- De Jong, E.; Williams, D.S.; Abdelmohsen, L.K.; Van Hest, J.C.; Zuhorn, I.S. A filter-free blood-brain barrier model to quantitatively study transendothelial delivery of nanoparticles by fluorescence spectroscopy. J. Control. Release 2018, 289, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Yasir, M.; Chauhan, I.; Haji, M.J.; Tura, A.J.; Saxena, P.K. Formulation and evaluation of glyceryl behenate based solid lipid nanoparticles for the delivery of donepezil to brain through nasal route. Res. J. Pharm. Technol. 2018, 11, 2836–2844. [Google Scholar] [CrossRef]
- Zheng, R.; Wu, Y.-H.; Jiang, D.-X.; Zhang, D. Determination of metabolite of nicergoline in human plasma by high-performance liquid chromatography and its application in pharmacokinetic studies. J. Pharm. Anal. 2012, 2, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Kozlovskaya, L.; Abou-Kaoud, M.; Stepensky, D. Quantitative analysis of drug delivery to the brain via nasal route. J. Control. Release 2014, 189, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Pallagi, E.; Ambrus, R.; Szabó-Révész, P.; Csóka, I. Adaptation of the quality by design concept in early pharmaceutical development of an intranasal nanosized formulation. Int. J. Pharm. 2015, 491, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mao, S. Application of quality by design in the current drug development. Asian J. Pharm. Sci. 2017, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Khames, A. Hexyl alginate derivative, an amphiphilic innovative buccal film-forming material of promising mechanical and release characteristics for the improvement of repaglinide bioavailability. Drug Des. Dev. Ther. 2019, 13, 925. [Google Scholar] [CrossRef] [Green Version]
- Christensen, R. Analysis of Variance, Design, and Regression: Linear Modeling for Unbalanced Data; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Khames, A. Preparation and Characterization of Sildenafil Loaded Solid Lipid Nanoparticles: Drug Delivery System Suitable for Nebulization Ahmed khames. Der Pharm. Lett. 2017, 9, 98–112. [Google Scholar]
- Lin, X.; Li, X.; Zheng, L.; Yu, L.; Zhang, Q.; Liu, W. Preparation and characterization of monocaprate nanostructured lipid carriers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 311, 106–111. [Google Scholar] [CrossRef]
- Das, S.; Ng, W.K.; Tan, R.B. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Pinho, S.C.; Souto, E.B.; Santana, M.H. Polymorphism, crystallinity and hydrophilic–lipophilic balance of stearic acid and stearic acid–capric/caprylic triglyceride matrices for production of stable nanoparticles. Colloids Surf. B Biointerfaces 2011, 86, 125–130. [Google Scholar] [CrossRef]
