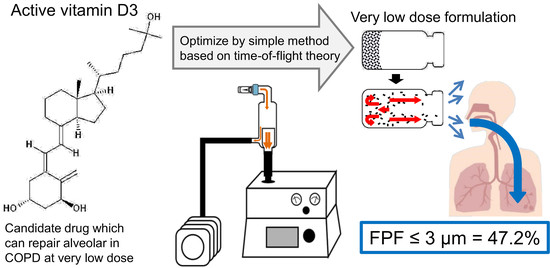
Optimization of Very Low-Dose Formulation of Vitamin D3 with Lyophilizate for Dry Powder Inhalation System by Simple Method Based on Time-of-Flight Theory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Freeze-Dried Cake for Dry Powder Inhalation of VD3
2.3. Visual Evaluation of the Cake Appearance
2.4. Measurement of Aerodynamic Particle Size Distribution
2.4.1. TOF Measurement
2.4.2. In Vitro Inhalation Characteristic Test by MSLI
2.5. Scanning Electron Microscopy
2.6. X-ray Diffractometry (XRD)
3. Results
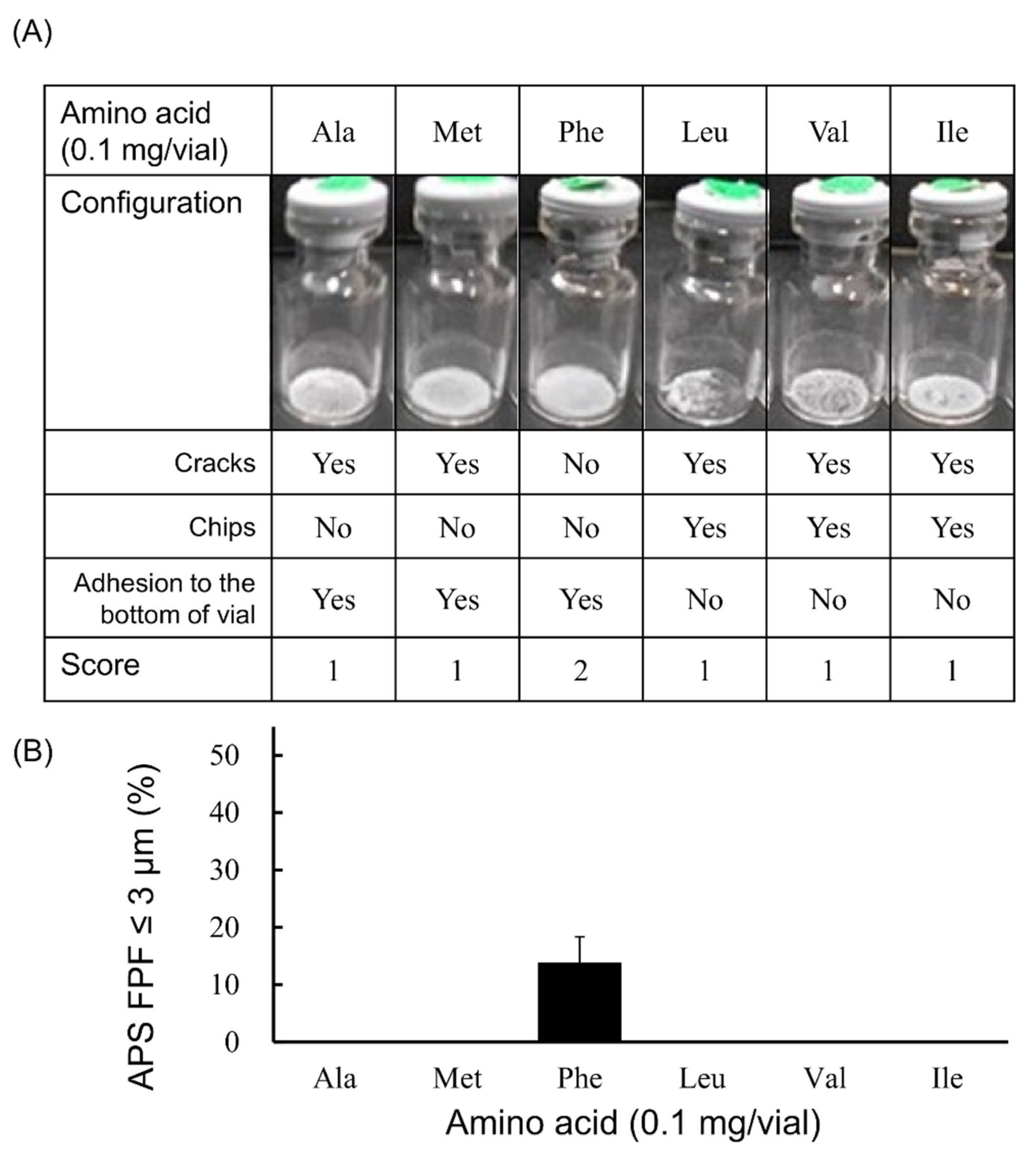
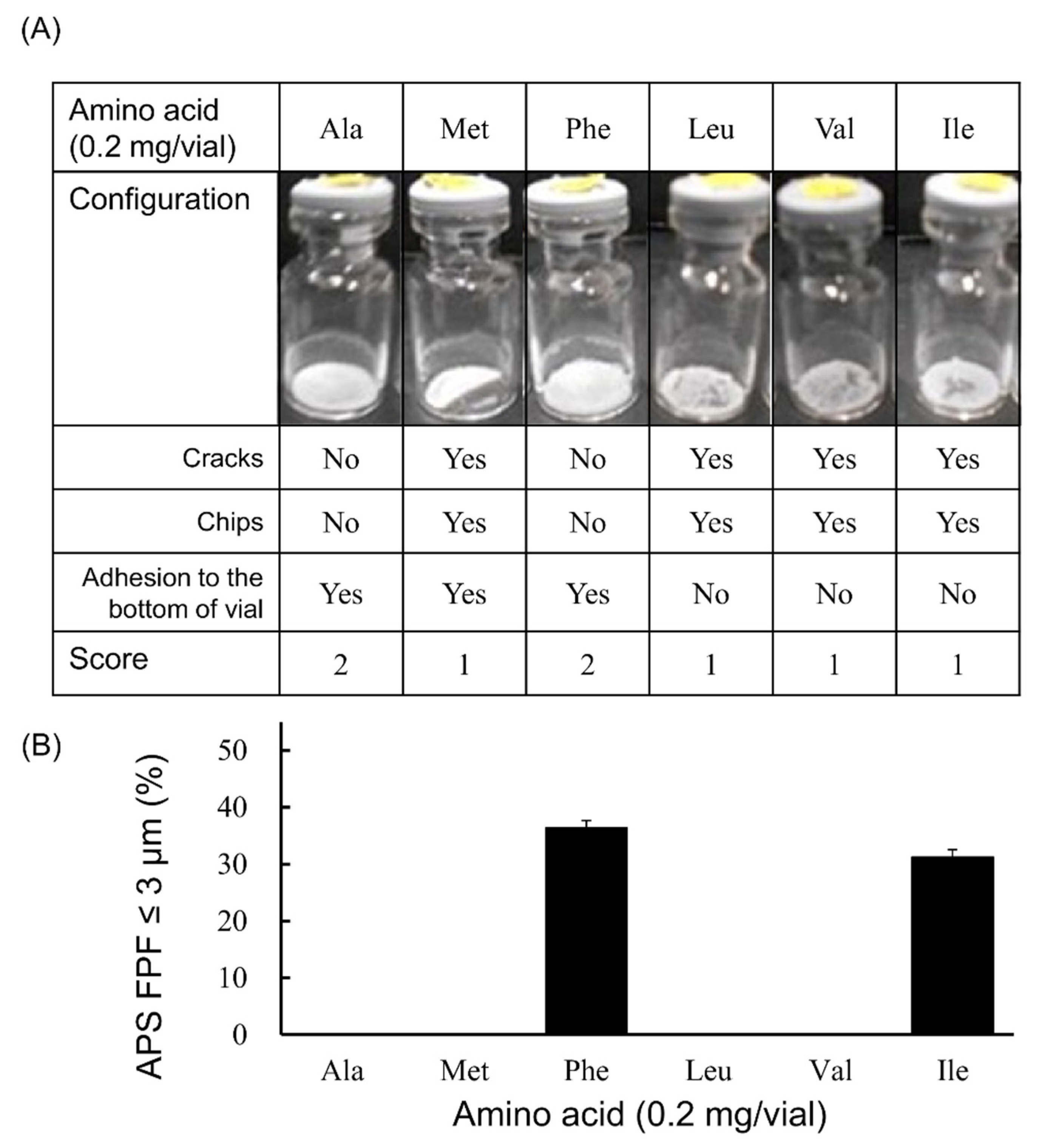
3.1. Selection of Excipients for LDPI Formulations
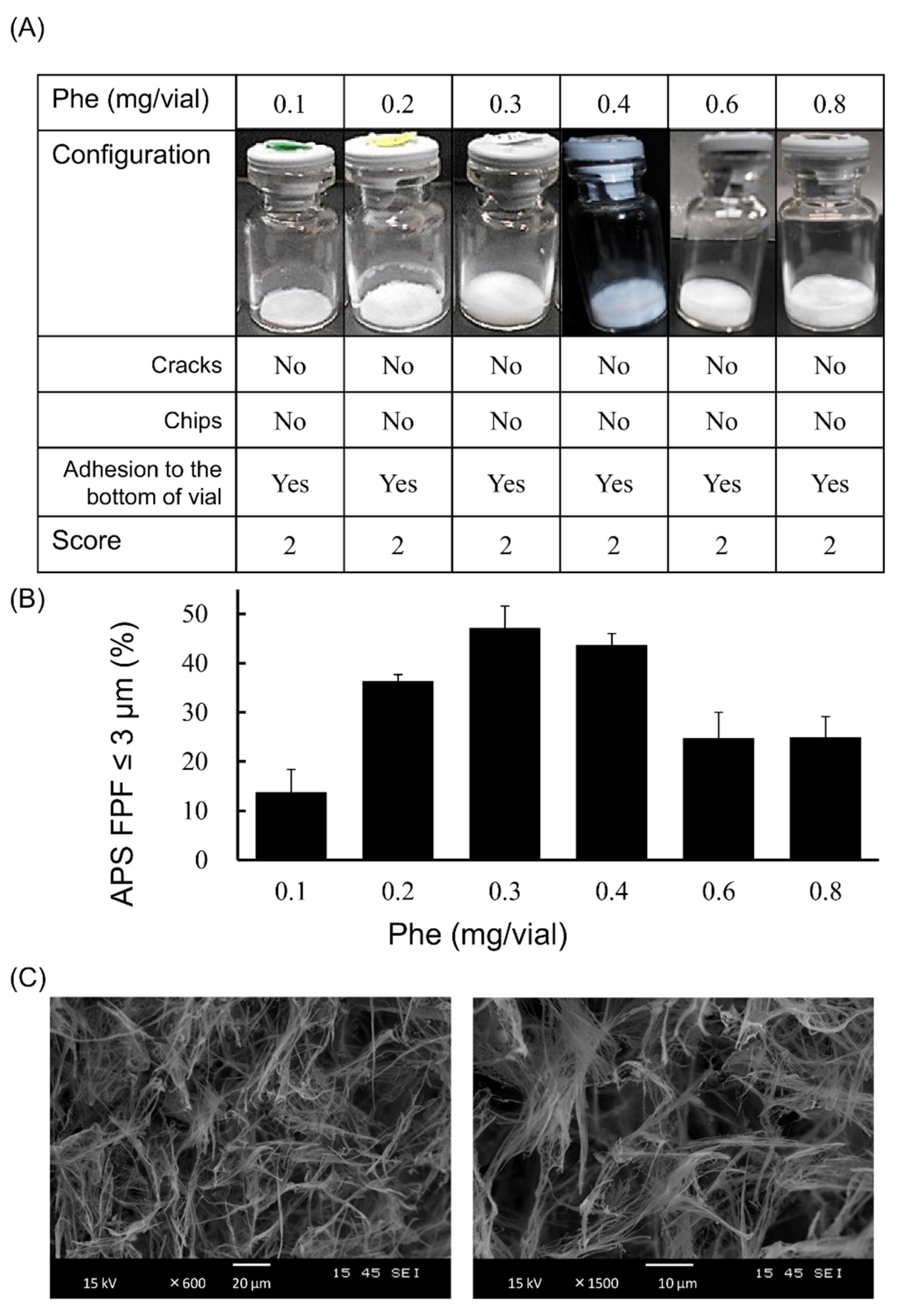
3.2. Optimization of Phe Amount in LDPI Formulation
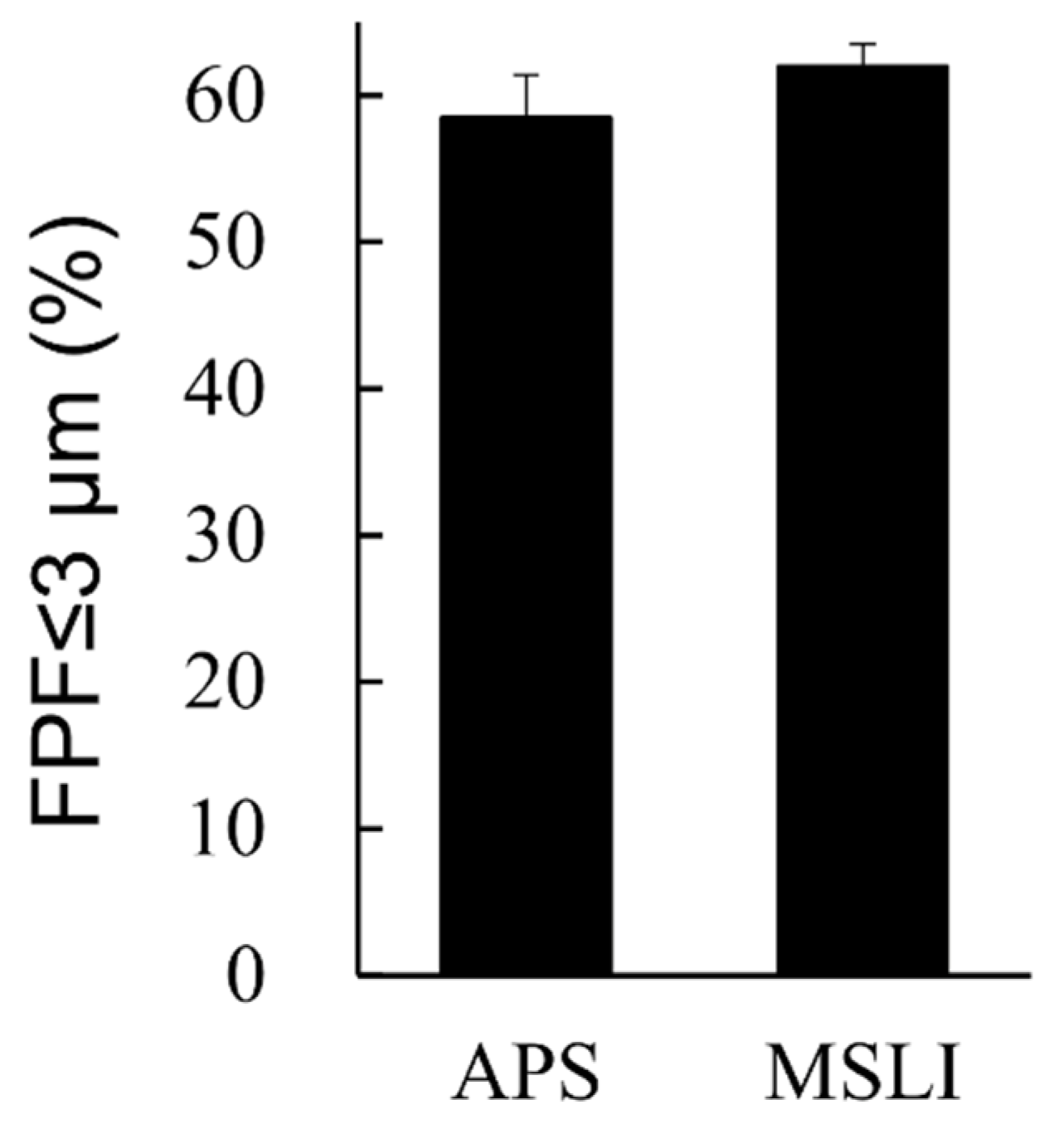
3.3. In Vitro Inhalation Characteristics of VD3 Formulations with LDPI System by MSLI
