Transdermal Film Loaded with Garlic Oil-Acyclovir Nanoemulsion to Overcome Barriers for Its Use in Alleviating Cold Sore Conditions
Abstract
:1. Introduction
2. Materials and Methods
3. Estimation of Acyclovir (ACV) Solubility in Various SNEDDS Components
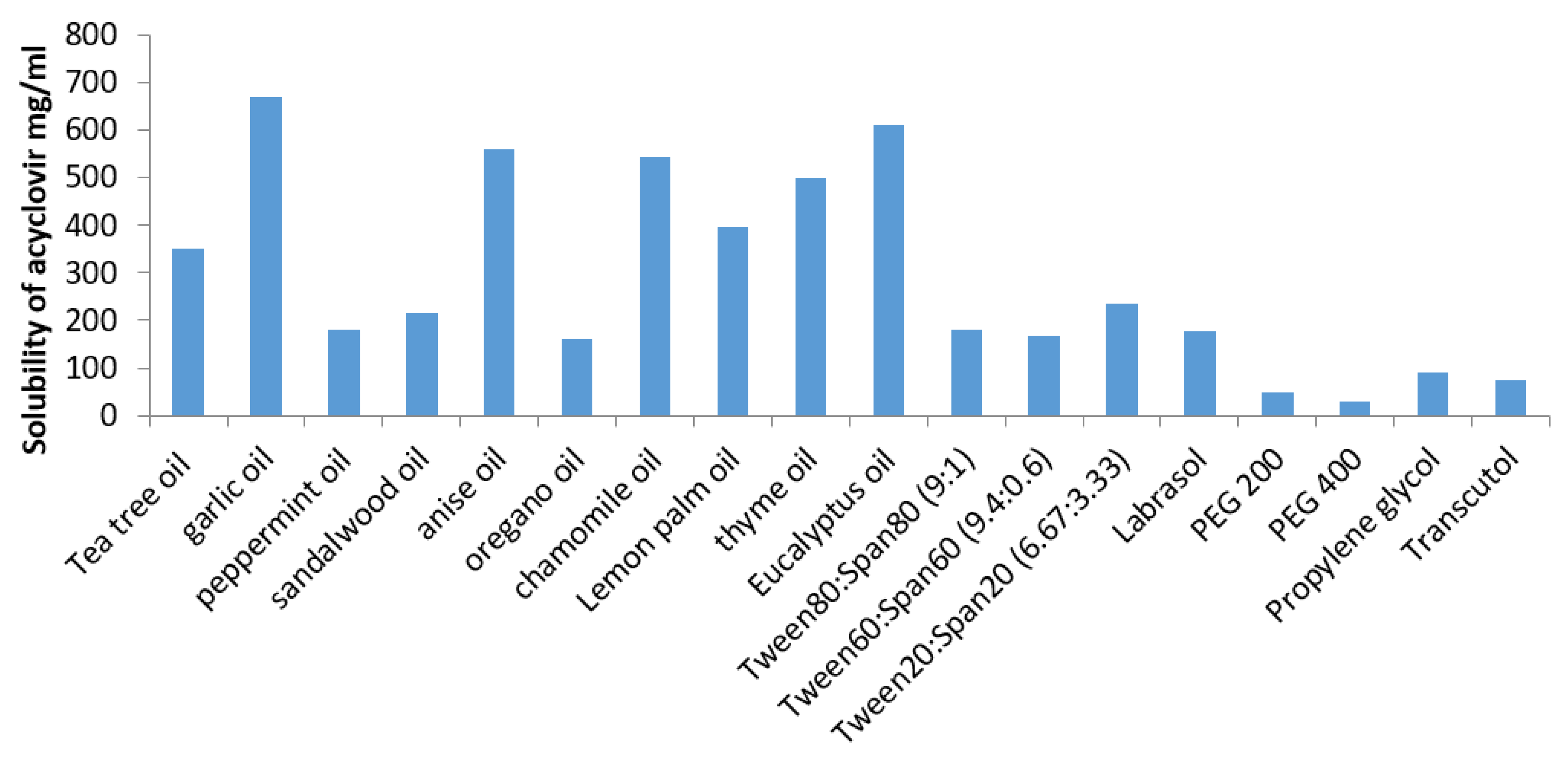
3.1. Screening of Oils
3.2. Screening of Surfactants and Co-Surfactants
3.3. Pseudo Ternary-Phase Diagram for Preparing ACV SNEDDS
3.4. Optimization of ACV-GO SNEDDS
3.5. Characterization of ACV-GO SNEDDS
3.5.1. Emulsification Ability
3.5.2. Determination of Globule Size of the ACV-GO SNEDDs
3.5.3. Evaluation of Stability of the Optimized ACV-GO SNEDDS
3.5.4. Preparation of ACV-GO SNEDDs Transdermal Films
3.6. Ex Vivo Skin Permeation Study of ACV-GO SNEDDS Transdermal Films
3.7. Pharmacokinetic Evaluation of the Optimized ACV-GO SNEDDs Transdermal Film
3.7.1. Animals
3.7.2. In Vivo Investigation of the Optimized ACV-GO SNEDDs Transdermal Film
3.8. Statistical Analysis
4. Results and Discussion
4.1. Estimation of Acyclovir (ACV) Solubility in Various SNEDDS Components
4.2. Pseudo Ternary-Phase Diagram
4.3. Optimization of ACV-GO SNEDDS
4.4. Effect of Formulation Variables on Particle Size
4.5. Emulsification Ability of ACV-GO SNEDDS
4.6. Evaluation of Stability of Optimized ACV-GO SNEDDS
4.7. Ex Vivo Skin Permeation Study of ACV-GO SNEDDS Transdermal Films
4.8. In Vivo Investigation of the Optimized ACV-GO SNEDDs Transdermal Film
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Looker, K.J.; Johnston, C.; Welton, N.J.; James, C.; Vickerman, P.; Turner, K.; Boily, M.C.; Gottlieb, S.L. The global and regional burden of genital ulcer disease due to herpes simplex virus: A natural history modelling study. BMJ Glob. Health 2020, 5, e001875. [Google Scholar] [CrossRef] [Green Version]
- Looker, K.J.; Welton, N.J.; Sabin, K.M.; Dalal, S.; Vickerman, P.; Turner, K.M.E.; Boily, M.C.; Gottlieb, S.L. Global and regional estimates of the contribution of herpes simplex virus type 2 infection to HIV incidence: A population attributable fraction analysis using published epidemiological data. Lancet Infect. Dis. 2020, 20, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Arduino, P.G.; Porter, S.R. Oral and perioral herpes simplex virus type 1 (HSV-1) infection: Review of its management. Oral Dis. 2006, 12, 254–270. [Google Scholar] [CrossRef]
