Current State of Breast Cancer Diagnosis, Treatment, and Theranostics
Abstract
:1. Introduction
2. Techniques for Diagnosis or Detection of Breast Cancer
2.1. Mammography
2.2. Magnetic Resonance Imaging
2.3. Dynamic Contrast Enhanced MRI (DCE-MRI)
2.4. Magnetic Resonance Elastography
2.5. Diffusion-Weighted Imaging
2.6. Magnetic Resonance Spectroscopy
2.7. Positron Emission Tomography (PET) Scanning and PET in Conjunction with Computer-aided Tomography (CT) Scanning (PET-CT)
2.8. Molecular Image-Guided Sentinel Node Biopsy
2.9. Breast Specific Gamma Imaging
2.10. Ultrasound
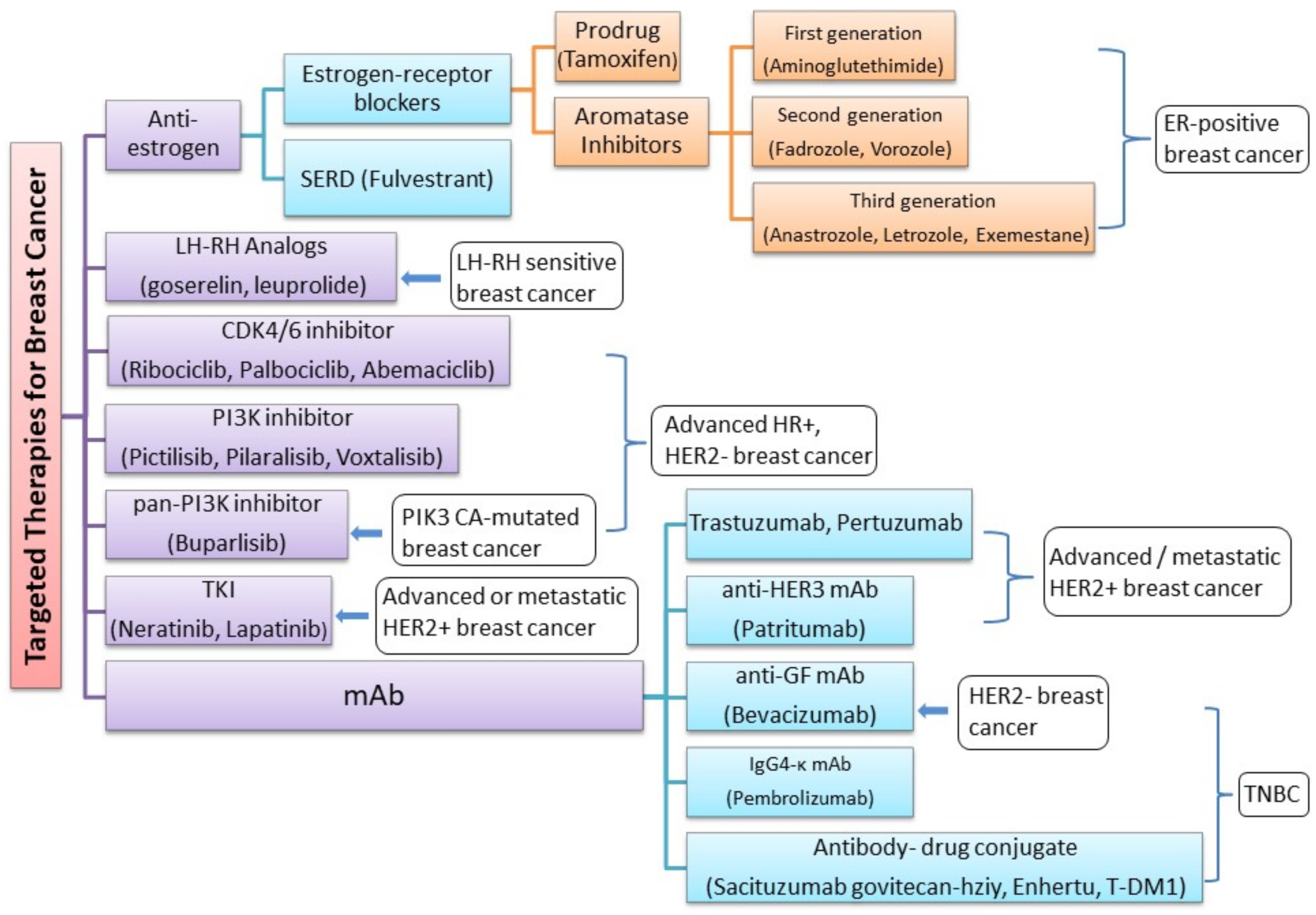
3. Current Treatment and Novel Therapies for Different Subtypes of Breast Cancer
- Luminal A breast cancer is low grade, HER2– and HR+ (estrogen- and/or progesterone-receptor positive), that has low levels of the protein Ki-67, which are responsible for controlling how fast cancer cells proliferate. Luminal A cancers tend to proliferate slowly with an excellent prognosis compared to other cancers.
