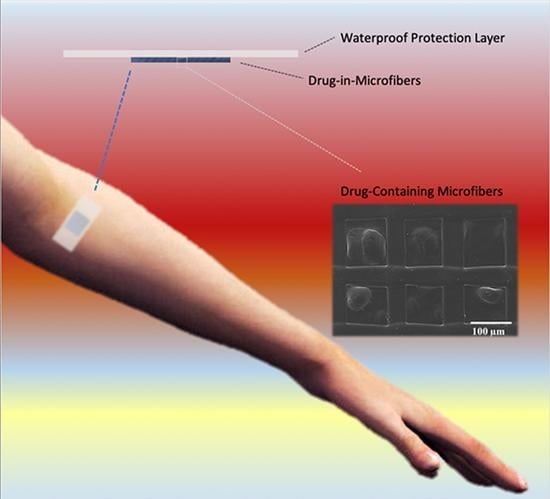
Kinetic Release Studies of Antibiotic Patches for Local Transdermal Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solutions
2.3. EHD-3D Printing
2.4. Characterization of Drug Patches
2.5. Drug Release Studies
2.6. Application of Drug Release Data to Mathematical Models
2.6.1. Zero Order Kinetics
2.6.2. First Order Kinetics
2.6.3. Higuchi Model
2.6.4. Korsmeyer–Peppas Model
2.7. Antibacterial Activity Assay
3. Results and Discussion
3.1. Characterization of Fabricated Samples
3.2. Drug Release and Mathematical Modeling of Release Mechanisms
3.3. Antibacterial Assessment of Fabricated Patches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misic, A.M.; Gardner, S.E.; Grice, E.A. The Wound Microbiome: Modern Approaches to Examining the Role of Microorganisms in Impaired Chronic Wound Healing. Adv. Wound Care 2014, 3, 502–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, S.; Sutton, J. Personalized medicine could transform healthcare. Biomed. Rep. 2017, 7, 3–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasubbu, D.A.; Smith, V.; Hayden, F.; Cronin, P. Systemic antibiotics for treating malignant wounds. Cochrane Database Syst. Rev. 2017, 8, CD011609. [Google Scholar] [CrossRef] [PubMed]
- Everts, R. How to Treat Wound Infection. Prevention and Treatment. 2016. Available online: https://www.acc.co.nz/assets/provider/treating-wound-infections.pdf (accessed on 7 April 2020).
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N. Antibiotic Delivery Strategies to Treat Skin Infections When Innate Antimicrobial Defense Fails. Antibiotics 2020, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, R. New methods of drug delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, W.M. Drug Delivery–Engineering Principles for Drug Therapy; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Berchane, N.; Jebrail, F.; Andrews, M. Optimization of PLG microspheres for tailored drug release. Int. J. Pharm. 2010, 383, 81–88. [Google Scholar] [CrossRef]
- Wei, L.; Cai, C.; Lin, J.; Chen, T. Dual-drug delivery system based on hydrogel/micelle composites. Biomaterials 2009, 30, 2606–2613. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Kee, I.; Krantz, W.B.; Chow, P.K.-H.; Wang, C.-H. Hydrogel Matrix Entrapping PLGA-Paclitaxel Microspheres: Drug Delivery with Near Zero-Order Release and Implantability Advantages for Malignant Brain Tumour Chemotherapy. Pharm. Res. 2009, 26, 2101–2114. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, S.; Wang, H.; Ducheyne, P. Polymer-coated mesoporous silica nanoparticles for the controlled release of macromolecules. Acta Biomater. 2012, 8, 3429–3435. [Google Scholar] [CrossRef]
- Akhtar, N.; Singh, V.; Yusuf, M.; Khan, R.A. Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed. Tech. Eng. 2020, 65, 243–272. [Google Scholar] [CrossRef] [Green Version]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [Green Version]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-U.; Lee, J.H.; Paik, U.; Lu, Y.; Rogers, J.A. Nanoscale Patterns of Oligonucleotides Formed by Electrohydrodynamic Jet Printing with Applications in Biosensing and Nanomaterials Assembly. Nano Lett. 2008, 8, 4210–4216. [Google Scholar] [CrossRef]