- Lotufo, M.A.; Tempestini Horliana, A.C.R.; Santana, T.; de Queiroz, A.C.; Gomes, A.O.; Motta, L.J.; Ferrari, R.A.M.; Dos Santos Fernandes, K.P.; Bussadori, S.K. Efficacy of photodynamic therapy on the treatment of herpes labialis: A systematic review. Photodiagn. Photodyn. Ther. 2020, 29, 101536. [Google Scholar] [CrossRef]
- Zupin, L.; Caracciolo, I.; Tricarico, P.M.; Ottaviani, G.; D’Agaro, P.; Crovella, S. Antiviral properties of blue laser in an in vitro model of HSV-1 infection. Microbiol. Immunol. 2018, 62, 477–479. [Google Scholar] [CrossRef] [Green Version]
- Attia, I.A.; El-Gizawy, S.A.; Fouda, M.A.; Donia, A.M. Influence of a niosomal formulation on the oral bioavailability of acyclovir in rabbits. AAPS Pharm. Sci. Tech. 2007, 8, E106. [Google Scholar] [CrossRef] [Green Version]
- Erlich, K.S. Management of herpes simplex and varicella-zoster virus infections. West. J. Med. 1997, 166, 211–215. [Google Scholar]
- Miranda, P.; Krasny, H.C.; Page, D.A.; Elion, C.B. Species differences in the disposition of acyclovir. Am. J. Med. 1982, 73, 31Y35. [Google Scholar] [CrossRef]
- de Miranda, P.; Blum, M.R. Pharmacokinetics of acyclovir after ntravenous and oral administration. J. Antimicrob. Chemother. 1983, 12, 29–37. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of n vitrodrug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Luengo, J.; Aranguiz, T.; Sepulveda, J. Preliminary pharmacokinetic study to different preparations of acyclovir with β-cyclodextrin. J. Pharm. Sci. 2002, 91, 2593–2598. [Google Scholar] [CrossRef]
- Krenitsky, T.A.; Hall, W.W.; de Miranda, P.; Bauchamp, L.M.; Schaeffer, H.J.; Whiteman, P. 6-Deoxyacyclovir: A xanthine oxidase-activated prodrug of acyclovir. Proc. Natl. Acad. Sci. USA 1984, 81, 3209–3213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubbinga, M.; Nguyen, M.A.; Staubach, P.; Teerenstra, S.; Langguth, L. The influence of chitosan on the oral bioavailability of acyclovir—a comparative bioavailability study in humans. Pharm. Res. 2015, 32, 2241–2249. [Google Scholar]
- Zhang, X.; Li, Y.; Zhou, H.; Fan, S.; Zhang, Z.; Wang, L.; Zhang, Y. Plasma metabolic profiling analysis of nephrotoxicity induced by acyclovir using metabonomics coupled with multivariate data analysis. J. Pharm. Biomed. Anal. C 2014, 97, 151–156. [Google Scholar] [CrossRef]