- Luminal B breast cancer is a molecular subtype of breast cancer in which the tumors are HR+ (progesterone-receptor and/or estrogen-receptor positive) and show elevated levels of the protein Ki-67 while being either HER2– or HER2+. Luminal B cancer subtype is associated with faster proliferation rate and tends to be more aggressive compared to Luminal A breast cancer, making its prognosis slightly worse [108].
- Triple-negative/basal-like breast cancer is HR– (estrogen-receptor and progesterone-receptor negative) and HER2–. Women with BRCA1 gene mutations are more prone to develop this form of cancer [109].
- HER2-enriched breast cancer is a molecular subtype of breast cancer in which tumors are HER2+ and HR– (i.e., negative for estrogen- and progesterone-receptor). This subtype is associated with a tendency to proliferate at a more rapid rate than luminal cancers [91]. However, patients are successfully treated with drugs targeting the HER2 protein, such as Tykerb (lapatinib), Herceptin (trastuzumab), Perjeta (pertuzumab), and Enhertu (fam-trastuzumab-deruxtecan-nxki) [110].
- Normal-like breast cancer is identical to luminal A cancer as it is HER2–, HR+ (estrogen- and/or progesterone-receptor positive) with reduced levels of the Ki-67 protein. The prognosis of normal-like breast cancer is, however, slightly worse than the luminal A cancer.
3.1. Cyclin-Dependent Kinases 4/6 (CDK4/6) Pathway
3.2. Phosphoinositide 3-kinase (PI3K) Pathway
3.3. Targeting HER2+ Breast Cancers
3.4. Treating Triple-Negative Breast Cancer
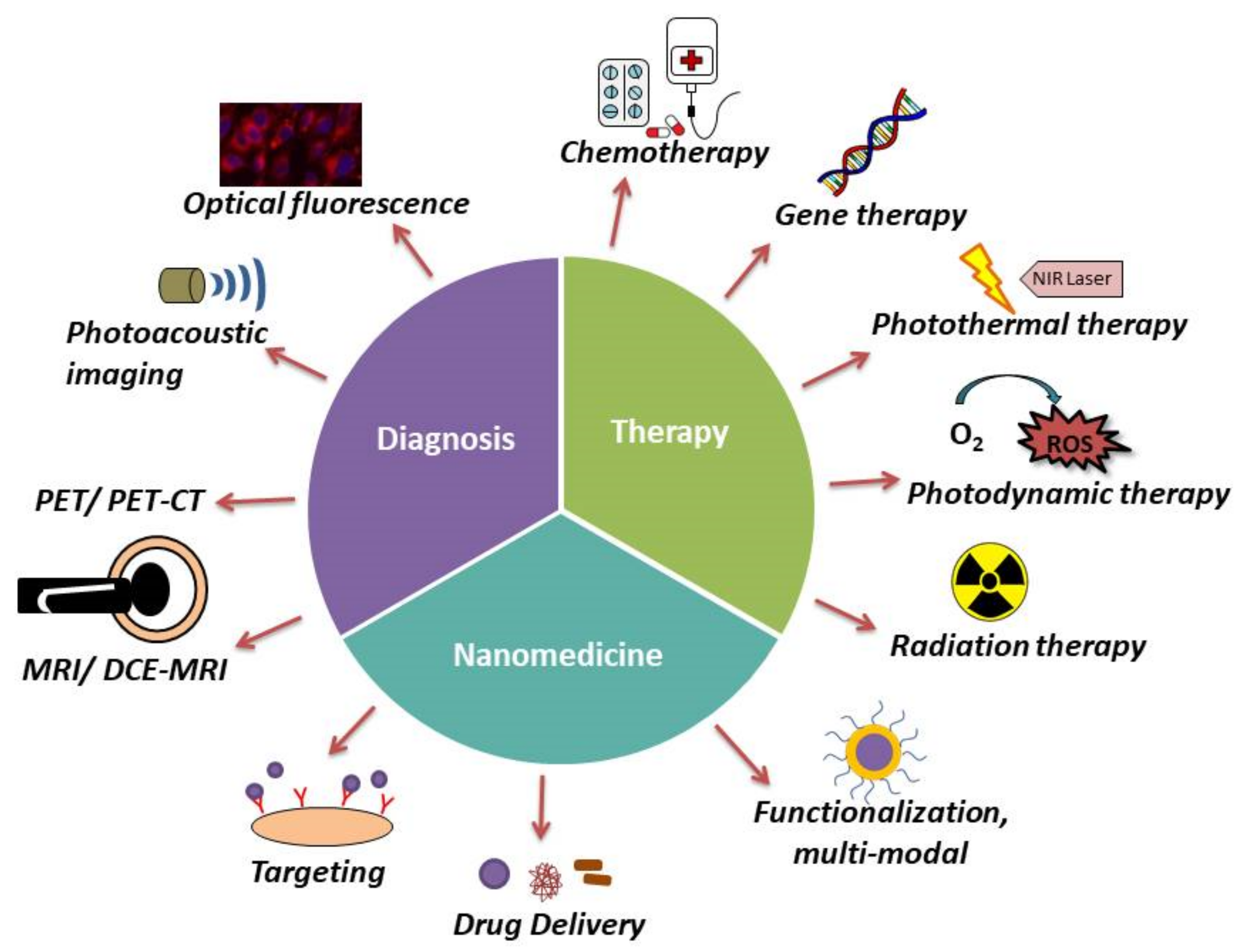
4. Recent Trends in Breast Cancer Theranostics
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebeid, N.I. Egyptian Medicine in the Days of the Pharaohs; General—Egyptian Book Organization: Cairo, Egypt, 1999; ISBN 9789770164228. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Duncan, W.; Kerr, G.R. The curability of breast cancer. Br. Med. J. 1976, 2, 781–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juanpere, S.; Perez, E.; Huc, O.; Motos, N.; Pont, J.; Pedraza, S. Imaging of breast implants-a pictorial review. Insights Into Imaging 2011, 2, 653–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basilion, J. Breast imaging technology: Current and future technologies for breast cancer imaging. Breast Cancer Res. 2001, 3, 13–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iranmakani, S.; Mortezazadeh, T.; Sajadian, F.; Ghaziani, M.F.; Ghafari, A.; Khezerloo, D.; Musa, A.E. A review of various modalities in breast imaging: Technical aspects and clinical outcomes. Egypt. J. Radiol. Nucl. Med. 2020, 51, 57. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-H.; Xiao, C. Diagnostic Value of Nineteen Different Imaging Methods for Patients with Breast Cancer: A Network Meta-Analysis. Cell. Physiol. Biochem. 2018, 46, 2041–2055. [Google Scholar] [CrossRef] [PubMed]
- Sant, M.; Allemani, C.; Berrino, F.; Coleman, M.P.; Aareleid, T.; Chaplain, G.; Coebergh, J.W.; Colonna, M.; Crosignani, P.; Danzon, A.; et al. Breast carcinoma survival in Europe and the United States. Cancer 2004, 100, 715–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeeshan, M.; Salam, B.; Khalid, Q.S.B.; Alam, S.; Sayani, R. Diagnostic Accuracy of Digital Mammography in the Detection of Breast Cancer. Cureus 2018, 10, e2448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Euler-Chelpin, M.; Lillholm, M.; Vejborg, I.; Nielsen, M.; Lynge, E. Sensitivity of screening mammography by density and texture: A cohort study from a population-based screening program in Denmark. Breast Cancer Res. 2019, 21, 111. [Google Scholar] [CrossRef] [Green Version]
- Devi, R.R.; Anandhamala, G. Recent Trends in Medical Imaging Modalities and Challenges for Diagnosing Breast Cancer. Biomed. Pharmacol. J. 2018, 11, 1649–1658. [Google Scholar] [CrossRef]
