Urea as a Cocrystal Former—Study of 3 Urea Based Pharmaceutical Cocrystals
Abstract
:1. Introduction
2. Materials and Methods
3. Results
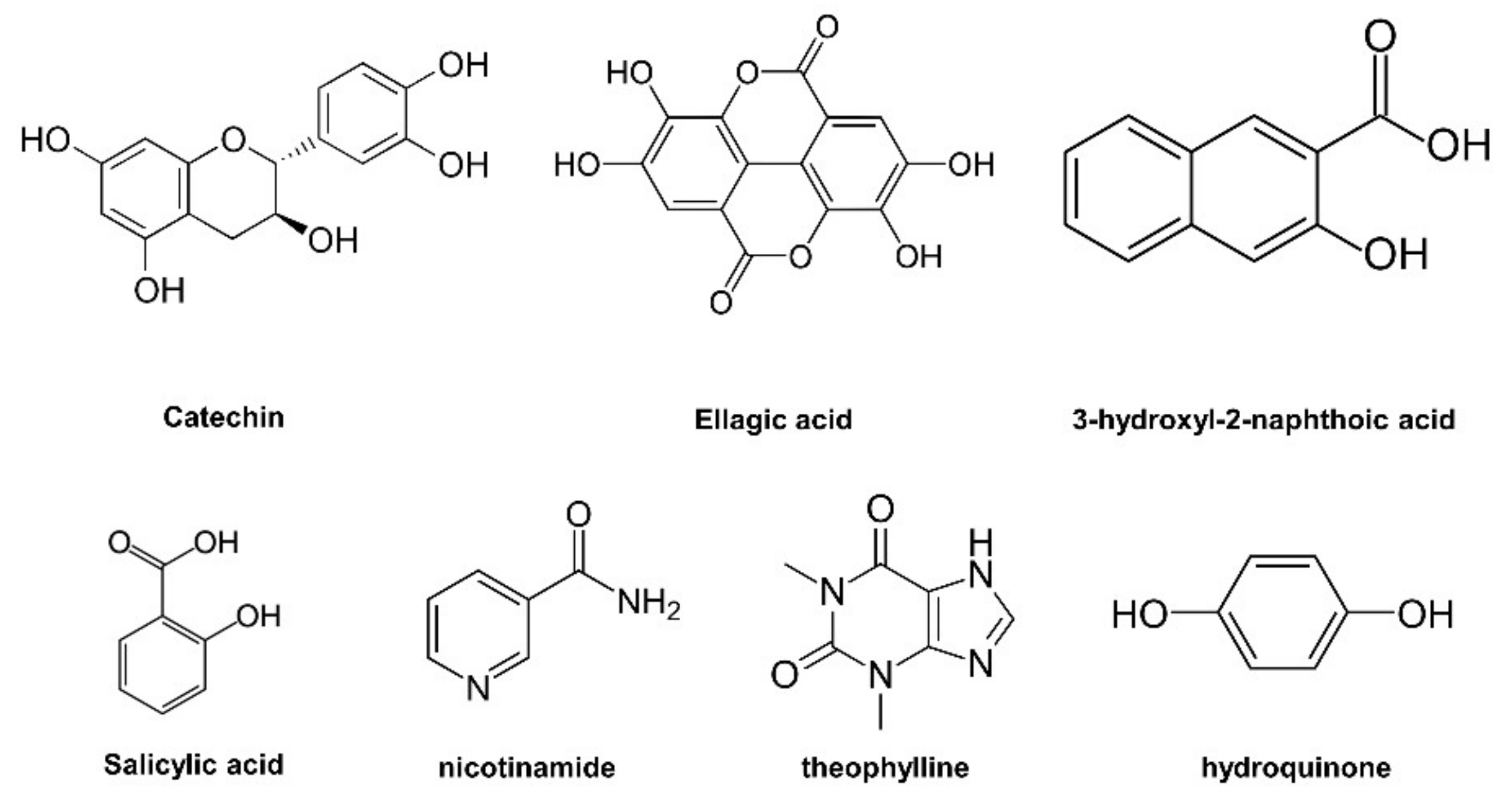
3.1. Cocrystal Screening
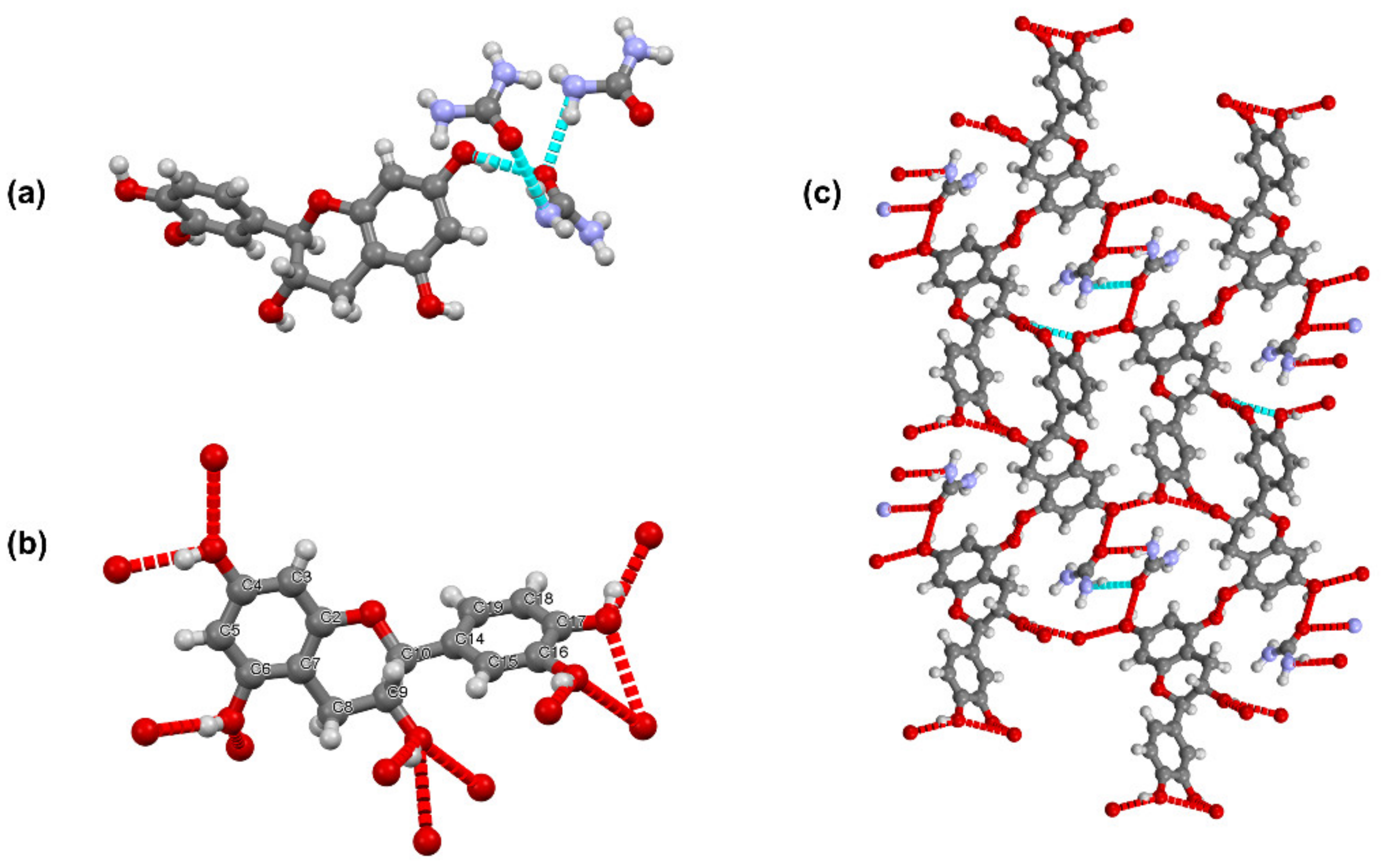
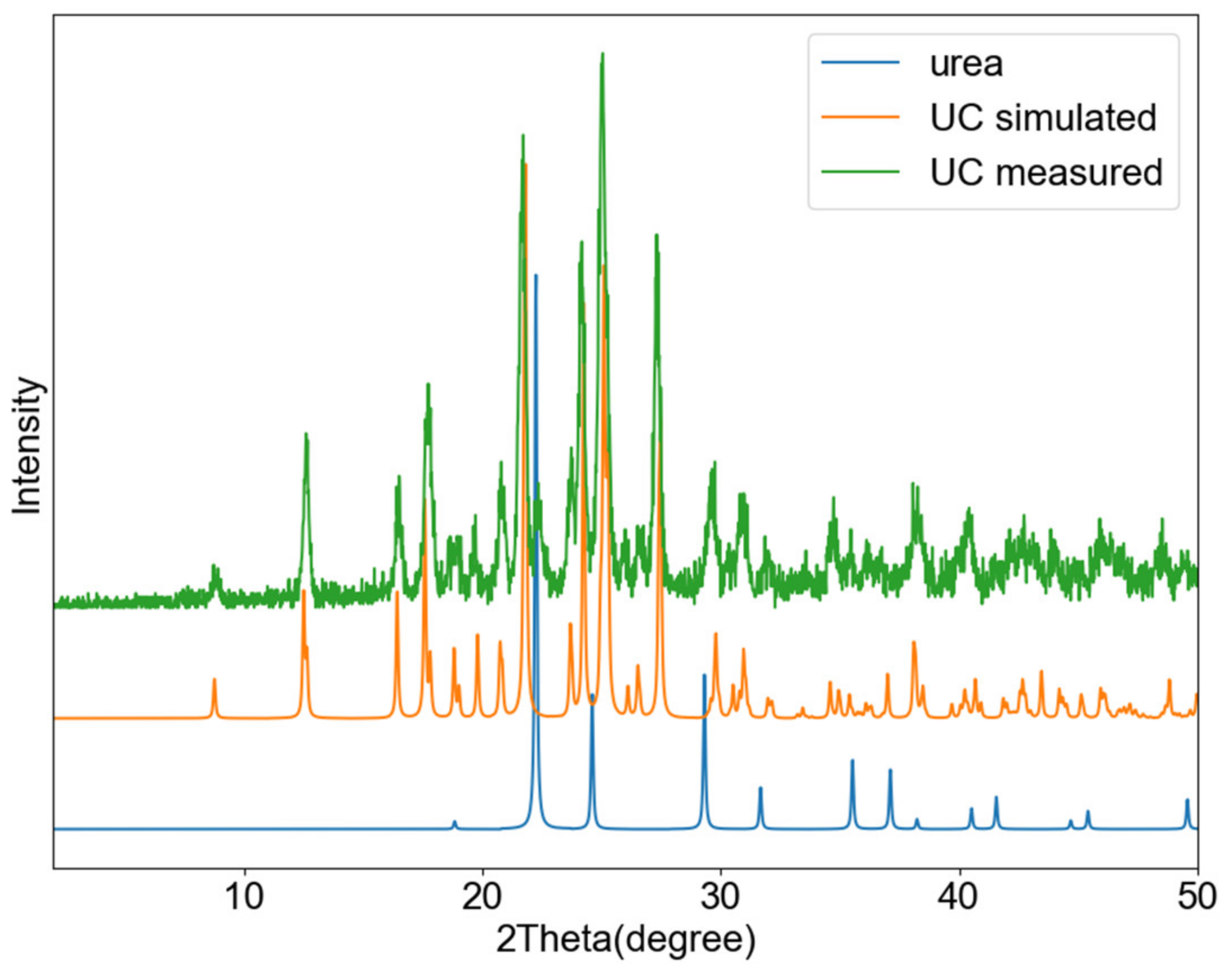
3.2. Urea-Catechin Cocrystal (UC)
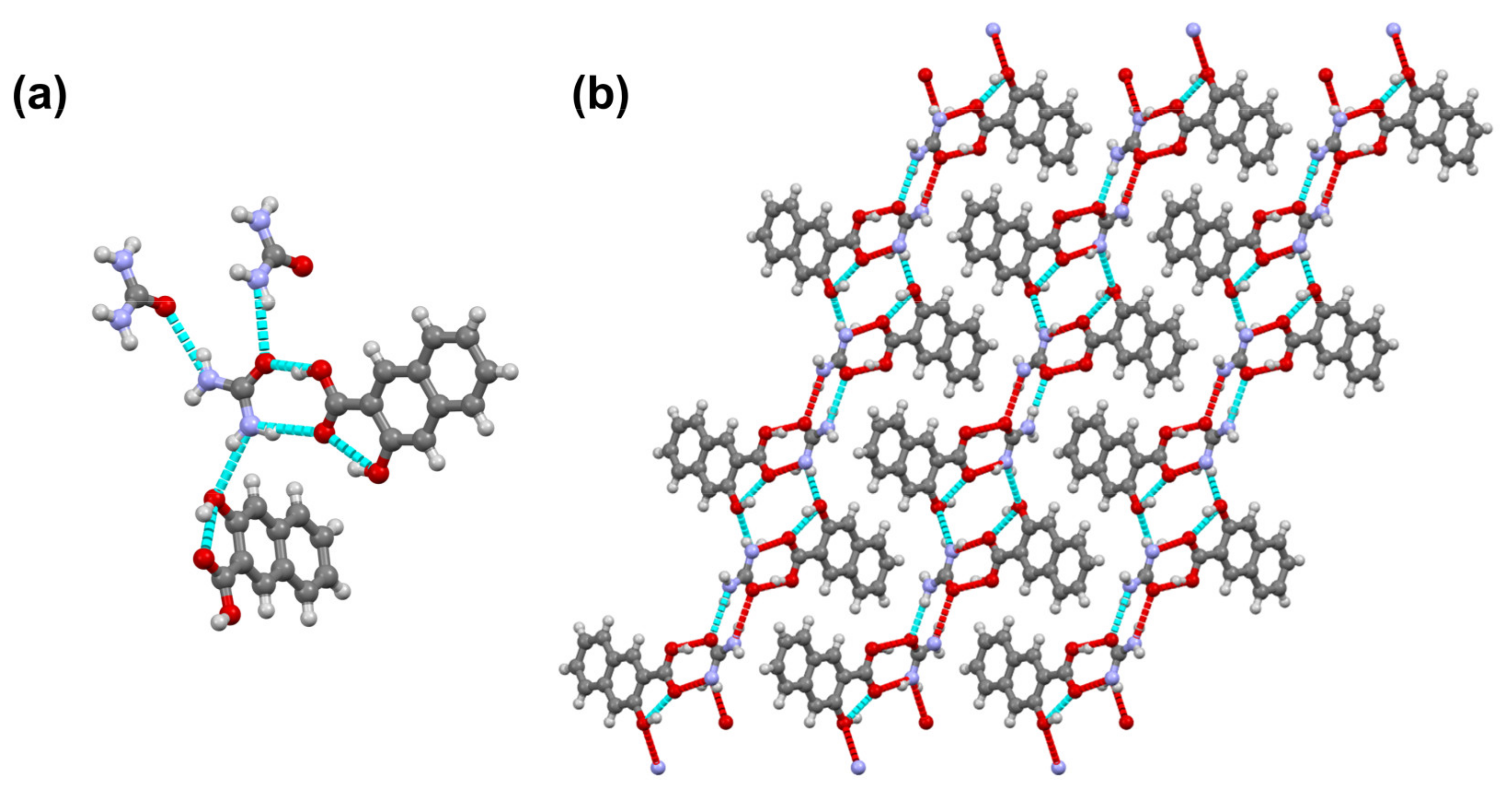
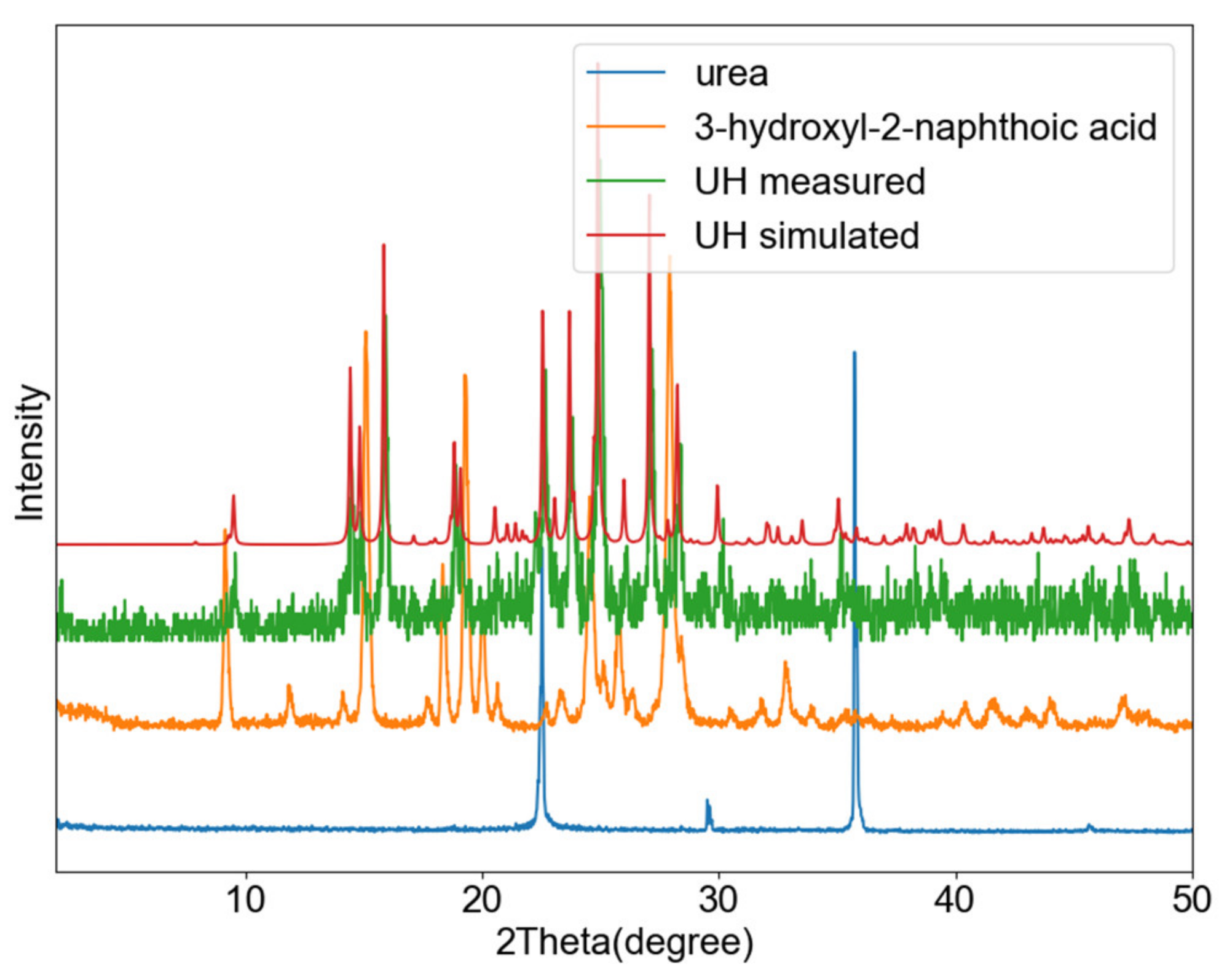
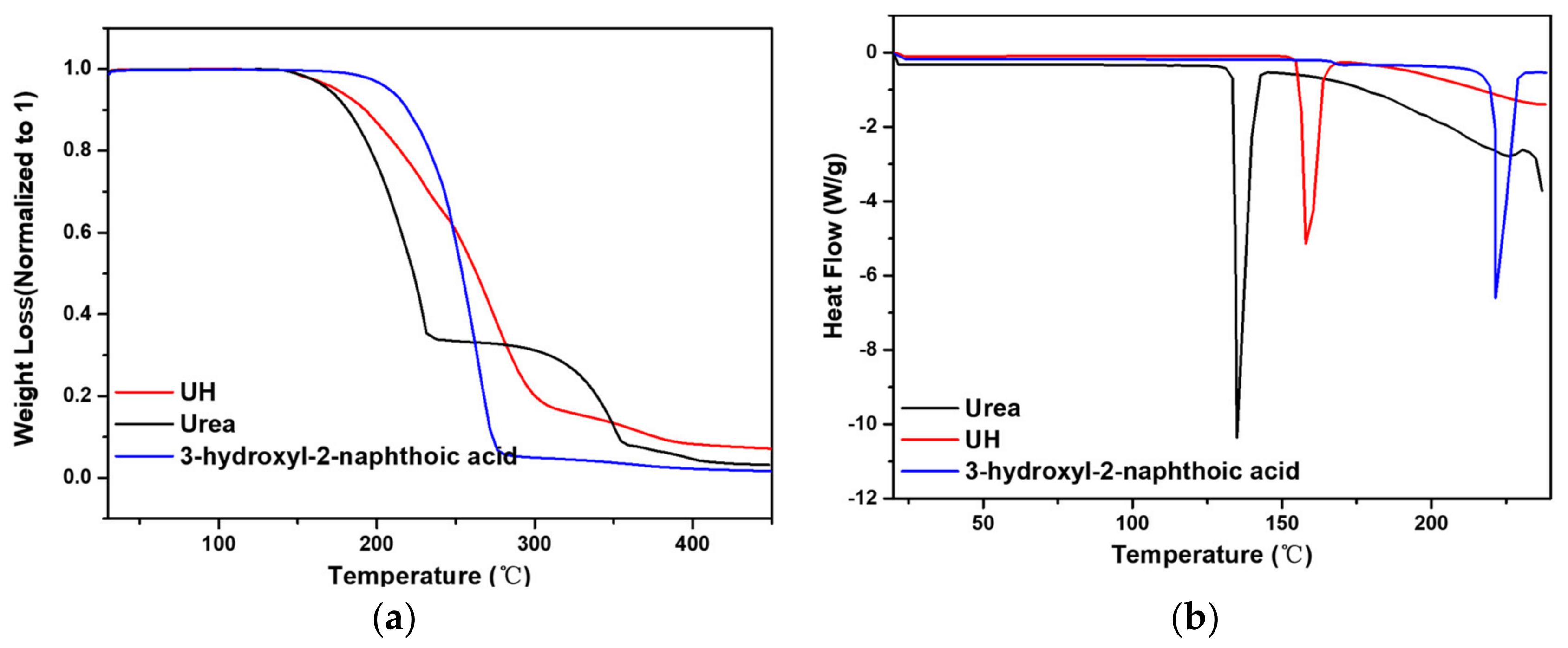
3.3. Urea-3-Hydroxyl-2-Naphtoic Acid (UH)
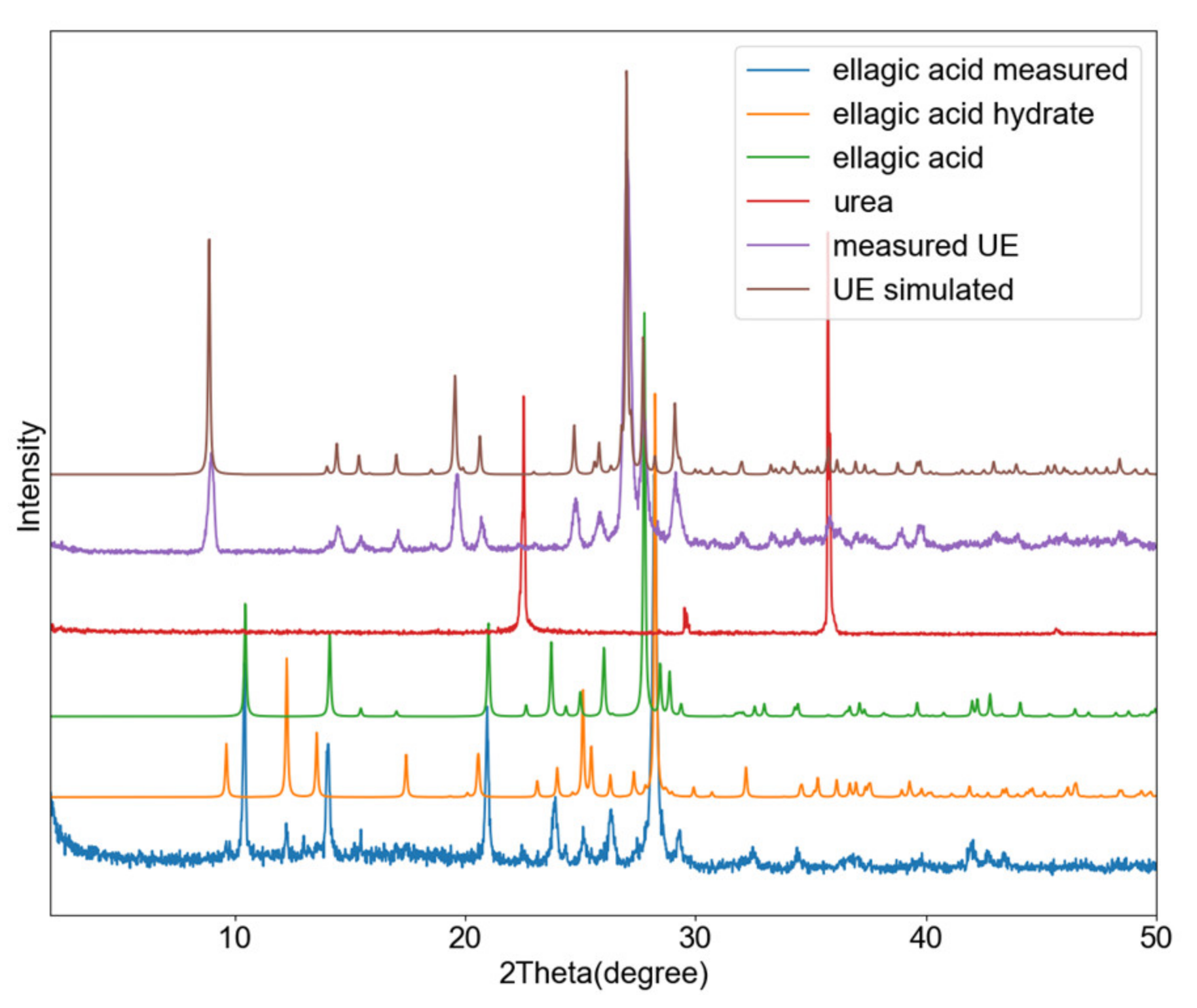
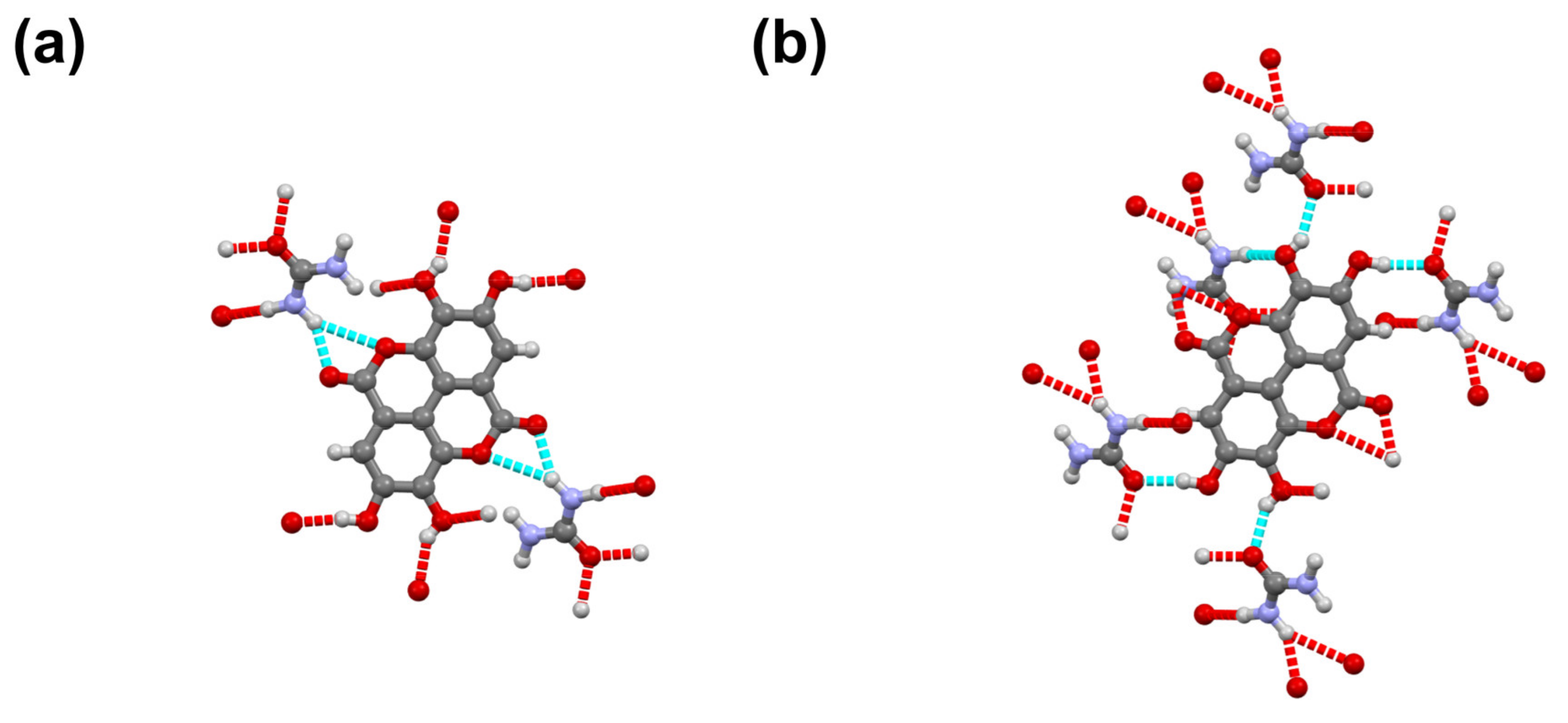
3.4. Urea-Ellagic Acid (UE)
3.5. Solution Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, C.C. Cocrystallization for successful drug delivery. Expert Opin. Drug Deliv. 2013, 10, 201–213. [Google Scholar] [CrossRef]
- Kuminek, G.; Cao, F.; Bahia de Oliveira da Rocha, A.; Goncalves Cardoso, S.; Rodriguez-Hornedo, N. Cocrystals to facilitate delivery of poorly soluble compounds beyond-rule-of-5. Adv. Drug Deliv. Rev. 2016, 101, 143–166. [Google Scholar] [CrossRef] [Green Version]