- Khames, A. Formulation and characterization of eplerenone nanoemulsion liquisolids, an oral delivery system with higher release rate and improved bioavailability. Pharmaceutics 2019, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Lockman, P.R.; Koziara, J.; Roder, K.E.; Paulson, J.; Abbruscato, T.J.; Mumper, R.J.; Allen, D.D. In vivo and in vitro assessment of baseline blood-brain barrier parameters in the presence of novel nanoparticles. Pharm. Res. 2003, 20, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Koziara, J.M.; Mumper, R.J.; Allen, D.D. Nanoparticle surface charges alter blood–brain barrier integrity and permeability. J. Drug Target. 2004, 12, 635–641. [Google Scholar] [CrossRef]
- Seyfoddin, A.; Al-Kassas, R. Development of solid lipid nanoparticles and nanostructured lipid carriers for improving ocular delivery of acyclovir. Drug Dev. Ind. Pharm. 2013, 39, 508–519. [Google Scholar] [CrossRef]
- Teeranachaideekul, V.; Souto, E.B.; Junyaprasert, V.B.; Müller, R.H. Cetyl palmitate-based NLC for topical delivery of Coenzyme Q10–Development, physicochemical characterization and in vitro release studies. Eur. J. Pharm. Biopharm. 2007, 67, 141–148. [Google Scholar] [CrossRef]
- Sawant, K.K.; Dodiya, S.S. Recent advances and patents on solid lipid nanoparticles. Recent Pat. Drug Deliv. Formul. 2008, 2, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Khames, A. Investigation of the effect of solubility increase at the main absorption site on bioavailability of BCS class II drug (risperidone) using liquisolid technique. Drug Deliv. 2017, 24, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Censi, R.; Martena, V.; Hoti, E.; Malaj, L.; Di Martino, P. Preformulation study of nicergoline solid dispersions. J. Therm. Anal. Calorim. 2014, 115, 2439–2446. [Google Scholar] [CrossRef]
- Agarwal, A.; Mittal, A.; Ikram, S.; Tyagi, L.; Gupta, C. Solubility Enhancement of Nicergoline Poorly Water Soluble Drug by Novel Melt Sonocrystallization Technique. Sch. Acad. J. Pharm. 2020, 9, 347–365. [Google Scholar]
- Bhatt, M.; Bhatt, G.K. An overview: Formulation and product development of nasal spray. World J. Pharm. Res. 2017, 6, 404–413. [Google Scholar] [CrossRef] [Green Version]
- Pereira, G.R.; Collett, J.H.; Garcia, S.B.; Thomazini, J.A.; Bentley, M.V.L.B. Glycerol monooleate/solvents systems for progesterone transdermal delivery: In vitro permeation and microscopic studies. Rev. Bras. Ciências Farm. 2002, 38, 55–62. [Google Scholar] [CrossRef]
- Gupta, S.; Kesarla, R.; Chotai, N.; Misra, A.; Omri, A. Systematic approach for the formulation and optimization of solid lipid nanoparticles of efavirenz by high pressure homogenization using design of experiments for brain targeting and enhanced bioavailability. BioMed Res. Int. 2017, 2017, 5984014. [Google Scholar] [CrossRef]