- Procz, S.; Roque, G.; Avila, C.; Racedo, J.; Rueda, R.; Santos, I.; Fiederle, M. Investigation of CdTe, GaAs, Se and Si as Sensor Materials for Mammography. IEEE Trans. Med. Imaging 2020, 39, 3766–3778. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.M.; Kuhl, C.K.; Moy, L. Contrast-enhanced MRI for breast cancer screening. J. Magn. Reson. Imaging 2019, 50, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Wallyn, J.; Anton, N.; Akram, S.; Vandamme, T.F. Biomedical Imaging: Principles, Technologies, Clinical Aspects, Contrast Agents, Limitations and Future Trends in Nanomedicines. Pharm. Res. 2019, 36, 78. [Google Scholar] [CrossRef] [PubMed]
- Grover, V.P.B.; Tognarelli, J.M.; Crossey, M.M.E.; Cox, I.J.; Taylor-Robinson, S.D.; McPhail, M.J.W. Magnetic Resonance Imaging: Principles and Techniques: Lessons for Clinicians. J. Clin. Exp. Hepatol. 2015, 5, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Fardanesh, R.; Marino, M.A.; Avendano, D.; Leithner, D.; Pinker, K.; Thakur, S.B. Proton MR spectroscopy in the breast: Technical innovations and clinical applications. J. Magn. Reson. Imaging 2019, 50, 1033–1046. [Google Scholar] [CrossRef]
- Hu, J.; Feng, W.; Hua, J.; Jiang, Q.; Xuan, Y.; Li, T.; Haacke, E.M. A high spatial resolution in vivo 1H magnetic resonance spectroscopic imaging technique for the human breast at 3 T. Med. Phys. 2009, 36, 4870–4877. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.S.; Sajjad, Z.; Hafeez, S.; Akhter, W. Magnetic resonance spectroscopy in focal brain lesions. J. Pak. Med. Assoc. 2011, 61, 540–543. [Google Scholar]
- Cai, H.; Liu, L.; Peng, Y.; Wu, Y.; Li, L. Diagnostic assessment by dynamic contrast-enhanced and diffusion-weighted magnetic resonance in differentiation of breast lesions under different imaging protocols. BMC Cancer 2014, 14, 366. [Google Scholar] [CrossRef] [Green Version]
- Jansen, S.A.; Fan, X.; Karczmar, G.S.; Abe, H.; Schmidt, R.A.; Giger, M.; Newstead, G.M. DCEMRI of breast lesions: Is kinetic analysis equally effective for both mass and nonmass-like enhancement? Med. Phys. 2008, 35, 3102–3109. [Google Scholar] [CrossRef]
- Tao, W.; Hu, C.; Bai, G.; Zhu, Y.; Zhu, Y. Correlation between the dynamic contrast-enhanced MRI features and prognostic factors in breast cancer: A retrospective case-control study. Medicine 2018, 97. [Google Scholar] [CrossRef]
- Pereira, N.P.; Curi, C.; Osório, C.A.B.T.; Marques, E.F.; Makdissi, F.B.; Pinker, K.; Bitencourt, A.G.V. Diffusion-Weighted Magnetic Resonance Imaging of Patients with Breast Cancer Following Neoadjuvant Chemotherapy Provides Early Prediction of Pathological Response—A Prospective Study. Sci. Rep. 2019, 9, 16372. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.; Berg, W.A. Dedicated Breast Gamma Camera Imaging and Breast PET: Current Status and Future Directions. PET Clin. 2018, 13, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, M.; Franceschini, G.; Douek, M. New techniques for sentinel node biopsy in breast cancer. Trans. Cancer Res. 2018. [Google Scholar] [CrossRef] [Green Version]
- Nandu, V.V.; Chaudhari, M.S. Efficacy of Sentinel Lymph Node Biopsy in Detecting Axillary Metastasis in Breast Cancer Using Methylene Blue. Indian J. Surg. Oncol. 2017, 8, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Brem, R.F.; Ruda, R.C.; Yang, J.L.; Coffey, C.M.; Rapelyea, J.A. Breast-Specific γ-Imaging for the Detection of Mammographically Occult Breast Cancer in Women at Increased Risk. J. Nucl. Med. 2016, 57, 678–684. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, A.; Newel, M.S. Alternative Screening for Women With Dense Breasts: Breast-Specific Gamma Imaging (Molecular Breast Imaging). Am. J. Roentgenol. 2015, 204, 252–256. [Google Scholar] [CrossRef]
- Liu, H.; Zhan, H.; Sun, D.; Zhang, Y. Comparison of BSGI, MRI, mammography, and ultrasound for the diagnosis of breast lesions and their correlations with specific molecular subtypes in Chinese women. BMC Med. Imaging 2020, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Screening for Breast Cancer:, U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 279–296. [Google Scholar] [CrossRef] [Green Version]
- Gøtzsche, P.C.; Jørgensen, K.J. Screening for breast cancer with mammography. Cochrane Database Syst. Rev. 2013, 2013, CD001877. [Google Scholar] [CrossRef]