- Bolla, G.; Nangia, A. Pharmaceutical cocrystals: Walking the talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating Cocrystals: A Review of Pharmaceutical Cocrystal Preparation Routes and Applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Kavanagh, O.N.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Pharmaceutical cocrystals: From serendipity to design to application. Drug Discov. Today 2019, 24, 796–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Good, D.J.; Rodriíguez-Hornedo, N. Solubility Advantage of Pharmaceutical Cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Machado, T.C.; Kuminek, G.; Cardoso, S.G.; Rodriguez-Hornedo, N. The role of pH and dose/solubility ratio on cocrystal dissolution, drug supersaturation and precipitation. Eur. J. Pharm. Sci. 2020, 152, 105422. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Gunawardana, C.A.; Aakeroy, C.B. Co-crystal synthesis: Fact, fancy, and great expectations. Chem. Commun. 2018, 54, 14047–14060. [Google Scholar] [CrossRef]
- Allu, S.; Bolla, G.; Tothadi, S.; Nangia, A.K. Novel Pharmaceutical Cocrystals and Salts of Bumetanide. Cryst. Growth Des. 2019, 20, 793–803. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Peng, B.; Zhu, B.; Wang, J.-R.; Mei, X. Improving Compliance and Decreasing Drug Accumulation of Diethylstilbestrol through Cocrystallization. Cryst. Growth Des. 2019, 19, 1942–1953. [Google Scholar] [CrossRef]
- Maddileti, D.; Jayabun, S.K.; Nangia, A. Soluble Cocrystals of the Xanthine Oxidase Inhibitor Febuxostat. Cryst. Growth Des. 2013, 13, 3188–3196. [Google Scholar] [CrossRef]
- Sanphui, P.; Kumar, S.S.; Nangia, A. Pharmaceutical Cocrystals of Niclosamide. Cryst. Growth Des. 2012, 12, 4588–4599. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, J.-M.; Geng, N.; Lu, T.-B. Improving the Solubility of Agomelatine via Cocrystals. Cryst. Growth Des. 2012, 12, 2226–2233. [Google Scholar] [CrossRef]