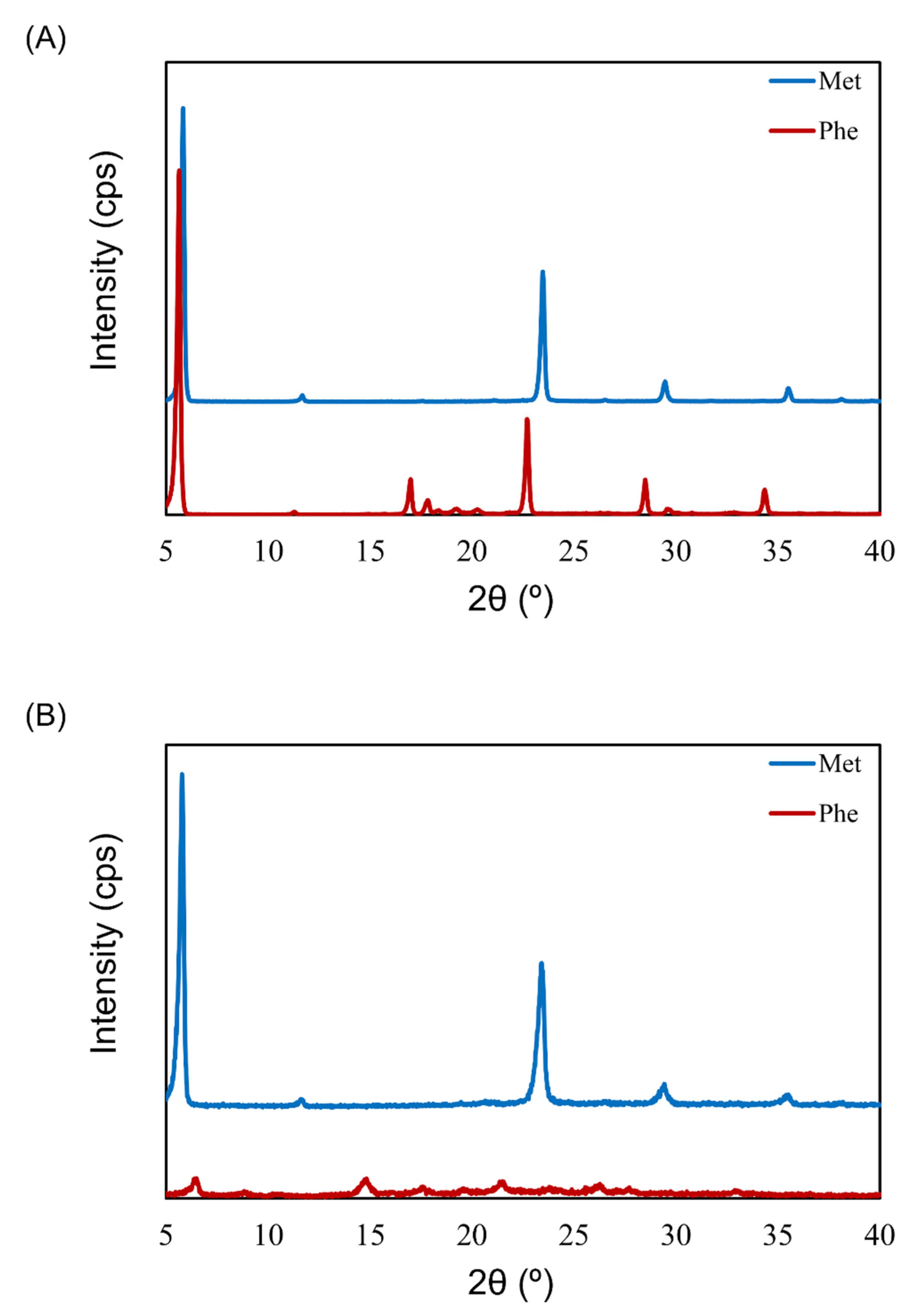
3.4. Crystallinity of Excipients in LDPI Formulations by XRD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [Green Version]
- Ellingsen, J.; Johansson, G.; Larsson, K.; Lisspers, K.; Malinovschi, A.; Ställberg, B.; Thuresson, M.; Janson, C. Impact of Comorbidities and Commonly Used Drugs on Mortality in COPD—Real-World Data from a Primary Care Setting. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Akita, T.; Hirokawa, M.; Yamashita, C. The effects of 1α,25-dihydroxyvitamin D3 on alveolar repair and bone mass in adiponectin-deficient mice. J. Steroid Biochem. Mol. Biol. 2020, 201, 105696. [Google Scholar] [CrossRef]
- Horiguchi, M.; Hirokawa, M.; Abe, K.; Kumagai, H.; Yamashita, C. Pulmonary administration of 1,25-dihydroxyvitamin D3 to the lungs induces alveolar regeneration in a mouse model of chronic obstructive pulmonary disease. J. Control. Release 2016, 233, 191–197. [Google Scholar] [CrossRef]