- Bhan, A. Comparative Analysis of Pre-Processing Techniques for Mammogram Image Enhancement. In Proceedings of the INCON VIII 2013: International Conference on Ongoing Research in Management and IT, Pune, India, 11–13 January 2013. [Google Scholar]
- 32. Sundaram, K.M.; Sasikala, D.; Rani, P.A. A Study On Preprocessing A MammogramImage Using Adaptive Median Filter. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 10333–10337. [Google Scholar]
- Mandelblatt, J.S.; Cronin, K.A.; Bailey, S.; Berry, D.A.; de Koning, H.J.; Draisma, G.; Huang, H.; Lee, S.J.; Munsell, M.; Plevritis, S.K.; et al. Effects of mammography screening under different screening schedules: Model estimates of potential benefits and harms. Ann. Intern. Med. 2009, 151, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; Tyne, K.; Naik, A.; Bougatsos, C.; Chan, B.K.; Humphrey, L. Screening for breast cancer: An update for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2009, 151, 727–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprague, B.L.; Conant, E.F.; Onega, T.; Garcia, M.P.; Beaber, E.F.; Herschorn, S.D.; Lehman, C.D.; Tosteson, A.N.A.; Lacson, R.; Schnall, M.D.; et al. Variation in Mammographic Breast Density Assessments Among Radiologists in Clinical Practice: A Multicenter Observational Study. Ann. Intern. Med. 2016, 165, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Yala, A.; Lehman, C.; Schuster, T.; Portnoi, T.; Barzilay, R. A Deep Learning Mammography-based Model for Improved Breast Cancer Risk Prediction. Radiology 2019, 292, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrisi, L.; Restuccia, N.; Torrisi, A. Study of gold nanoparticles for mammography diagnostic and radiotherapy improvements. Rep. Pract. Oncol. Radiother. J. Greatpoland Cancer Cent. Pozn. Pol. Soc. Radiat. Oncol. 2019, 24, 450–457. [Google Scholar] [CrossRef]
- Graves, M.J.; Zhu, C. Basic Principles of Magnetic Resonance Imaging. In 3D Imaging Technologies in Atherosclerosis; Trivedi, R., Saba, L., Suri, J.S., Eds.; Springer: Boston, MA, USA, 2015; pp. 153–169. ISBN 978-1-4899-7618-5. [Google Scholar]
- Jethava, A.; Ali, S.; Wakefield, D.; Crowell, R.; Sporn, J.; Vrendenburgh, J. Diagnostic Accuracy of MRI in Predicting Breast Tumor Size: Comparative Analysis of MRI vs Histopathological Assessed Breast Tumor Size. Conn. Med. 2015, 79, 261–267. [Google Scholar]
- Grimsby, G.M.; Gray, R.; Dueck, A.; Carpenter, S.; Stucky, C.-C.; Aspey, H.; Giurescu, M.E.; Pockaj, B. Is there concordance of invasive breast cancer pathologic tumor size with magnetic resonance imaging? Am. J. Surg. 2009, 198, 500–504. [Google Scholar] [CrossRef]
- Goldsmith, M.; Koutcher, J.A.; Damadian, R. NMR in cancer, XIII: Application of the NMR malignancy index to human mammary tumours. Br. J. Cancer 1978, 38, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Niell, B.L.; Gavenonis, S.C.; Motazedi, T.; Chubiz, J.C.; Halpern, E.P.; Rafferty, E.A.; Lee, J.M. Auditing a Breast MRI Practice: Performance Measures for Screening and Diagnostic Breast MRI. J. Am. Coll. Radiol. 2014, 11, 883–889. [Google Scholar] [CrossRef] [Green Version]
- Michel, S.C.A.; Keller, T.M.; Fröhlich, J.M.; Fink, D.; Caduff, R.; Seifert, B.; Marincek, B.; Kubik-Huch, R.A. Preoperative Breast Cancer Staging: MR Imaging of the Axilla with Ultrasmall Superparamagnetic Iron Oxide Enhancement. Radiology 2002, 225, 527–536. [Google Scholar] [CrossRef]
- Ayat, N.R.; Vaidya, A.; Yeung, G.A.; Buford, M.N.; Hall, R.C.; Qiao, P.L.; Yu, X.; Lu, Z.-R. Effective MR Molecular Imaging of Triple Negative Breast Cancer With an EDB-Fibronectin-Specific Contrast Agent at Reduced Doses. Front. Oncol. 2019, 9, 1351. [Google Scholar] [CrossRef] [Green Version]
- Leithner, D.; Moy, L.; Morris, E.A.; Marino, M.A.; Helbich, T.H.; Pinker, K. Abbreviated MRI of the Breast: Does It Provide Value? J. Magn. Reson. Imaging 2019, 49, e85–e100. [Google Scholar] [CrossRef]