- Ceci, C.; Graziani, G.; Faraoni, I.; Cacciotti, I. Strategies to improve ellagic acid bioavailability: From natural or semisynthetic derivatives to nanotechnological approaches based on innovative carriers. Nanotechnology 2020, 31, 382001. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Khopde, S.M.; Kumar, S.S.; Mohan, H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J. Agric. Food Chem. 2002, 50, 2200–2206. [Google Scholar] [CrossRef]
- Park, S.M.; Choi, J.; Nam, T.G.; Ku, J.M.; Jeong, K. Anti-diabetic effect of 3-hydroxy-2-naphthoic acid, an endoplasmic reticulum stress-reducing chemical chaperone. Eur. J. Pharmacol. 2016, 779, 157–167. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Q.Y.; Tsang, D.; Huang, Y. Degradation of green tea catechins in tea drinks. J. Agric. Food Chem. 2001, 49, 477–482. [Google Scholar] [CrossRef]
- De Araujo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2020, 338, 127535. [Google Scholar] [CrossRef]
- Li, N.; Taylor, L.S.; Ferruzzi, M.G.; Mauer, L.J. Kinetic study of catechin stability: Effects of pH, concentration, and temperature. J. Agric. Food Chem. 2012, 60, 12531–12539. [Google Scholar] [CrossRef]
- Labbé, D.; Têtu, B.; Trudel, D.; Bazinet, L. Catechin stability of EGC- and EGCG-enriched tea drinks produced by a two-step extraction procedure. Food Chem. 2008, 111, 139–143. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, version 1.171.37.35; Rigaku Corporation: Wroclaw, Poland, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Boultif, A.; Louër, D. Powder pattern indexing with the dichotomy method. J. Appl. Cryst. 2004, 37, 724–731. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Buol, X.; Robeyns, K.; Caro Garrido, C.; Tumanov, N.; Collard, L.; Wouters, J.; Leyssens, T. Improving Nefiracetam Dissolution and Solubility Behavior Using a Cocrystallization Approach. Pharmaceutics 2020, 12, 653. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Wallwork, S.C. The Crystal Structure of the 1:1 Complex between Quinol and Urea. Acta Cryst. 1975, B31, 338. [Google Scholar] [CrossRef]
- Wiedenfeld, H.; Knoch, F. Zur Kristallstruktur des Theophyllin-Harnstoff-Komplexes. Arch. Pharm. 1986, 319, 654–659. [Google Scholar] [CrossRef]
- Preut, H.; Haupt, H.-J. Packing analyses on organometallic compounds. Acta Crystallogr. Sect. A 1981, 37, C93. [Google Scholar] [CrossRef] [Green Version]
- Mazzei, L.; Broll, V.; Casali, L.; Silva, M.; Braga, D.; Grepioni, F.; Baltrusaitis, J.; Ciurli, S. Multifunctional urea cocrystal with combined ureolysis and nitrification inhibiting capabilities for enhanced nitrogen management. ACS Sustain. Chem. Eng. 2019, 7, 13369–13378. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Ascacio, A.; Rodríguez-Herrera, R.; Aguilera-Carbó, A.; Aguilar, C.N. Ellagic acid: Biological properties and biotechnological development for production processes. Afr. J. Biotechnol. 2011, 10, 4518–4523. [Google Scholar]
- Dudek, M.K.; Paluch, P.; Śniechowska, J.; Nartowski, K.P.; Day, G.M.; Potrzebowski, M.J. Crystal structure determination of an elusive methanol solvate—Hydrate of catechin using crystal structure prediction and NMR crystallography. CrystEngComm 2020, 22, 4969–4981. [Google Scholar] [CrossRef]



| Compound | UC Cocrystal | UH Cocrystal |
|---|---|---|
| Formula | C16H18N2O7 | C12H12N2O4 |
| Dcalc./g cm−3 | 1.544 | 1.398 |
| m/mm−1 | 0.123 | 0.107 |
| Formula Weight | 350.32 | 248.24 |
| Colour | Brown | colourless |
| Shape | needle | rod |
| Size/mm3 | 0.35 × 0.02 × 0.02 | 0.30 × 0.10 × 0.05 |
| T/K | 150(2) | 293(2) |
| Crystal System | monoclinic | monoclinic |
| Space Group | P21 | C2/c |
| a/Å | 10.7771(12) | 24.353(2) |
| b/Å | 5.0024(5) | 5.0996(4) |
| c/Å | 14.960(3) | 20.7056(19) |
| α/° | 90 | 90 |
| β/° | 110.849(17) | 113.490(11) |
| γ/° | 90 | 90 |
| V/Å3 | 753.68(19) | 2358.3(4) |
| Z | 2 | 8 |
| Z’ | 1 | 1 |
| Wavelength/Å | 0.71073 | 0.71073 |