- Lu, H.; Han, Y.J.; Xu, J.D.; Xing, W.M.; Chen, J. Proteomic characterization of acyclovir-induced nephrotoxicity in a mouse model. PLoS ONE 2014, 9, e103185. [Google Scholar] [CrossRef] [Green Version]
- Kamel, A.O.; Awad, G.A.S.; Geneidi, A.S.; Mortada, N.D. Preparation of intravenous stealthy acyclovir nanoparticles with increased mean residence time. AAPS Pharm. Sci. Tech. 2009, 10, 1427–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B. Quantification of uptake and clearance of acyclovir in skin layers. Antivir. Ther. 2015, 21, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Glaxo Operations UK. ZOVIRAX Labialis, 50 mg/g, Crème Aciclovir (Package Insert); Glaxo Operations: Brentford, UK, 2012. [Google Scholar]
- U.S. Food and Drug Administration. ZOVIRAX (Acyclovir) Cream 5% for Topical Use—Reference ID: 3481551; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2014.
- Ahmed, O.A.A.; Afouna, M.I.; El-Say, K.M.; Abdel-Naim, A.B.; Khedr, A.; Banjar, Z.M. Optimization of self-nanoemulsifying systems for the enhancement of in vivo hypoglycemic efficacy of glimepiride transdermal patches. Expert Opin. Drug Deliv. 2014, 11, 1005–1013. [Google Scholar]
- Date, A.A.; Nagarsenker, M.S. Design and evaluation of selfnanoemulsifying drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int. J. Pharm. 2007, 329, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Vanani, R.; Azarpira, N.; Heli, H.; Karimian, K.; Sattarahmady, N. A novel self-nanoemulsifying formulation for sunitinib: Evaluation of anticancer efficacy. Colloids Surf. B Biointerfaces 2017, 160, 65–72. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Kaz, M.; Hadi Albgomi, M.; Ahad, A.; Raish, M. Development and optimization of self-nanoemulsifying drug delivery systems (SNEDDS) for curcumin transdermal delivery: An anti-inflammatory exposure. Drug Dev. Ind. Pharm. 2019, 45, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.M.; Taha, E.I.; Tayel, M.M.; Al-Suwayeh, S. A Ultra-fine self nanoemulsifying drug delivery system for transdermal delivery of meloxicam: Dependency on the type of surfactants. J. Mol. Liq. 2014, 190, 16–22. [Google Scholar] [CrossRef]
- Cho, C.W.; Choi, J.S.; Yang, K.H.; Shin, S.C. Enhanced transdermal controlled delivery of glimepiride from the ethylene-vinyl acetate matrix. Drug Deliv. 2009, 16, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.D.; Shinde, P.; Girkar, K.; Madage, R.; Chowdhary, A. Assessment of anti-influenza activity and hemagglutination inhibition of Plumbago indica and Allium sativum extracts. Pharmacogn. Res. 2016, 8, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Chou, T.W.; Cheng, L.H.; Ho, C.W. In-vitro anti-adenoviral activity of five Allium plants. J. Taiwan Inst. Chem. Eng. 2011, 42, 228–232. [Google Scholar] [CrossRef]