- Peters, N.H.G.M.; Borel Rinkes, I.H.M.; Zuithoff, N.P.A.; Mali, W.P.T.M.; Moons, K.G.M.; Peeters, P.H.M. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology 2008, 246, 116–124. [Google Scholar] [CrossRef]
- Sardanelli, F.; Podo, F.; D’Agnolo, G.; Verdecchia, A.; Santaquilani, M.; Musumeci, R.; Trecate, G.; Manoukian, S.; Morassut, S.; de Giacomi, C.; et al. Multicenter comparative multimodality surveillance of women at genetic-familial high risk for breast cancer (HIBCRIT study): Interim results. Radiology 2007, 242, 698–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahbar, H.; Partridge, S.C. Multiparametric MR Imaging of Breast Cancer. Magn. Reson. Imaging Clin. North Am. 2016, 24, 223–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teifke, A.; Behr, O.; Schmidt, M.; Victor, A.; Vomweg, T.W.; Thelen, M.; Lehr, H.-A. Dynamic MR imaging of breast lesions: Correlation with microvessel distribution pattern and histologic characteristics of prognosis. Radiology 2006, 239, 351–360. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, N.; Kim, S.J.; Cha, J.H.; Cho, K.S.; Ko, E.S.; Moon, W.K. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J. Radiol. 2008, 9, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.J.; Choi, H.; Choi, S.A.; Youk, J.H. Dynamic contrast-enhanced breast magnetic resonance imaging for the prediction of early and late recurrences in breast cancer. Medicine 2016, 95, e5330. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, Y.; Li, H.; Xie, Y.; Wang, X.; Wan, W. Preliminary study on identification of estrogen receptor-positive breast cancer subtypes based on dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) texture analysis. Gland Surg. 2020, 9, 622–628. [Google Scholar] [CrossRef]
- Gillman, J.; Toth, H.K.; Moy, L. The Role of Dynamic Contrast-Enhanced Screening Breast MRI in Populations at Increased Risk for Breast Cancer. Women’s Health 2014, 10, 609–622. [Google Scholar] [CrossRef] [Green Version]
- Turnbull, L.W. Dynamic contrast-enhanced MRI in the diagnosis and management of breast cancer. NMR Biomed. 2009, 22, 28–39. [Google Scholar] [CrossRef]
- Amornsiripanitch, N.; Bickelhaupt, S.; Shin, H.J.; Dang, M.; Rahbar, H.; Pinker, K.; Partridge, S.C. Diffusion-weighted MRI for Unenhanced Breast Cancer Screening. Radiology 2019, 293, 504–520. [Google Scholar] [CrossRef]
- Glaser, K.J.; Manduca, A.; Ehman, R.L. Review of MR elastography applications and recent developments. J. Magn. Reson. Imaging 2012, 36, 757–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, B.K.; Samreen, N.; Zhou, Y.; Chen, J.; Brandt, K.; Ehman, R.; Pepin, K. MR Elastography of the Breast: Evolution of Technique, Case Examples, and Future Directions. Clin. Breast Cancer 2021, 21, e102–e111. [Google Scholar] [CrossRef]
- Hawley, J.R.; Kalra, P.; Mo, X.; Raterman, B.; Yee, L.D.; Kolipaka, A. Quantification of breast stiffness using MR elastography at 3 Tesla with a soft sternal driver: A reproducibility study. J. Magn. Reson. Imaging 2017, 45, 1379–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKnight, A.L.; Kugel, J.L.; Rossman, P.J.; Manduca, A.; Hartmann, L.C.; Ehman, R.L. MR Elastography of Breast Cancer: Preliminary Results. Am. J. Roentgenol. 2002, 178, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Pepin, K.M.; Ehman, R.L.; McGee, K.P. Magnetic resonance elastography (MRE) in cancer: Technique, analysis, and applications. Prog. Nucl. Magn. Reson. Spectrosc. 2015, 90–91, 32–48. [Google Scholar] [CrossRef] [Green Version]
- Sinkus, R.; Siegmann, K.; Xydeas, T.; Tanter, M.; Claussen, C.; Fink, M. MR elastography of breast lesions: Understanding the solid/liquid duality can improve the specificity of contrast-enhanced MR mammography. Magn. Reson. Med. 2007, 58, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Manduca, A.; Oliphant, T.E.; Dresner, M.A.; Mahowald, J.L.; Kruse, S.A.; Amromin, E.; Felmlee, J.P.; Greenleaf, J.F.; Ehman, R.L. Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Med. Image Anal. 2001, 5, 237–254. [Google Scholar] [CrossRef]