- Lavorini, F.; Corrigan, C.J.; Barnes, P.J.; Dekhuijzen, P.R.N.; Levy, M.L.; Pedersen, S.; Roche, N.; Vincken, W.; Crompton, G.K. Retail sales of inhalation devices in European countries: So much for a global policy. Respir. Med. 2011, 105, 1099–1103. [Google Scholar] [CrossRef] [Green Version]
- Sorino, C.; Negri, S.; Spanevello, A.; Visca, D.; Scichilone, N. Inhalation therapy devices for the treatment of obstructive lung diseases: The history of inhalers towards the ideal inhaler. Eur. J. Intern. Med. 2020, 75, 15–18. [Google Scholar] [CrossRef] [PubMed]
- De Boer, A.H.; Gjaltema, D.; Hagedoorn, P.; Frijlink, H.W. Characterization of inhalation aerosols: A critical evaluation of cascade impactor analysis and laser diffraction technique. Int. J. Pharm. 2002, 249, 219–231. [Google Scholar] [CrossRef]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Scheuch, G.; Siekmeier, R. Novel approaches to enhance pulmonary delivery of proteins and peptides. J. Physiol. Pharmacol. 2007, 58 (Suppl. S5), 615–625. [Google Scholar]
- Feeley, J.C.; York, P.; Sumby, B.S.; Dicks, H. Determination of surface properties and flow characteristics of salbutamol sulphate, before and after micronisation. Int. J. Pharm. 1998, 172, 89–96. [Google Scholar] [CrossRef]
- Telko, M.J.; Hickey, A.J. Dry powder inhaler formulation. Respir. Care 2005, 50, 1209–1227. [Google Scholar] [PubMed]
- Yamashita, C.; Ibaragi, S.; Fukunaga, Y.; Akagi, A. Composition, Vessel, Dry Powder Inhalation System, and Related Methods for Transpulmonary Administration. U.S. Patent No 7,448,379, 11 November 2008. [Google Scholar]
- Yamashita, C.; Fukunaga, Y.; Akagi, A. Dry Powder Inhalation System for Transpulmonary Administration. U.S. Patent No 7,735,485, 15 June 2010. [Google Scholar]