- Mistry, A.; Glud, S.Z.; Kjems, J.; Randel, J.; Howard, K.A.; Stolnik, S.; Illum, L. Effect of physicochemical properties on intranasal nanoparticle transit into murine olfactory epithelium. J. Drug Target. 2009, 17, 543–552. [Google Scholar] [CrossRef]
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef]
- Bourganis, V.; Kammona, O.; Alexopoulos, A.; Kiparissides, C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur. J. Pharm. Biopharm. 2018, 128, 337–362. [Google Scholar] [CrossRef]
- Selvaraj, K.; Gowthamarajan, K.; Karri, V.V.S.R. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]




| Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Independent variables | |||
| A: Oil: Total lipid (% w/w) | 10 | 20 | 30 |
| B: Surfactant concentration (% w/w) | 1 | 2 | 3 |
| C: Sonication time (minutes) | 2 | 4 | 6 |
| Dependent variables | Constraint | Importance | |
| R1: Particle size (nm) | Minimize | 4 | |
| R2: Zeta potential (mV) | −(15–20) | 5 | |
| R3: Entrapment efficacy (%) | Maximize | 5 | |
| Formula | A Oil (% w/w) | B Surfactant (% w/w) | C ST (min) | R1 PS ± SD (nm) | R2 ZP ± SD (mV) | R3 E.E ± SD (%) | PDI ± SD |
|---|---|---|---|---|---|---|---|
| F1 | 20 | 3 | 2 | 112 ± 3.2 | −17.91 ± 1.2 | 86.7 ± 3.2 | 0.224 ± 0.02 |
| F2 | 20 | 2 | 4 | 126 ± 4.1 | −19.86 ± 2.3 | 80.6 ± 4.1 | 0.251 ± 0.02 |
| F3 | 20 | 2 | 4 | 132 ± 3.8 | −20.04 ± 0.7 | 81.3 ± 2.6 | 0.213 ± 0.04 |
| F4 | 20 | 3 | 6 | 107 ± 2.7 | −15.96 ± 0.9 | 85.1 ± 3.7 | 0.212 ± 0.04 |
| F5 | 30 | 2 | 2 | 127 ± 3 | −21.52 ± 1.2 | 90.6 ± 5.2 | 0.188 ± 0.05 |
| F6 | 10 | 1 | 4 | 159 ± 5.1 | −34.52 ± 1.7 | 70.9 ± 2.9 | 0.214 ± 0.03 |
| F7 | 20 | 2 | 4 | 134 ± 4.6 | −23.77 ± 2.3 | 81.9 ± 3.1 | 0.293 ± 0.04 |
| F8 | 20 | 1 | 6 | 147 ± 3.9 | −33.18 ± 3.1 | 83.7 ± 1.6 | 0.305 ± 0.02 |
| F9 | 20 | 1 | 2 | 153 ± 3.9 | −33.68 ± 1.6 | 73.6 ± 3.1 | 0.261 ± 0.01 |
| F10 | 30 | 3 | 4 | 103 ± 2.7 | −14.43 ± 0.8 | 94.2 ± 2.2 | 0.225 ± 0.05 |
| F11 | 20 | 2 | 4 | 119 ± 2.2 | −18.59 ± 1.5 | 80.08 ± 1.4 | 0.206 ± 0.04 |
| F12 | 10 | 2 | 2 | 143 ± 5.7 | −25.84 ± 2.1 | 75.1 ± 0.9 | 0.161 ± 0.03 |
| F13 | 10 | 2 | 6 | 140 ± 5.3 | −25.01 ± 1.3 | 74.6 ± 1.7 | 0.142 ± 0.05 |
| F14 | 10 | 3 | 4 | 123 ± 3.1 | −17.71 ± 0.7 | 79.2 ± 3.2 | 0.270 ± 0.03 |
| F15 | 30 | 1 | 4 | 150 ± 6.2 | −31.77 ± 4.1 | 86.17 ± 1.7 | 0.128 ± 0.01 |
| F16 | 20 | 2 | 4 | 136 ± 4.1 | −21.99 ± 1.1 | 83.56 ± 1.3 | 0.229 ± 0.02 |
| F17 | 30 | 2 | 6 | 129 ± 2.9 | −20.81 ± 2.2 | 91.18 ± 3.9 | 0.211 ± 0.03 |
| Formula | NIC–NLC Formulations | Remarks | |||||
|---|---|---|---|---|---|---|---|
| R Squared | Adjusted R Squared | Predicted R Squared | SD | CV% | Adequate Precision for ANOVA | ||
| R1 (Particle size) | |||||||
| Linear model | 0.9180 | 0.8990 | 0.8797 | 5.09 | 3.86 | 22.2677 | Suggested |
| 2FI model | 0.9269 | 0.8831 | 0.8383 | 5.48 | |||