- Lorenzen, J.; Sinkus, R.; Lorenzen, M.; Dargatz, M.; Leussler, C.; Röschmann, P.; Adam, G. MR elastography of the breast:preliminary clinical results. Rofo Fortschr. Geb. Rontgenstrahlen Nukl. 2002, 174, 830–834. [Google Scholar] [CrossRef]
- Malayeri, A.A.; El Khouli, R.H.; Zaheer, A.; Jacobs, M.A.; Corona-Villalobos, C.P.; Kamel, I.R.; Macura, K.J. Principles and Applications of Diffusion-weighted Imaging in Cancer Detection, Staging, and Treatment Follow-up. RadioGraphics 2011, 31, 1773–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petralia, G.; Bonello, L.; Priolo, F.; Summers, P.; Bellomi, M. Breast MR with special focus on DW-MRI and DCE-MRI. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2011, 11, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Baron, P.; Dorrius, M.D.; Kappert, P.; Oudkerk, M.; Sijens, P.E. Diffusion-weighted imaging of normal fibroglandular breast tissue: Influence of microperfusion and fat suppression technique on the apparent diffusion coefficient. NMR Biomed. 2010, 23, 399–405. [Google Scholar] [CrossRef]
- Durur-Subasi, I. DW-MRI of the breast: A pictorial review. Insights Into Imaging 2019, 10, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baltzer, P.A.T.; Kapetas, P.; Sodano, C.; Dietzel, M.; Pinker, K.; Helbich, T.H.; Clauser, P. Contrast agent-free breast MRI: Advantages and potential disadvantages. Der Radiol. 2019, 59, 510–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baltzer, P.; Mann, R.M.; Iima, M.; Sigmund, E.E.; Clauser, P.; Gilbert, F.J.; Martincich, L.; Partridge, S.C.; Patterson, A.; Pinker, K.; et al. Diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur. Radiol. 2020, 30, 1436–1450. [Google Scholar] [CrossRef] [Green Version]
- Bolan, P.J.; Meisamy, S.; Baker, E.H.; Lin, J.; Emory, T.; Nelson, M.; Everson, L.I.; Yee, D.; Garwood, M. In vivo quantification of choline compounds in the breast with 1H MR spectroscopy. Magn. Reson. Med. 2003, 50, 1134–1143. [Google Scholar] [CrossRef]
- Jagannathan, N.R.; Kumar, M.; Seenu, V.; Coshic, O.; Dwivedi, S.N.; Julka, P.K.; Srivastava, A.; Rath, G.K. Evaluation of total choline from in-vivo volume localized proton MR spectroscopy and its response to neoadjuvant chemotherapy in locally advanced breast cancer. Br. J. Cancer 2001, 84, 1016–1022. [Google Scholar] [CrossRef] [Green Version]
- Meisamy, S.; Bolan, P.J.; Baker, E.H.; Pollema, M.G.; Le, C.T.; Kelcz, F.; Lechner, M.C.; Luikens, B.A.; Carlson, R.A.; Brandt, K.R.; et al. Adding in Vivo Quantitative 1H MR Spectroscopy to Improve Diagnostic Accuracy of Breast MR Imaging: Preliminary Results of Observer Performance Study at 4.0 T. Radiology 2005, 236, 465–475. [Google Scholar] [CrossRef]
- Stanwell, P.; Mountford, C. In Vivo Proton MR Spectroscopy of the Breast. RadioGraphics 2007, 27, S253–S266. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Flavell, R.R.; Naeger, D.M.; Mari Aparici, C.; Hawkins, R.A.; Pampaloni, M.H.; Behr, S.C. Malignancies with Low Fluorodeoxyglucose Uptake at PET/CT: Pitfalls and Prognostic Importance: Resident and Fellow Education Feature. RadioGraphics 2016, 36, 293–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawada, K.; Iwamoto, M.; Sakai, Y. Mechanisms underlying (18)F-fluorodeoxyglucose accumulation in colorectal cancer. World J. Radiol. 2016, 8, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, T.; Aogi, K.; Kiyoto, S.; Masumoto, N.; Sugawara, Y.; Okada, M. Role of maximum standardized uptake value in fluorodeoxyglucose positron emission tomography/computed tomography predicts malignancy grade and prognosis of operable breast cancer: A multi-institute study. Breast Cancer Res. Treat. 2013, 141, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.; Kim, S.-B.; Ahn, J.-H.; Jung, K.H.; Ahn, S.H.; Son, B.H.; Lee, J.W.; Gong, G.; Kim, H.O.; Moon, D.H. 18 F-fluorodeoxyglucose uptake predicts pathological complete response after neoadjuvant chemotherapy for breast cancer: A retrospective cohort study. J. Surg. Oncol. 2013, 107, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.; Madsen, K.S.; Kalinyak, J.E.; Berg, W.A. Interpretation of Positron Emission Mammography and MRI by Experienced Breast Imaging Radiologists: Performance and Observer Reproducibility. Am. J. Roentgenol. 2011, 196, 971–981. [Google Scholar] [CrossRef] [Green Version]