- Yamashita, C.; Matsushita, H.; Ibaragi, S.; Akagi, A. Inhalation Device for Transpulmonary Administration. U.S. Patent No 7,708,014, 4 May 2010. [Google Scholar]
- Weers, J.G.; Miller, D.P. Formulation Design of Dry Powders for Inhalation. J. Pharm. Sci. 2015, 104, 3259–3288. [Google Scholar] [CrossRef]
- Jehangir, M.; Ahmed, M.; Shafiq, M.I.; Samad, A. Iftikhar-Ul-Haq UHPLC-PDA Assay for Simultaneous Determination of Vitamin D3 and Menaquinone-7 in Pharmaceutical Solid Dosage Formulation. J. Anal. Methods Chem. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, E.M.; Moorkoth, S.; Rane, P.D.; Lewis, L.; Rao, P. Cost-effective HPLC-UV method for quantification of Vitamin D2 and D3 in dried blood spot: A potential adjunct to newborn screening for prophylaxis of intractable paediatric seizures. Chem. Pharm. Bull. 2019, 67, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Oberson, J.M.; Bénet, S.; Redeuil, K.; Campos-Giménez, E. Quantitative analysis of vitamin D and its main metabolites in human milk by supercritical fluid chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Shekunov, B.Y.; Chattopadhyay, P.; Tong, H.H.Y.; Chow, A.H.L. Particle Size Analysis in Pharmaceutics: Principles, Methods and Applications. Pharm. Res. 2007, 24, 203–227. [Google Scholar] [CrossRef]
- Mitchell, J.; Nagel, M. Particle Size Analysis of Aerosols from Medicinal Inhalers. KONA Powder Part. J. 2004, 22, 32–65. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, K.; Taga, H.; Akita, T.; Yamashita, C. Simple Method to Measure the Aerodynamic Size Distribution of Porous Particles Generated on Lyophilizate for Dry Powder Inhalation. Pharmaceutics 2020, 12, 976. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estimating-maximum-safe-starting-dose-initial-clinical-trials-therapeutics-adult-healthy-volunteers (accessed on 19 March 2021).