| Quadratic model | 0.9502 | 0.8861 | 0.8747 | 5.41 | |||
| Cubic model | 0.9535 | 0.8139 | - | 6.91 | Aliased | ||
| R2 (Zeta potential) | |||||||
| Linear model | 0.8953 | 0.8711 | 0.8498 | 2.31 | |||
| 2FI model | 0.8962 | 0.8339 | 0.7534 | 2.26 | |||
| Quadratic model | 0.9734 | 0.9391 | 0.9355 | 1.59 | 6.8 | 16.7850 | Suggested |
| Cubic model | 0.9749 | 0.8998 | - | 2.04 | Aliased | ||
| R3 (EE) | |||||||
| Linear model | 0.9158 | 0.8963 | 0.8385 | 2.08 | |||
| 2FI model | 0.9673 | 0.9476 | 0.8933 | 1.48 | 1.8 | 24.5089 | Suggested |
| Quadratic model | 0.9747 | 0.9422 | 0.7518 | 1.56 | |||
| Cubic model | 0.9892 | 0.9566 | - | 1.35 | Aliased | ||
| Source | R1 (Particle size) Linear Model | R2 (Zeta Potential) Quadratic Model | R3 (E.E.%) 2FI Model | |||
|---|---|---|---|---|---|---|
| F-Values | p-Values | F-Values | p-Values | F-Values | p-Values | |
| Model | 48.49 | <0.0001 * | 28.41 | 0.0001 * | 49.26 | <0.0001 * |
| A | 15.12 | 0.0019 * | 10.52 | 0.0142 * | 221.49 | <0.0001 * |
| B | 129.67 | <0.0001 * | 223.90 | <0.0001 * | 54.15 | <0.0001 * |
| C | 0.6942 | 0.4198 | 0.7907 | 0.4034 | 4.19 | 0.0677 |
| AB | 0.0279 | 0.8721 | 0.0083 | 0.9292 | ||
| AC | 0.0014 | 0.9709 | 0.1329 | 0.7230 | ||
| BC | 0.2089 | 0.6615 | 15.60 | 0.0027 * | ||
| A2 | 1.46 | 0.2658 | ||||
| B2 | 13.33 | 0.0082 * | ||||
| C2 | 3.81 | 0.0917 | ||||
| Lack of fit | 0.3390 | 0.9180 | 0.0842 | 0.9651 | 1.35 | 0.4038 |
| Formula | Composition | Response | Predicted | Observed | Prediction Error (%) | Desirability | Drug Loading (%) | PDI ± SD | ||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||||
| F1 | 30 | 3 | 4.17 | R1 R2 R3 | 104.14 −14.16 93.7 | 111.18 −15.41 95.11 | 6.33 8.11 1.48 | 0.987 | 4.6% | 0.251 ± 0.04 |
| F2 | 30 | 3 | 4.36 | R1 R2 R3 | 103.99 −14.20 93.56 | 106.04 −14.88 91.99 | 1.93 4.56 1.67 | 0.985 | 4.1% | 0.273 ± 0.02 |
| F3 | 30 | 3 | 4.79 | R1 | 103.67 | 115.11 | 9.93 | 0.986 | 3.8% | 0.279 ± 0.04 |
| R2 | −14.17 | −13.27 | 6.78 | |||||||
| R3 | 93.21 | 94.61 | 1.47 | |||||||
| Organ/. tissue | AR | Formula | Pharmacokinetic Parameters | |||||
|---|---|---|---|---|---|---|---|---|
| C max (µg/mL) | Tmax (h) | AUC (0-t) (µg.h/mL) | AUC (0-∞) (µg.h/mL) | Kel (h−1) | t1/2 (h) | |||
| Plasma | IN | NIC–NLC | 1.68 | 2 | 8.65 | 9.43 | 0.218 | 3.17 |
| NIC–SOL | 1.08 | 2 | 5.33 | 5.62 | 0.476 | 1.46 | ||
| IV | NIC–NLC | 9.46 | - | 10.87 | 10.99 | 0.932 | 0.74 | |
| NIC–SOL | 9.84 | - | 7.71 | 7.76 | 0.903 | 0.77 | ||
| Brain | IN | NIC–NLC | 1.46 | 2 | 11.61 | 13.53 | 0.129 | 3.62 |
| NIC–SOL | 0.75 | 2 | 2.78 | 2.96 | 0.461 | 1.51 | ||
| IV | NIC–NLC | 1.37 | 1 | 8.04 | 8.42 | 0.291 | 2.38 | |
| NIC–SOL | 1.53 | 0.75 | 4.36 | 4.41 | 0.673 | 1.03 | ||
| Parameter | Source | Sum of Squares | df | Mean Square | F | Significance |