| Radiation type | MoKα | MoKα |
| Measured Refl’s. | 3867 | 8859 |
| Indep’t Refl’s | 2127 | 2341 |
| Refl’s I ≥ 2 s(I) | 1217 | 1938 |
| Rint | 0.1191 | 0.0365 |
| GooF | 1.026 | 1.063 |
| wR2 (all data) | 0.1510 | 0.1192 |
| wR2 | 0.1240 | 0.1125 |
| R1 (all data) | 0.1472 | 0.0511 |
| R1 | 0.0775 | 0.0421 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, F.; Robeyns, K.; Leyssens, T. Urea as a Cocrystal Former—Study of 3 Urea Based Pharmaceutical Cocrystals. Pharmaceutics 2021, 13, 671. https://doi.org/10.3390/pharmaceutics13050671
Leng F, Robeyns K, Leyssens T. Urea as a Cocrystal Former—Study of 3 Urea Based Pharmaceutical Cocrystals. Pharmaceutics. 2021; 13(5):671. https://doi.org/10.3390/pharmaceutics13050671
Chicago/Turabian StyleLeng, Fucheng, Koen Robeyns, and Tom Leyssens. 2021. "Urea as a Cocrystal Former—Study of 3 Urea Based Pharmaceutical Cocrystals" Pharmaceutics 13, no. 5: 671. https://doi.org/10.3390/pharmaceutics13050671







