Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride
Abstract
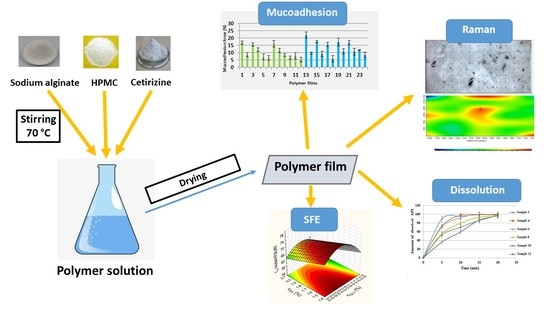
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Films
2.3. Thickness of Films
2.4. Tensile Strength (Hardness) of Films
2.5. In Vitro Mucoadhesion Test
2.6. Contact Angle and Surface Free Energy (SFE) Measurement
2.7. FTIR Spectroscopy Measurement
2.8. Raman Spectroscopy Measurement
2.9. Dissolution Test
2.10. Statistical Analysis
3. Results
3.1. Thickness and Tensile Strength of the Films
R2 = 0.9218
R2 = 0.9784
R2 = 0.9461
R2 = 0.9972
(2% polymer films containing CTZ)
y2 = 25.32 + 1.72∙x1 − 5.93∙x2 + 1.32∙x22
(3% polymer films containing CTZ)
R2 = 0.9784
(2% polymer films without CTZ)
R2 = 0.9955
(3% polymer films without CTZ)
R2 = 0.9979
3.2. In Vitro Mucoadhesion Test
(2% polymer films containing CTZ)
R2 = 0.6133
(3% polymer films containing CTZ)
y3 = 18.96 − 1.21∙x1 − 4.47∙x2 + 0.44∙x1∙x2
(2% polymer films without CTZ)
R2 = 0.8408
(3% polymer films without CTZ)
R2 = 0.9120
3.3. Contact Angle and Surface Free Energy (SFE) Measurement
(2% polymer films containing CTZ)
R2 = 0.9638
R2 = 0.9054
y4 = 57.81 + 3.19∙x1 + 5.67∙x2 + 0.84∙x22
(2% polymer films without CTZ)
R2 = 0.7854
(3% polymer films without CTZ)
R2 = 0.7237
3.4. FTIR Measurement
3.5. Raman Spectroscopy Measurement
3.6. Dissolution Test
(2% polymer films containing CTZ)
R2 = 0.9671
(3% polymer films containing CTZ)
R2 = 0.9996
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Boetker, J.; Rantanen, J.; Jacobsen, J.; Vizirianakis, I.S.; Fatouros, D.G. Fabrication of Mucoadhesive Buccal Films for Local Administration of Ketoprofen and Lidocaine Hydrochloride by Combining Fused Deposition Modeling and Inkjet Printing. J. Pharm. Sci. 2020, 109, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Koradia, H.; Chaudhari, K. Formulation of unidirectional buccal tablet of Mirtazapine: An in vitro and ex vivo evaluation. J. Drug Deliv. Sci. Technol. 2018, 43, 233–242. [Google Scholar] [CrossRef]
- Marques, A.C.; Rocha, A.I.; Leal, P.; Estanqueiro, M.; Lobo, J.M.S. Development and characterization of mucoadhesive buccal gels containing lipid nanoparticles of ibuprofen. Int. J. Pharm. 2017, 533, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhubiab, B.E.; Nair, A.; Kumria, R.; Attimarad, M.; Harsha, S. Development and evaluation of buccal films impregnated with selegiline-loaded nanospheres. Drug Deliv. 2014, 23, 2154–2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itin, C.; Komargodski, R.; Domb, A.J.; Hoffman, A. Controlled Delivery of Apomorphine Through Buccal Mucosa, Towards a Noninvasive Administration Method in Parkinson’s Disease: A Preclinical Mechanistic Study. J. Pharm. Sci. 2020, 109, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Orlu, M.; Ranmal, S.R.; Sheng, Y.; Tuleu, C.; Seddon, P. Acceptability of orodispersible films for delivery of medicines to infants and preschool children. Drug Deliv. 2017, 24, 1243–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chachlioutaki, K.; Tzimtzimis, E.K.; Tzetzis, D.; Chang, M.-W.; Ahmad, Z.; Karavasili, C.; Fatouros, D.G. Electrospun Orodispersible Films of Isoniazid for Pediatric Tuberculosis Treatment. Pharmacy 2020, 12, 470. [Google Scholar] [CrossRef]