|---|---|---|---|---|---|---|
| C max (µg/mL) | Between Groups | 412.099 | 3 | 137.366 | 188923.61 | <0.001 |
| Within Groups | 0.015 | 20 | 0.001 | |||
| Total | 412.114 | 23 | ||||
| AUC (0–24) (µg·h/mL) | Between Groups | 94.677 | 3 | 31.559 | 664514.72 | |
| Within Groups | 0.001 | 20 | 0.000 | |||
| Total | 94.678 | 23 | ||||
| AUC (0–α) (µg·h/mL) | Between Groups | 95.387 | 3 | 31.796 | 383621.32 | |
| Within Groups | 0.002 | 20 | 0.000 | |||
| Total | 95.389 | 23 | ||||
| Kel (h−1) | Between Groups | 2.155 | 3 | 0.718 | 12655.19 | |
| Within Groups | 0.001 | 20 | 0.000 | |||
| Total | 2.156 | 23 | ||||
| t1/2 (h) | Between Groups | 35.308 | 3 | 11.769 | 62118.63 | |
| Within Groups | 0.004 | 20 | 0.000 | |||
| Total | 35.311 | 23 |
| Parameter | Group | Mean Difference | Confidence Interval | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| C max (µg/mL) | NIC–NLC formula (IN) | NIC–SOL (IN) | 0.602667 * | 0.55909 | 0.64624 |
| NIC–NLC (IV) | −7.780000 * | −7.82357 | −7.73643 | ||
| NIC–SOL (IV) | −8.161667 * | −8.20524 | −8.11809 | ||
| AUC (0−24) (µg.h/mL) | NIC–SOL (IN) | 3.317333 * | 3.30620 | 3.32847 | |
| NIC–NLC (IV) | −2.220500 * | −2.23164 | −2.20936 | ||
| NIC–SOL (IV) | 0.937333 * | 0.92620 | 0.94847 | ||
| AUC (0-α) (µg.h/mL) | NIC–SOL (IN) | 3.810500 * | 3.79579 | 3.82521 | |
| NIC–NLC (IV) | −1.559667 * | −1.57438 | −1.54495 | ||
| NIC–SOL (IV) | 1.669833 * | 1.65512 | 1.68455 | ||
| Kel (h−1) | NIC–SOL (IN) | −0.2580333 * | −0.270207 | −0.245860 | |
| NIC–NLC (IV) | −0.7140333 * | −0.726207 | −0.701860 | ||
| NIC–SOL (IV) | −0.6848667 * | −0.697040 | −0.672693 | ||
| t1/2 (h) | NIC–SOL (IN) | 2.25133333 * | 2.2290903 | 2.2735764 | |
| NIC–NLC (IV) | 2.97071667 * | 2.9484736 | 2.9929597 | ||
| NIC–SOL (IV) | 2.94116667 * | 2.9189236 | 2.9634097 | ||
| Parameter | Source | Sum of Squares | df | Mean Square | F | Significance |
|---|---|---|---|---|---|---|
| C max (µg/mL) | Between Groups | 2.309 | 3 | 0.770 | 2710.53 | <0.001 |
| Within Groups | 0.006 | 20 | 0.000 | |||
| Total | 2.315 | 23 | ||||
| Tmax (h) | Between Groups | 7.781 | 3 | 2.594 | - | |
| Within Groups | 0.000 | 20 | 0.000 | |||
| Total | 7.781 | 23 | ||||
| AUC (0−24) (µg·hr/mL) | Between Groups | 280.430 | 3 | 93.477 | 62140.72 | |
| Within Groups | 0.030 | 20 | 0.002 | |||
| Total | 280.460 | 23 | ||||
| AUC (0-α) (µg·hr/mL) | Between Groups | 417.873 | 3 | 139.291 | 214847.56 | |
| Within Groups | 0.013 | 20 | 0.001 | |||
| Total | 417.886 | 23 | ||||
| K (h−1) | Between Groups | 0.981 | 3 | 0.327 | 34365.89 | |
| Within Groups | 0.000 | 20 | 0.000 | |||
| Total | 0.982 | 23 | ||||
| t1/2 (h) | Between Groups | 23.314 | 3 | 7.771 | 101699.49 | |
| Within Groups | 0.002 | 20 | 0.000 | |||
| Total | 23.316 | 23 |