- Huang, Y.; Bu, N.; Duan, Y.; Pan, Y.; Liu, H.; Yin, Z.; Xiong, Y. Electrohydrodynamic direct-writing. Nanoscale 2013, 5, 12007–12017. [Google Scholar] [CrossRef]
- Meier, W. Polymer nanocapsules. Chem. Soc. Rev. 2000, 29, 295–303. [Google Scholar] [CrossRef]
- Park, J.U. High-Resolution Electrohydrodynamic Jet Printing Methods for Applications in Electronics and Biotechnology. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2009. [Google Scholar]
- Wang, K.; Cai, L.; Zhang, L.; Dong, J.; Wang, S. Biodegradable Photo-Crosslinked Polymer Substrates with Concentric Microgrooves for Regulating MC3T3-E1 Cell Behavior. Adv. Heal. Mater. 2012, 1, 292–301. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lee, H.J.; Kim, G.H. Polycaprolactone Scaffolds Fabricated with an Advanced Electrohydrodynamic Direct-Printing Method for Bone Tissue Regeneration. Biomacromolecules 2011, 12, 4256–4263. [Google Scholar] [CrossRef]
- Altun, E.; Akyol, S.; Ekren, N.; Kilic, O.; Gündüz, O.; Kılıç, O. Electrohydrodynamic (EHD) Bioprinting of Polycaprolactone Scaffolds. Mater. Sci. Forum 2018, 923, 93–97. [Google Scholar] [CrossRef]
- Béduneau, A.; Saulnier, P.; Anton, N.; Hindré, F.; Passirani, C.; Rajerison, H.; Noiret, N.; Benoit, J.-P. Pegylated Nanocapsules Produced by an Organic Solvent-Free Method: Evaluation of their Stealth Properties. Pharm. Res. 2006, 23, 2190–2199. [Google Scholar] [CrossRef]
- Albrecht, L.D.; Sawyer, S.W.; Soman, P. Developing 3D Scaffolds in the Field of Tissue Engineering to Treat Complex Bone Defects. 3D Print. Addit. Manuf. 2016, 3, 106–112. [Google Scholar] [CrossRef]
- Bernards, D.A.; Lance, K.D.; Ciaccio, N.A.; Desai, T.A. Nanostructured Thin Film Polymer Devices for Constant-Rate Protein Delivery. Nano Lett. 2012, 12, 5355–5361. [Google Scholar] [CrossRef] [Green Version]
- Altun, E.; Aydogdu, M.O.; Koc, F.; Crabbe-Mann, M.; Brako, F.; Kaur-Matharu, R.; Ozen, G.; Kuruca, S.E.; Edirisinghe, U.; Gunduz, O.; et al. Novel Making of Bacterial Cellulose Blended Polymeric Fiber Bandages. Macromol. Mater. Eng. 2018, 303, 1700607. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef]
- Qiu, Y.; Qiu, L.; Cui, J.; Wei, Q. Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater. Sci. Eng. C 2016, 59, 303–309. [Google Scholar] [CrossRef]
- Altun, E.; Ekren, N.; Kuruca, S.E.; Gunduz, O. Cell studies on Electrohydrodynamic (EHD)-3D-bioprinted Bacterial Cellulose\Polycaprolactone scaffolds for tissue engineering. Mater. Lett. 2019, 234, 163–167. [Google Scholar] [CrossRef]
- Puppi, D.; Piras, A.M.; Detta, N.; Dinucci, D.; Chiellini, F. Poly(lactic-co-glycolic acid) electrospun fibrous meshes for the controlled release of retinoic acid. Acta Biomater. 2010, 6, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Samuelov, Y.; Donbrow, M.; Friedman, M. Sustained Release of Drugs from Ethylcellulose-Polyethylene Glycol Films and Kinetics of Drug Release. J. Pharm. Sci. 1979, 68, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Aydogdu, M.O.; Altun, E.; Crabbe-Mann, M.; Brako, F.; Koç, F.; Ozen, G.; Kuruca, S.E.; Edirisinghe, U.; Luo, C.; Gündüz, O.; et al. Cellular interactions with bacterial cellulose: Polycaprolactone nanofibrous scaffolds produced by a portable electrohydrodynamic gun for point-of-need wound dressing. Int. Wound J. 2018, 15, 789–797. [Google Scholar] [CrossRef]