- Macedo, A.S.; Castro, P.M.; Roque, L.; Thomé, N.G.; Reis, C.P.; Pintado, M.E.; Fonte, P. Novel and revisited approaches in nanoparticle systems for buccal drug delivery. J. Control. Release 2020, 320, 125–141. [Google Scholar] [CrossRef]
- Patel, V.F.; Liu, F.; Brown, M.B. Advances in oral transmucosal drug delivery. J. Control. Release 2011, 153, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Serrano, D.R.; Fernandez-Garcia, R.; Mele, M.; Healy, A.M.; Lalatsa, A. Designing Fast-Dissolving Orodispersible Films of Amphotericin B for Oropharyngeal Candidiasis. Pharmaceutics 2019, 11, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karagianni, A.; Peltonen, L. Production of Itraconazole Nanocrystal-Based Polymeric Film Formulations for Immediate Drug Release. Pharmaceutics 2020, 12, 960. [Google Scholar] [CrossRef]
- Kumria, R.; Nair, A.B.; Goomber, G.; Gupta, S. Buccal films of prednisolone with enhanced bioavailability. Drug Deliv. 2014, 23, 471–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, C.; Liu, H.; Lu, Y.; Zhang, L. Blend Films from Sodium Alginate and Gelatin Solutions. J. Macromol. Sci. Part A 2001, 38, 317–328. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Khan, S.; Boateng, J.S.; Mitchell, J.; Trivedi, V. Formulation, Characterisation and Stabilisation of Buccal Films for Paediatric Drug Delivery of Omeprazole. AAPS PharmSciTech 2015, 16, 800–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okeke, O.C.; Boateng, J.S. Nicotine stabilization in composite sodium alginate based wafers and films for nicotine replacement therapy. Carbohydr. Polym. 2017, 155, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Juliano, C.C.A.; Cossu, M.; Pigozzi, P.; Rassu, G.; Giunchedi, P. Preparation, In Vitro Characterization and Preliminary In Vivo Evaluation of Buccal Polymeric Films Containing Chlorhexidine. AAPS PharmSciTech 2008, 9, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelemen, A.; Katona, B.; Módra, S.; Aigner, Z.; Sebe, I.; Pintye-Hódi, K.; Zelkó, R.; Regdon, J.G.; Kristó, K. Effects of Sucrose Palmitate on the Physico-Chemical and Mucoadhesive Properties of Buccal Films. Molecules 2020, 25, 5248. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Ando, M.; Nagata, N.; Goto, E.; Yoshimura, N.; Takeuchi, T.; Noda, T.; Ozeki, T. Fabrication of Naftopidil-Loaded Tablets Using a Semisolid Extrusion-Type 3D Printer and the Characteristics of the Printed Hydrogel and Resulting Tablets. J. Pharm. Sci. 2019, 108, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, G.; Liang, W.; Chow, M. Prediction of drug release from HPMC matrices: Effect of physicochemical properties of drug and polymer concentration. J. Control. Release 2004, 95, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Viridén, A.; Wittgren, B.; Larsson, A. Investigation of critical polymer properties for polymer release and swelling of HPMC matrix tablets. Eur. J. Pharm. Sci. 2009, 36, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; das Neves, J.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014, 39, 2030–2075. [Google Scholar] [CrossRef]
- Kumria, R.; Nair, A.; Al-Dhubiab, B.E. Loratidine buccal films for allergic rhinitis: Development and evaluation. Drug Dev. Ind. Pharm. 2014, 40, 625–631. [Google Scholar] [CrossRef]