- Choi, H.J. Chemical constituents of essential oils possessing anti-influenza A/ WS/33 virus activity. Osong Public Health Res. Perspect. 2018, 9, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.D.; Andersen, D.O.; North, J.A.; Murray, B.K.; Lawson, L.D.; Hughes, B.G. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992, 58, 417–423. [Google Scholar] [CrossRef]
- Rouf, R.; Uddin, S.J.; Sarker, D.K.; Islam, M.T.; Ali, E.S.; Shilpi, J.A.; Sarker, S.D. Anti-viral potential of garlic (Allium sativum) and it’s organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 2020, 104, 219–234. [Google Scholar] [CrossRef]
- AlHakamy, N.; Fahmy, U.A.; Ahmed, O.A.A.; Almohammadi, E.A.; Alotaibi, S.A.; Aljohani, R.A.; Alfaifi, M.Y. Development of an optimized Febuxostat self-nanoemulsified loaded transdermal film: In-vitro, ex-vivo and in-vivo evaluation. Pharm. Dev. Technol. 2019, 25, 326–331. [Google Scholar] [CrossRef]
- Basalious, E.B.; Shawky, N.; Badr-Eldin, S.M. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: Development and optimization. Int. J. Pharm. 2010, 391, 203–211. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; El-Massik, M.A.; Abdallah, O.Y. Self-nanoemulsifying drug delivery systems of tamoxifen citrate: Design and optimization. Int. J. Pharm. 2009, 380, 133–141. [Google Scholar] [CrossRef]
- Huang, Y.B.; Tsai, Y.H.; Lee, S.H.; Chang, J.S.; Wu, P.C. Optimization of pH-independent release of nicardipine hydrochloride extended-release matrix tablets using response surface methodology. Int. J. Pharm. 2005, 289, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lia, P.; Ghosha, A.; Wagnera, R.F.; Krillb, S.; Joshia, Y.M.; Serajuddin, A.T.M. Effect of combined use of nonionic surfactant on formation of oil-in-water microemulsions. Int. J. Pharm. 2005, 288, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mustafa Kiyani, M.; Sohail, M.F.; Shahnaz, G.; Rehman, H.; Akhtar, M.F.; Nawaz, I.; Mahmood, T.; Manzoor, M.; Bokhari, I.; Ali, S. Evaluation of Turmeric Nanoparticles as Anti-Gout Agent: Modernization of a Traditional Drug. Medicina 2019, 55, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanna, V.; Gavini, E.; Cossu, M.; Rassu, G.; Giunchedi, P. Solid lipid nanoparticles (SLN) as carriers for the topical delivery of econazole nitrate: In-vitro characterization, ex-vivo and in-vivo studies. J. Pharm. Pharmacol. 2007, 59, 1057–1064. [Google Scholar] [CrossRef]