- Roldán, G.J.C.; Bolivariana, U.P.; Martínez, Y.Q.; Gómez, L.M.A.; Vinasco, L.F.R.; Palacio, L.M.H. Influence of the molecular weight of polymer, solvents and operational condition in the electrospinning of polycaprolactone. Revista Facultad de Ingeniería Universidad de Antioquia 2017, 84, 35–45. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospun porous cellulose acetate fibers from volatile solvent mixture. Mater. Lett. 2011, 65, 2291–2294. [Google Scholar] [CrossRef] [Green Version]
- Wendorff, J.H.; Agarwal, S.; Greiner, A. Medicinal Applications for Electrospun Nanofibers. Electrospinning; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 217–236. [Google Scholar]
- Currey, J.D. Biomechanics of Mineralized Skeletons. In Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends; Carter, J.G., Ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C.; Morril, T.C. Spectrometric Identification of Organic Compounds; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Altun, E.; Aydogdu, M.O.; Koc, F.; Kutlu, O.; Gozuacik, D.; Yucel, S.; Gunduz, O. Amoxicillin Loaded Hollow Microparticles in the Treatment of Osteomyelitis Disease Using Single-Nozzle Electrospinning. BioNanoScience 2018, 8, 790–801. [Google Scholar] [CrossRef]
- Wang, J.-C.; Chang, M.-W.; Ahmad, Z.; Li, J.-S. Fabrication of patterned polymer-antibiotic composite fibers via electrohydrodynamic (EHD) printing. J. Drug Deliv. Sci. Technol. 2016, 35, 114–123. [Google Scholar] [CrossRef]
- Niinomi, M.; Hattori, T.; Niwa, S.; Yaszemski, M.; Trantolo, D.; Lewandrowski, K.-U.; Hasirci, V.; Altobelli, D.; Wise, D. Material Characteristics and Biocompatibility of Low Rigidity Titanium Alloys for Biomedical Applications. In Biomaterials in Orthopedics; Yaszemski, M., Trantolo, D., Lewandrowski, K., Hasirci, V., Altobelli, D., Wise, D., Eds.; Marcel Dekker, INC.: Basel, Switzerland, 2004; pp. 41–62. [Google Scholar]
- Griffin, M.; Premakumar, Y.; Seifalian, A.; Butler, P.E.; Szarko, M. Biomechanical Characterization of Human Soft Tissues Using Indentation and Tensile Testing. J. Vis. Exp. 2016, 118, e54872. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Galán, A.; Franco, L.; Puiggalí, J.; García, M.L.F. Biodegradable Polyurethanes and Poly(ester amide)s. In Handbook of Biodegradable Polymers: Isolation, Synthesis, Characterization and Applications; Lendlein, A., Sisson, A., Eds.; Wiley: Hoboken, NJ, US, 2011; Chapter 6; pp. 133–154. [Google Scholar]
- Dash, T.K.; Konkimalla, V.B. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Altun, E.; Aydogdu, M.O.; Togay, S.O.; Sengil, A.Z.; Ekren, N.; Haskoylu, M.E.; Oner, E.T.; Altuncu, N.A.; Ozturk, G.; Crabbe-Mann, M.; et al. Bioinspired scaffold induced regeneration of neural tissue. Eur. Polym. J. 2019, 114, 98–108. [Google Scholar] [CrossRef]
- Valarezo, E.; Tammaro, L.; González, S.; Malagón, O.; Vittoria, V. Fabrication and sustained release properties of poly(ε-caprolactone) electrospun fibers loaded with layered double hydroxide nanoparticles intercalated with amoxicillin. Appl. Clay Sci. 2013, 72, 104–109. [Google Scholar] [CrossRef]