- Peh, K.K.; Wong, C.F. Polymeric films as vehicle for buccal delivery: Swelling, mechanical, and bioadhesive properties. J. Pharm. Pharm. Sci. 2000, 2, 53–61. [Google Scholar]
- Castán, H.; Ruiz, M.; Clares, B.; Morales, M. Design, development and characterization of buccal bioadhesive films of Doxepin for treatment of odontalgia. Drug Deliv. 2015, 22, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.R.; Khadheer Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. 3-Biopolymer composites with high dielectric performance: Interface engineering. In Biopolymer Composites in Electronics; Sadasivuni, K.K., Ponnamma, D., Kim, J., Cabibihan, J.-J., AlMaadeed, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. [Google Scholar] [CrossRef]
- Ghavami-Lahiji, M.; Shafiei, F.; Najafi, F.; Erfan, M. Drug-loaded polymeric films as a promising tool for the treatment of periodontitis. J. Drug Deliv. Sci. Technol. 2019, 52, 122–129. [Google Scholar] [CrossRef]
- Paolicelli, P.; Petralito, S.; Varani, G.; Nardoni, M.; Pacelli, S.; Di Muzio, L.; Tirillò, J.; Bartuli, C.; Cesa, S.; Casadei, M.A.; et al. Effect of glycerol on the physical and mechanical properties of thin gellan gum films for oral drug delivery. Int. J. Pharm. 2018, 547, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velazquez, G.; Vázquez, M. Novel composite films from regenerated cellulose-glycerol-polyvinyl alcohol: Mechanical and barrier properties. Food Hydrocoll. 2019, 89, 481–491. [Google Scholar] [CrossRef]
- Gao, W.; Liu, P.; Li, X.; Qiu, L.; Hou, H.; Cui, B. The co-plasticization effects of glycerol and small molecular sugars on starch-based nanocomposite films prepared by extrusion blowing. Int. J. Biol. Macromol. 2019, 133, 1175–1181. [Google Scholar] [CrossRef]
- Schmid, Y.; Navarini, A.; Thomas, Z.-R.M.; Pfleiderer, B.; Krähenbühl, S.; Mueller, S.M. Sex differences in the pharmacology of itch therapies—a narrative review. Curr. Opin. Pharmacol. 2019, 46, 122–142. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Feldman, S.R.; Fleischer, A.B. An assessment of the use of antihistamines in the management of atopic dermatitis. J. Am. Acad. Dermatol. 2018, 79, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Ahmad, M.; Akhtar, N.; Laffleur, F.; Bernkop-Schnürch, A. Thiolated α-Cyclodextrin: The Invisible Choice to Prolong Ocular Drug Residence Time. J. Pharm. Sci. 2016, 105, 2848–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Balen, G.P.; Caron, G.; Ermondi, G.; Pagliara, A.; Grandi, T.; Bouchard, G.; Fruttero, R.; Carrupt, P.; Testa, B. Lipophilicity Behaviour of the Zwitterionic Antihistamine Cetirizine in Phosphatidylcholine Liposomes/Water Systems. Pharm. Res. 2001, 18, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Testa, B.; Pagliara, A.; Carrupt, P.A. The molecular behaviour of cetirizine. Clin. Exp. Allergy 1997, 27, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Alrasbi, M.; Sheikh, A. Comparison of international guidelines for the emergency medical management of anaphylaxis. Allergy 2007, 62, 838–841. [Google Scholar] [CrossRef]