- Gupta, S.; Chavhan, S.; Sawant, K.K. Self-nanoemulsifying drug delivery system for adefovir dipivoxil: Design, characterization, in vitro and ex vivo evaluation. Colloids Surf. Physicochem. Eng. Asp. 2011, 392, 145–155. [Google Scholar] [CrossRef]
- El-Say, K.M.; Ahmad, T.A.; Badr-Eldin, S.M.; Fahmy, U.; Aldawsari, H.; Ahmed, O.A.A. Enhanced permeation parameters of optimized nanostructured simvastatin transdermal films: Ex vivo and in vivo evaluation. Pharm. Dev. Technol. 2015, 20, 919–926. [Google Scholar] [CrossRef]
- Thacharodi, D.; Panduranga, R.K. Transdermal absorption of nifedipine from microemulsions of lipophilic skin penetration enhancers. Int. J. Pharm. 1994, 111, 235–240. [Google Scholar] [CrossRef]
- Pathan, I.B.; Setty, C.M. Enhancement of transdermal delivery of tamoxifen citrate using nanoemulsion vehicle. Int. J. Pharm. Technol. Res. 2011, 3, 287–297. [Google Scholar]


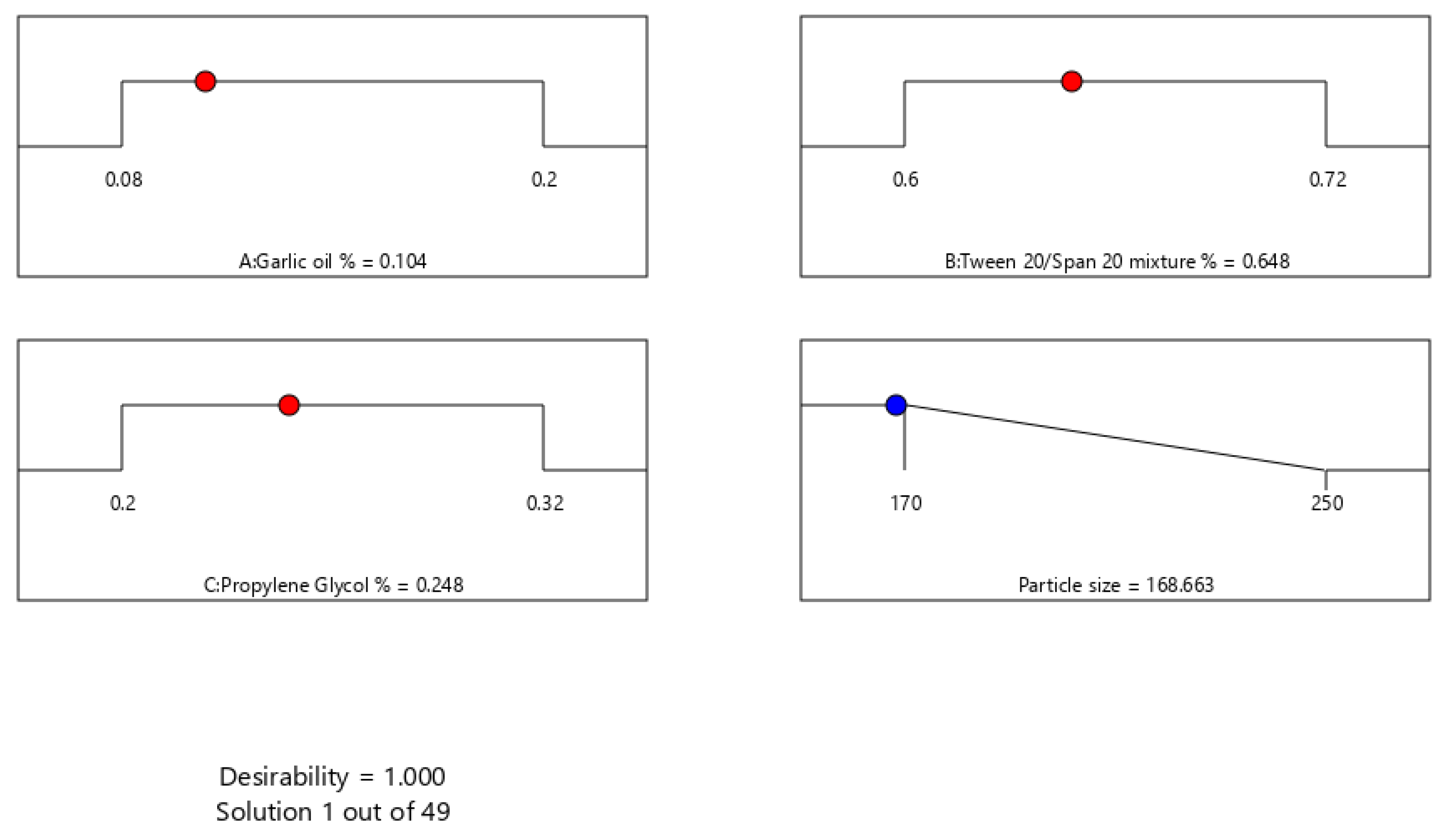

| Formulations | A:Garlic Oil (%w/w) | B:Tween 20/Span 20 Mixture (%w/w) | C:Propylene Glycol % (w/w) | Particle Size nm ± SD |
|---|---|---|---|---|
| 1 | 0.08 | 0.60 | 0.32 | 250 ± 5.54 |
| 2 | 0.08 | 0.66 | 0.26 | 200 ± 11.53 |
| 3 | 0.08 | 0.60 | 0.32 | 248 ± 7.12 |
| 4 | 0.10 | 0.62 | 0.28 | 192 ± 15.79 |
| 5 | 0.08 | 0.72 | 0.20 | 215 ± 12.97 |
| 6 | 0.16 | 0.62 | 0.22 | 200 ± 4.98 |
| 7 | 0.20 | 0.60 | 0.20 | 240 ± 17.43 |
| 8 | 0.20 | 0.60 | 0.20 | 239 ± 6.95 |
| 9 | 0.14 | 0.60 | 0.26 | 180 ± 10.11 |
| 10 | 0.14 | 0.66 | 0.20 | 202 ± 19.38 |
| 11 | 0.14 | 0.66 | 0.20 | 200 ± 18.11 |
| 12 | 0.10 | 0.68 | 0.22 | 177 ± 9.41 |
| 13 | 0.08 | 0.72 | 0.20 | 215 ± 12.09 |
| 14 | 0.12 | 0.64 | 0.24 | 170 ± 11.65 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9095.51 | 8 | 1136.94 | 915.85 | <0.0001 |
| Linear Mixture | 992.28 | 2 | 496.14 | 399.66 | <0.0001 |
| AB | 917.05 | 1 | 917.05 | 738.72 | <0.0001 |
| AC | 3318.03 | 1 | 3318.03 | 2672.82 | <0.0001 |
| BC | 819.49 | 1 | 819.49 | 660.13 | <0.0001 |
| A²BC | 85.02 | 1 | 85.02 | 68.48 | 0.0004 |
| AB²C | 204.01 | 1 | 204.01 | 164.34 | <0.0001 |