- Gimeno, M.; Pinczowski, P.; Pérez, M.; Giorello, A.; Martínez, M.Á.; Santamaría, J.; Arruebo, M.; Luján, L. A controlled antibiotic release system to prevent orthopedic-implant associated infections: An in vitro study. Eur. J. Pharm. Biopharm. 2015, 96, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.M.; Hohman, M.M.; Brenner, M.P.; Rutledge, G.C. Electrospinning: A whipping fluid jet generates submicron polymer fibers. Appl. Phys. Lett. 2001, 78, 1149–1151. [Google Scholar] [CrossRef]
- Chou, S.-F.; Carson, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-C.; Zheng, H.; Chang, M.-W.; Ahmad, Z.; Li, J.-S. Preparation of active 3D film patches via aligned fiber electrohydrodynamic (EHD) printing. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Peppas, N.A.; Duncan, R.; Wnek, G.E.; Hoffman, A.S.; Gao, G.H.; Kim, S.W.; Lee, D.S.; Hadjiargyrou, M.; Touitou, E.; Ainbinder, D.; et al. Highly cited research articles in Journal of Controlled Release: Commentaries and perspectives by authors. J. Control. Release 2014, 190, 29–74. [Google Scholar] [CrossRef]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]











| Sample Name (wt. ratio) | Viscosity (Pa.S) | Electrical Conductivity (µ.S cm−1) | Density (kg m−3) | Surface Tension (mN m−1) |
|---|---|---|---|---|
| BC/PCL (5:95) | 363 ± 0.5 | 1.20 ± 0.03 | 1445 ± 2 | 52.8 ± 0.1 |
| BC/PCL (5:95) +AMX | 368 ± 0.3 | 1.20 ± 0.02 | 1448 ± 1 | 52.9 ± 0.1 |
| BC/PCL (5:95) +AMP | 371 ± 0.1 | 1.20 ± 0.02 | 1452 ± 1 | 53.2 ± 0.5 |
| BC/PCL (5:95) +KAN | 376 ± 0.2 | 1.20 ± 0.01 | 1459 ± 1 | 53.4 ± 0.3 |
| Sample Name (wt. ratio) | Pore Size (µm2) | Porosity (%) |
|---|---|---|
| BC/PCL (5:95) | 2.22 ± 0.85 | 11.52 ± 1.14 |
| BC/PCL (5:95) +AMX | 10.20 ± 0.35 | 32.43 ± 1.36 |
| BC/PCL (5:95) +AMP | 6.18 ± 0.12 | 53.21 ± 1.76 |
| BC/PCL (5:95) +KAN | 3.06 ± 1.09 | 19.11 ± 1.44 |
| Sample Name (wt. ratio) | Yield (%) | Drug Loading (%) | Encapsulation Efficiency (%) |
|---|---|---|---|
| BC/PCL (5:95) | 98.4 ± 0.6 | - | - |
| BC/PCL (5:95) +AMX | 98.2 ± 1.3 | 21.9 ± 0.3 | 97.4 ± 0.9 |
| BC/PCL (5:95) +AMP | 96.3 ± 2.1 | 21.7 ± 0.7 | 96.8 ± 1.6 |
| BC/PCL (5:95) +KAN | 97.9 ± 1.9 | 21.9 ± 0.5 | 98.6 ± 1.4 |
| Sample | BC/PCL | BC/PCL+AMX | BC/PCL+AMP | BC/PCL+KAN | |
|---|---|---|---|---|---|
| Bacterial Strain | |||||
| S. aureus |  |  |  |  | |
| E. coli |  |  |  |  | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altun, E.; Yuca, E.; Ekren, N.; Kalaskar, D.M.; Ficai, D.; Dolete, G.; Ficai, A.; Gunduz, O. Kinetic Release Studies of Antibiotic Patches for Local Transdermal Delivery. Pharmaceutics 2021, 13, 613. https://doi.org/10.3390/pharmaceutics13050613
Altun E, Yuca E, Ekren N, Kalaskar DM, Ficai D, Dolete G, Ficai A, Gunduz O. Kinetic Release Studies of Antibiotic Patches for Local Transdermal Delivery. Pharmaceutics. 2021; 13(5):613. https://doi.org/10.3390/pharmaceutics13050613
Chicago/Turabian StyleAltun, Esra, Esra Yuca, Nazmi Ekren, Deepak M. Kalaskar, Denisa Ficai, Georgiana Dolete, Anton Ficai, and Oguzhan Gunduz. 2021. "Kinetic Release Studies of Antibiotic Patches for Local Transdermal Delivery" Pharmaceutics 13, no. 5: 613. https://doi.org/10.3390/pharmaceutics13050613
APA StyleAltun, E., Yuca, E., Ekren, N., Kalaskar, D. M., Ficai, D., Dolete, G., Ficai, A., & Gunduz, O. (2021). Kinetic Release Studies of Antibiotic Patches for Local Transdermal Delivery. Pharmaceutics, 13(5), 613. https://doi.org/10.3390/pharmaceutics13050613