- Hsu, D.F.C.; Freese, D.L.; Levin, C.S. Breast-Dedicated Radionuclide Imaging Systems. J. Nucl. Med. 2016, 57, 40S–45S. [Google Scholar] [CrossRef] [Green Version]
- Giammarile, F.; Castellucci, P.; Dierckx, R.; Estrada Lobato, E.; Farsad, M.; Hustinx, R.; Jalilian, A.; Pellet, O.; Rossi, S.; Paez, D. Non-FDG PET/CT in Diagnostic Oncology: A pictorial review. Eur. J. Hybrid Imaging 2019, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Blanchet, E.M.; Millo, C.; Martucci, V.; Maass-Moreno, R.; Bluemke, D.A.; Pacak, K. Integrated whole-body PET/MRI with 18F-FDG, 18F-FDOPA, and 18F-FDA in paragangliomas in comparison with PET/CT: NIH first clinical experience with a single-injection, dual-modality imaging protocol. Clin. Nucl. Med. 2014, 39, 243–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wibmer, A.G.; Hricak, H.; Ulaner, G.A.; Weber, W. Trends in oncologic hybrid imaging. Eur. J. Hybrid Imaging 2018, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Escalona, S.; Blasco, J.A.; Reza, M.M.; Andradas, E.; Gómez, N. A systematic review of FDG-PET in breast cancer. Med. Oncol. 2010, 27, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.T.; Norregaard, K.; Simón Martín, M.; Oddershede, L.B.; Kjaer, A. Non-invasive Early Response Monitoring of Nanoparticle-assisted Photothermal Cancer Therapy Using (18)F-FDG, (18)F-FLT, and (18)F-FET PET/CT Imaging. Nanotheranostics 2018, 2, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyman, G.H.; Somerfield, M.R.; Bosserman, L.D.; Perkins, C.L.; Weaver, D.L.; Giuliano, A.E. Sentinel Lymph Node Biopsy for Patients With Early-Stage Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2016, 35, 561–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, C.E.; Kiluk, J.V.; Riker, A.I.; Cox, J.M.; Allred, N.; Ramos, D.C.; Dupont, E.L.; Vrcel, V.; Diaz, N.; Boulware, D. Significance of sentinel lymph node micrometastases in human breast cancer. J. Am. Coll. Surg. 2008, 206, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Iddings, D.M.; Scheri, R.P.; Bilchik, A.J. Lymphatic mapping and sentinel node analysis: Current concepts and applications. CA A Cancer J. Clin. 2006, 56, 292–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Chang, J.H.; Kim, S.M.; Lee, H.J.; Kim, H.; Wilson, B.C.; Song, T.-K. Real-time sentinel lymph node biopsy guidance using combined ultrasound, photoacoustic, fluorescence imaging: In vivo proof-of-principle and validation with nodal obstruction. Sci. Rep. 2017, 7, 45008. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, K.; Krishnan, S.; Thanki, R. Breast Cancer Detection and Diagnosis Using AI. In Artificial Intelligence in Breast Cancer Early Detection and Diagnosis; Springer International Publishing: Cham, Switzerland, 2021; pp. 79–92. ISBN 978-3-030-59208-0. [Google Scholar]
- Liu, H.; Zhan, H.; Sun, D. Comparison of 99mTc-MIBI scintigraphy, ultrasound, and mammography for the diagnosis of BI-RADS 4 category lesions. BMC Cancer 2020. [Google Scholar] [CrossRef]
- Gong, Z.; Williams, M.B. Comparison of breast specific gamma imaging and molecular breast tomosynthesis in breast cancer detection: Evaluation in phantoms. Med. Phys. 2015, 42, 4250–4259. [Google Scholar] [CrossRef] [Green Version]
- Rechtman, L.R.; Lenihan, M.J.; Lieberman, J.H.; Teal, C.B.; Torrente, J.; Rapelyea, J.A.; Brem, R.F. Breast-Specific Gamma Imaging for the Detection of Breast Cancer in Dense Versus Nondense Breasts. Am. J. Roentgenol. 2014, 202, 293–298. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Wang, X.; Yu, X.; Zhu, Y.; Zhan, H.; Chen, Z.; Li, B.; Huang, J. Breast-specific gamma imaging or ultrasonography as adjunct imaging diagnostics in women with mammographically dense breasts. Eur. Radiol. 2020, 30, 6062–6071. [Google Scholar] [CrossRef]