| ABC² | 6.86 | 1 | 6.86 | 5.52 | 0.0655 |
| Residual | 6.21 | 5 | 1.24 | - | - |
| Lack of Fit | 1.71 | 1 | 1.71 | 1.52 | 0.2855 |
| Pure Error | 4.5 | 4 | 1.13 | - | - |
| Cor Total | 9101.71 | 13 | - | - | - |
| Parameters of Permeation | F1 | F2 | F3 | Commercial ACV (5%) Cream |
|---|---|---|---|---|
| Cumulative amount permeated (μg/cm2) | 11327 ± 977 | 4811 ± 333 | 8933 ± 741 | 7772 ± 568 |
| Steady state flux, Jss, (μg/cm2/min) | 47.128 ± 6.2 | 17.433 ± 2.4 | 34.167 ± 4.9 | 29.308 ± 3.1 |
| Permeability coefficient, Pc, (cm/min) | (3.9 ± 0.3) × 10−3 | (1.5 ± 0.2) × 10−3 | (2.9 ± 0.4) × 10−3 | (2.1 ± 0.2) × 10−3 |
| Diffusion coefficient, D, (cm2/min) | (12.3 ± 0.9) × 10−3 | (4.6 ± 0.5) × 10−3 | (8.5 ± 0.7) × 10−3 | (5.9 ± 0.6) × 10−3 |
| Relative permeation rate (RPR) | 1.457 ± 0.6 | 0.619 ± 0.3 | 1.149 ± 0.5 | - |
| Enhancement factor (EF) | 2.35 ± 0.4 | - | 1.85 ± 0.3 | 1.61 ± 0.4 |
| PK Parameters | Raw ACV-HPC Film | Optimized ACV-GO SNEDDs Film | Marketed ACV 5% Cream |
|---|---|---|---|
| Cmax (ng/mL) | 305 ± 42 | 993 ± 101 | 410 ± 65 |
| Tmax (min) | 120 ± 30 | 240 ± 30 | 180 ± 30 |
| AUC0–t (ng/mL h) | 4213 ± 509 | 11,234.1 ± 1312.6 | 5718.3 ±811.2 |
| AUC0–inf (ng/mL h) | 4566 ± 619 | 13,711.4 ± 1845.2 | 6218.4 ± 918.6 |
| Kel (h−1) | 0.133 ± 0.041 | 0.086 ± 0.021 | 0.118 ± 0.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almehmady, A.M.; Ali, S.A. Transdermal Film Loaded with Garlic Oil-Acyclovir Nanoemulsion to Overcome Barriers for Its Use in Alleviating Cold Sore Conditions. Pharmaceutics 2021, 13, 669. https://doi.org/10.3390/pharmaceutics13050669
Almehmady AM, Ali SA. Transdermal Film Loaded with Garlic Oil-Acyclovir Nanoemulsion to Overcome Barriers for Its Use in Alleviating Cold Sore Conditions. Pharmaceutics. 2021; 13(5):669. https://doi.org/10.3390/pharmaceutics13050669
Chicago/Turabian StyleAlmehmady, Alshaimaa M., and Sarah A. Ali. 2021. "Transdermal Film Loaded with Garlic Oil-Acyclovir Nanoemulsion to Overcome Barriers for Its Use in Alleviating Cold Sore Conditions" Pharmaceutics 13, no. 5: 669. https://doi.org/10.3390/pharmaceutics13050669
APA StyleAlmehmady, A. M., & Ali, S. A. (2021). Transdermal Film Loaded with Garlic Oil-Acyclovir Nanoemulsion to Overcome Barriers for Its Use in Alleviating Cold Sore Conditions. Pharmaceutics, 13(5), 669. https://doi.org/10.3390/pharmaceutics13050669