- Patel, S.M.; Nail, S.L.; Pikal, M.J.; Geidobler, R.; Winter, G.; Hawe, A.; Davagnino, J.; Rambhatla Gupta, S. Lyophilized Drug Product Cake Appearance: What Is Acceptable? J. Pharm. Sci. 2017, 106, 1706–1721. [Google Scholar] [CrossRef]
- Edwards, D.A.; Ben-jebria, A.; Langer, R. Recent advances in pulmonary drug delivery using large, porous inhaled particles. J. Appl. Physiol. 1998, 85, 379–385. [Google Scholar] [CrossRef]
- Claus, S.; Schoenbrodt, T.; Weiler, C.; Friess, W. Novel dry powder inhalation system based on dispersion of lyophilisates. Eur. J. Pharm. Sci. 2011, 43, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Claus, S.; Weiler, C.; Schiewe, J.; Friess, W. Optimization of the fine particle fraction of a lyophilized lysozyme formulation for dry powder inhalation. Pharm. Res. 2013, 30, 1698–1713. [Google Scholar] [CrossRef] [PubMed]
- Ohori, R.; Kiuchi, S.; Sugiyama, S.; Miyamoto, K.; Akita, T.; Yamashita, C. Efficient optimization of high-dose formulation of novel lyophilizates for dry powder inhalation by the combination of response surface methodology and time-of-flight measurement. Int. J. Pharm. 2020, 581, 119255. [Google Scholar] [CrossRef]
- Kaialy, W. A review of factors affecting electrostatic charging of pharmaceuticals and adhesive mixtures for inhalation. Int. J. Pharm. 2016, 503, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Mitaku, S.; Hirokawa, T.; Tsuji, T. Amphiphilicity index of polar amino acids as an aid in the characterization of amino acid preference at membrane–water interfaces. Bioinformatics 2002, 18, 608–616. [Google Scholar] [CrossRef] [Green Version]
- Rau, J.L. The inhalation of drugs: Advantages and problems. Respir. Care 2005, 50, 367–382. [Google Scholar]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ishibashi, Y.; Akita, T.; Yamashita, C. Systemic Delivery of hGhrelin Derivative by Lyophilizate for Dry Powder Inhalation System in Monkeys. Pharmaceutics 2021, 13, 233. [Google Scholar] [CrossRef]
- Griffith, E.C.; Vaida, V. Ionization state of L-Phenylalanine at the Air—Water Interface. J. Am. Chem. Soc. 2013, 135. [Google Scholar] [CrossRef] [PubMed]
- Watry, M.R.; Richmond, G.L. Orientation and Conformation of Amino Acids in Monolayers Adsorbed at an Oil/Water Interface As Determined by Vibrational Sum-Frequency Spectroscopy. J. Phys. Chem. B 2002, 106, 12517–12523. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyamoto, K.; Yanagisawa, M.; Taga, H.; Yamaji, H.; Akita, T.; Yamashita, C. Optimization of Very Low-Dose Formulation of Vitamin D3 with Lyophilizate for Dry Powder Inhalation System by Simple Method Based on Time-of-Flight Theory. Pharmaceutics 2021, 13, 632. https://doi.org/10.3390/pharmaceutics13050632
Miyamoto K, Yanagisawa M, Taga H, Yamaji H, Akita T, Yamashita C. Optimization of Very Low-Dose Formulation of Vitamin D3 with Lyophilizate for Dry Powder Inhalation System by Simple Method Based on Time-of-Flight Theory. Pharmaceutics. 2021; 13(5):632. https://doi.org/10.3390/pharmaceutics13050632
Chicago/Turabian StyleMiyamoto, Kahori, Misato Yanagisawa, Hiroaki Taga, Hiromichi Yamaji, Tomomi Akita, and Chikamasa Yamashita. 2021. "Optimization of Very Low-Dose Formulation of Vitamin D3 with Lyophilizate for Dry Powder Inhalation System by Simple Method Based on Time-of-Flight Theory" Pharmaceutics 13, no. 5: 632. https://doi.org/10.3390/pharmaceutics13050632
APA StyleMiyamoto, K., Yanagisawa, M., Taga, H., Yamaji, H., Akita, T., & Yamashita, C. (2021). Optimization of Very Low-Dose Formulation of Vitamin D3 with Lyophilizate for Dry Powder Inhalation System by Simple Method Based on Time-of-Flight Theory. Pharmaceutics, 13(5), 632. https://doi.org/10.3390/pharmaceutics13050632