- Soar, J.; Pumphrey, R.; Cant, A.; Clarke, S.; Corbett, A.; Dawson, P.; Ewan, P.; Foëx, B.; Gabbott, D.; Griffiths, M.; et al. Emergency treatment of anaphylactic reactions—Guidelines for healthcare providers. Resuscitation 2008, 77, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Phalguna, Y.; Pasupulati, H.; Rudra, S. Formulation, Characterization and In-Vitro Evaluation of Fast Dissolving Oral Films of Cetirizine HCL. J. Drug Deliv. Ther. 2019, 9, 122–125. [Google Scholar] [CrossRef]
- Baniya, D.P.; Pandey, G.; Bajaracharya, M.; Dhungana, B.R. Formulation and Evaluation of Fast Dissolving Oral Films of Cetirizine Hydrochloride. Eur. J. Med. Sci. 2020, 2, 23–29. [Google Scholar] [CrossRef]
- Ibrahim, Y.H.-E.; Regdon, G.; Kristó, K.; Kelemen, A.; Adam, M.E.; Hamedelniel, E.I.; Sovány, T. Design and characterization of chitosan/citrate films as carrier for oral macromolecule delivery. Eur. J. Pharm. Sci. 2020, 146, 105270. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, A.; Gottnek, M.; Regdon, G.; Pintye-Hódi, K.; Regdon, J.G. New equipment for measurement of the force of adhesion of mucoadhesive films. J. Adhes. Sci. Technol. 2015, 29, 1360–1367. [Google Scholar] [CrossRef] [Green Version]
- Gottnek, M.; Süvegh, K.; Pintye-Hódi, K.; Regdon, G.; Regdon, J.G. Effects of excipients on the tensile strength, surface properties and free volume of Klucel® free films of pharmaceutical importance. Radiat. Phys. Chem. 2013, 89, 57–63. [Google Scholar] [CrossRef]
- Patel, V.F.; Liu, F.; Brown, M.B. Modeling the oral cavity: In vitro and in vivo evaluations of buccal drug delivery systems. J. Control. Release 2012, 161, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, M.; Mizera, M.; Lewandowska, K.; Kozak, M.; Miklaszewski, A.; Cielecka-Piontek, J. Effects of inclusion of cetirizine hydrochloride in β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.S.; Hassan, S.; Hussain, A.; Abbas, N.; Kucuk, I.; Nazari, K.; Ali, R.; Ramzan, S.; Alqahtani, A.; Andriotis, E.G.; et al. Improved transdermal delivery of cetirizine hydrochloride using polymeric microneedles. DARU J. Pharm. Sci. 2019, 27, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Sellimi, S.; Younes, I.; Ben Ayed, H.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Centkowska, K.; Ławrecka, E.; Sznitowska, M. Technology of Orodispersible Polymer Films with Micronized Loratadine—Influence of Different Drug Loadings on Film Properties. Pharmaceutics 2020, 12, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Total Polymer (%) | Samples | SA (w/w %) | HPMC (w/w %) | GLY (w/w %) | CTZ (10 mg) |
|---|---|---|---|---|---|
| 2 | 1 | 1 | 1 | 1 | − |
| 2 | 1 | 1 | 1 | + | |
| 3 | 1 | 1 | 3 | − | |
| 4 | 1 | 1 | 3 | + | |
| 5 | 1 | 1 | 5 | − | |
| 6 | 1 | 1 | 5 | + | |
| 7 | 1.33 | 0.66 | 1 | − | |
| 8 | 1.33 | 0.66 | 1 | + | |
| 9 | 1.33 | 0.66 | 3 | − | |
| 10 | 1.33 | 0.66 | 3 | + | |
| 11 | 1.33 | 0.66 | 5 | − | |
| 12 | 1.33 | 0.66 | 5 | + | |
| 3 | 13 | 1.5 | 1.5 | 1 | − |
| 14 | 1.5 | 1.5 | 1 | + | |
| 15 | 1.5 | 1.5 | 3 | − | |
| 16 | 1.5 | 1.5 | 3 | + | |
| 17 | 1.5 | 1.5 | 5 | − | |
| 18 | 1.5 | 1.5 | 5 | + | |
| 19 | 2 | 1 | 1 | − | |
| 20 | 2 | 1 | 1 | + | |
| 21 | 2 | 1 | 3 | − | |
| 22 | 2 | 1 | 3 | + | |
| 23 | 2 | 1 | 5 | − | |
| 24 | 2 | 1 | 5 | + |
| Total Polymer Concentration (%) | Factors | Low Level | Zero Level | High Level |
|---|---|---|---|---|
| 2% | Concentration of SA (x1) | 1 | - | 1.33 |
| Concentration of GLY (x2) | 1 | 3 | 5 | |