| Parameter | Group | Mean Difference | Confidence Interval | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| C max (µg/mL) | NIC–NLC formula (IN) | NIC–SOL (IN) | 0.711000 * | 0.68377 | 0.73823 |
| NIC–NLC (IV) | 0.091167 * | 0.06393 | 0.11840 | ||
| NIC–SOL (IV) | −0.070333 * | −0.09757 | −0.04310 | ||
| AUC (0–24) (µg.h/mL) | NIC–SOL (IN) | 8.829833 * | 8.76716 | 8.89251 | |
| NIC–NLC (IV) | 3.569333 * | 3.50666 | 3.63201 | ||
| NIC–SOL (IV) | 7.248333 * | 7.18566 | 7.31101 | ||
| AUC (0–α) (µg.h/mL) | NIC–SOL (IN) | 10.838333 * | 10.79719 | 10.87948 | |
| NIC–NLC (IV) | 5.109333 * | 5.06819 | 5.15048 | ||
| NIC–SOL (IV) | 9.118833 * | 9.07769 | 9.15998 | ||
| Kel (h−1) | NIC–SOL (IN) | −0.3319667 * | −0.336953 | −0.326981 | |
| NIC–NLC (IV) | −0.1599000 * | −0.164886 | −0.154914 | ||
| NIC–SOL (IV) | −0.5442167 * | −0.549203 | −0.539231 | ||
| t1/2 (h) | NIC–SOL (IN) | 2.11368333 * | 2.0995572 | 2.1278095 | |
| NIC–NLC (IV) | 1.24011667 * | 1.2259905 | 1.2542428 | ||
| NIC–SOL (IV) | 2.59192500 * | 2.5777989 | 2.6060511 | ||
| Storage Conditions | LC ± SD (%) | ZP ± SD (mV) | PS ± SD (nm) | PDI ± SD |
|---|---|---|---|---|
| Fresh | 4.6 ± 0.34 | −15.41 ± 0.71 | 111.18 ± 4.71 | 0.251 ± 0.04 |
| 25 °C | 4.3 ± 0.31 | −19.45 ± 0.92 | 132.65 ± 5.29 | 0.273 ± 0.01 |
| 4 °C | 4.5 ± 0.21 | −15.89 ± 0.38 | 117.42 ± 2.11 | 0.257 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abourehab, M.A.S.; Khames, A.; Genedy, S.; Mostafa, S.; Khaleel, M.A.; Omar, M.M.; El Sisi, A.M. Sesame Oil-Based Nanostructured Lipid Carriers of Nicergoline, Intranasal Delivery System for Brain Targeting of Synergistic Cerebrovascular Protection. Pharmaceutics 2021, 13, 581. https://doi.org/10.3390/pharmaceutics13040581
Abourehab MAS, Khames A, Genedy S, Mostafa S, Khaleel MA, Omar MM, El Sisi AM. Sesame Oil-Based Nanostructured Lipid Carriers of Nicergoline, Intranasal Delivery System for Brain Targeting of Synergistic Cerebrovascular Protection. Pharmaceutics. 2021; 13(4):581. https://doi.org/10.3390/pharmaceutics13040581
Chicago/Turabian StyleAbourehab, Mohammed A. S., Ahmed Khames, Samar Genedy, Shahin Mostafa, Mohammad A. Khaleel, Mahmoud M. Omar, and Amani M. El Sisi. 2021. "Sesame Oil-Based Nanostructured Lipid Carriers of Nicergoline, Intranasal Delivery System for Brain Targeting of Synergistic Cerebrovascular Protection" Pharmaceutics 13, no. 4: 581. https://doi.org/10.3390/pharmaceutics13040581
APA StyleAbourehab, M. A. S., Khames, A., Genedy, S., Mostafa, S., Khaleel, M. A., Omar, M. M., & El Sisi, A. M. (2021). Sesame Oil-Based Nanostructured Lipid Carriers of Nicergoline, Intranasal Delivery System for Brain Targeting of Synergistic Cerebrovascular Protection. Pharmaceutics, 13(4), 581. https://doi.org/10.3390/pharmaceutics13040581