| 3% | Concentration of SA (x1) | 1.5 | - | 2 |
| Concentration of GLY (x2) | 1 | 3 | 5 |
| Samples | Thickness (µm) | Tensile Strength (N) | Mucoadhesion (N) |
|---|---|---|---|
| 1 | 56.25 ± 8.54 | 5.61 ± 0.01 | 16.94 ± 1.20 |
| 2 | 104.75 ± 7.27 | 13.89 ± 0.92 | 8.56 ± 1.52 |
| 3 | 128.00 + 7.7 | 6.07 ± 1.07 | 15.70 ± 1.32 |
| 4 | 142.32 ± 8.52 | 9.90 ± 1.19 | 11.94 ± 1.31 |
| 5 | 159.14 ± 6.32 | 5.50 ± 1.95 | 7.13 ± 2.04 |
| 6 | 174.43 ± 6.02 | 6.33 ± 0.75 | 6.38 ± 1.59 |
| 7 | 81.50 ± 6.66 | 18.15 ± 1.45 | 16.02 ± 2.20 |
| 8 | 86.74 ± 6.34 | 27.37 ± 0.28 | 11.57 ± 1.45 |
| 9 | 105.31 ± 8.18 | 12.85 ± 2.37 | 8.54 ± 1.10 |
| 10 | 146.00 ± 6.06 | 14.56 ± 2.18 | 6.63 ± 1.25 |
| 11 | 166.52 ± 3.87 | 9.10 ± 1.91 | 7.97 ± 2.23 |
| 12 | 179.19 ± 4.99 | 16.92 ± 1.46 | 5.37 ± 1.64 |
| 13 | 93.53 ± 6.35 | 8.53 ± 0.57 | 22.12 ± 2.11 |
| 14 | 125.29 ± 5.56 | 28.06 ± 3.76 | 9.66 ± 0.70 |
| 15 | 146.22 ± 5.91 | 11.39 ± 0.91 | 17.59 ± 0.63 |
| 16 | 164.79 ± 5.88 | 26.47 ± 1.42 | 9.07 ± 1.95 |
| 17 | 188.03 ± 2.71 | 4.58 ± 0.37 | 15.64 ± 1.41 |
| 18 | 200.22 ± 8.14 | 16.28 ± 2.92 | 5.83 ± 2.11 |
| 19 | 87.23 ± 1.26 | 17.96 ± 1.52 | 17.32 ± 2.74 |
| 20 | 102.00 ± 2.71 | 32.68 ± 3.03 | 10.82 ± 1.99 |
| 21 | 145.12 ± 3.46 | 23.47 ± 2.49 | 16.87 ± 1.51 |
| 22 | 169.20 ± 2.49 | 27.68 ± 3.65 | 10,7 ± 2.33 |
| 23 | 212.55 ± 9.98 | 16.96 ± 1.95 | 11.95 ± 0.30 |
| 24 | 246.65 ± 5.51 | 20.75 ± 0.22 | 8.56 ± 1.87 |
| Samples | γtot (mN/m) | γd (mN/m) | γp (mN/m) | Polarity (%) |
|---|---|---|---|---|
| 1 | 49.59 | 32.54 | 17.05 | 34.38 |
| 2 | 74.88 | 38.85 | 36.03 | 48.12 |
| 3 | 51.65 | 36.34 | 15.31 | 29.64 |
| 4 | 76.33 | 37.44 | 38.89 | 50.95 |
| 5 | 62.60 | 36.35 | 26.25 | 41.93 |
| 6 | 75.72 | 35.60 | 40.12 | 52.98 |
| 7 | 53.56 | 32.28 | 21.29 | 39.75 |
| 8 | 76.86 | 39.17 | 37.69 | 49.04 |
| 9 | 66.21 | 31.85 | 34.36 | 51.90 |
| 10 | 76.89 | 40.57 | 36.32 | 47.24 |
| 11 | 63.23 | 28.15 | 35.08 | 55.48 |
| 12 | 75.98 | 38.26 | 37.73 | 49.66 |
| 13 | 54.32 | 33.12 | 21.20 | 39.03 |
| 14 | 68.61 | 38.80 | 29.87 | 43.54 |
| 15 | 57.36 | 36.21 | 21.15 | 36.87 |
| 16 | 73.78 | 35.14 | 38.65 | 52.39 |
| 17 | 50.84 | 33.59 | 17.25 | 33.93 |
| 18 | 73.72 | 36.07 | 37.65 | 51.07 |
| 19 | 47.99 | 33.56 | 14.43 | 30.07 |
| 20 | 61.05 | 35.11 | 25.93 | 42.47 |
| 21 | 48.72 | 31.61 | 17.11 | 35.12 |
| 22 | 71.73 | 37.12 | 34.61 | 48.25 |
| 23 | 52.68 | 36.01 | 16.67 | 31.64 |
| 24 | 65.12 | 38.40 | 26.72 | 41.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pamlényi, K.; Kristó, K.; Jójárt-Laczkovich, O.; Regdon, G., Jr. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics 2021, 13, 619. https://doi.org/10.3390/pharmaceutics13050619
Pamlényi K, Kristó K, Jójárt-Laczkovich O, Regdon G Jr. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics. 2021; 13(5):619. https://doi.org/10.3390/pharmaceutics13050619
Chicago/Turabian StylePamlényi, Krisztián, Katalin Kristó, Orsolya Jójárt-Laczkovich, and Géza Regdon, Jr. 2021. "Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride" Pharmaceutics 13, no. 5: 619. https://doi.org/10.3390/pharmaceutics13050619
APA StylePamlényi, K., Kristó, K., Jójárt-Laczkovich, O., & Regdon, G., Jr. (2021). Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics, 13(5), 619. https://doi.org/10.3390/pharmaceutics13050619