- Surti, S. Radionuclide methods and instrumentation for breast cancer detection and diagnosis. Semin. Nucl. Med. 2013, 43, 271–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbano, N.; Scimeca, M.; Tancredi, V.; Bonanno, E.; Schillaci, O. 99mTC-sestamibi breast imaging: Current status, new ideas and future perspectives. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Candelaria, R.; Fornage, B.D. Second-look US examination of MR-detected breast lesions. J. Clin. Ultrasound 2011, 39, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, A.; Eisenbrey, J.R.; Machado, P.; Ojeda-Fournier, H.; Wilkes, A.; Sevrukov, A.; Mattrey, R.F.; Wallace, K.; Chalek, C.L.; Thomenius, K.E.; et al. Quantitative analysis of vascular heterogeneity in breast lesions using contrast-enhanced 3-D harmonic and subharmonic ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 502–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, S.S. Automated whole breast ultrasound. Radiol. Clin. North Am. 2014, 52, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Skerl, K.; Vinnicombe, S.; Thomson, K.; McLean, D.; Giannotti, E.; Evans, A. Anisotropy of Solid Breast Lesions in 2D Shear Wave Elastography is an Indicator of Malignancy. Acad. Radiol. 2016, 23, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamber, J.; Cosgrove, D.; Dietrich, C.F.; Fromageau, J.; Bojunga, J.; Calliada, F.; Cantisani, V.; Correas, J.-M.; D’Onofrio, M.; Drakonaki, E.E.; et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall in der Medizin 2013, 34, 169–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, C.F.; Du, J.; Fang, H.; Li, F.H.; Zhu, J.S.; Liu, Q. Enhancement patterns and parameters of breast cancers at contrast-enhanced US: Correlation with prognostic factors. Radiology 2012, 262, 450–459. [Google Scholar] [CrossRef]
- Gill, M.R.; Menon, J.U.; Jarman, P.J.; Owen, J.; Skaripa-Koukelli, I.; Able, S.; Thomas, J.A.; Carlisle, R.; Vallis, K.A. (111)In-labelled polymeric nanoparticles incorporating a ruthenium-based radiosensitizer for EGFR-targeted combination therapy in oesophageal cancer cells. Nanoscale 2018, 10, 10596–10608. [Google Scholar] [CrossRef] [Green Version]
- Sood, R.; Rositch, A.F.; Shakoor, D.; Ambinder, E.; Pool, K.-L.; Pollack, E.; Mollura, D.J.; Mullen, L.A.; Harvey, S.C. Ultrasound for Breast Cancer Detection Globally: A Systematic Review and Meta-Analysis. J. Glob. Oncol. 2019, 5, 1–17. [Google Scholar] [CrossRef]
- Polyak, K. Heterogeneity in breast cancer. J. Clin. Investig. 2011, 121, 3786–3788. [Google Scholar] [CrossRef] [Green Version]
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch. Pathol. Lab. Med. 2014, 138, 241–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagawa, M.; Ikemot, K.; Kawauchi, S.; Furuya, T.; Yamamoto, S.; Oka, M.; Oga, A.; Nagashima, Y.; Sasaki, K. Luminal A and luminal B (HER2 negative) subtypes of breast cancer consist of a mixture of tumors with different genotype. BMC Res. Notes 2012, 5, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado-Vázquez, G.; Gámez-Pozo, A.; Trilla-Fuertes, L.; Arevalillo, J.M.; Zapater-Moros, A.; Ferrer-Gómez, M.; Díaz-Almirón, M.; López-Vacas, R.; Navarro, H.; Maín, P.; et al. A novel approach to triple-negative breast cancer molecular classification reveals a luminal immune-positive subgroup with good prognoses. Sci. Rep. 2019, 9, 1538. [Google Scholar] [CrossRef]
- Wang, J.; Xu, B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct. Target. Ther. 2019, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Love, R.R.; Koroltchouk, V. Tamoxifen therapy in breast cancer control worldwide. Bull. World Health Organ. 1993, 71, 795–803. [Google Scholar] [PubMed]
- Meiser, B.; Wong, W.K.T.; Peate, M.; Julian-Reynier, C.; Kirk, J.; Mitchell, G. Motivators and barriers of tamoxifen use as risk-reducing medication amongst women at increased breast cancer risk: A systematic literature review. Hered. Cancer Clin. Pract. 2017, 15, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuzick, J.; Sestak, I.; Bonanni, B.; Costantino, J.P.; Cummings, S.; DeCensi, A.; Dowsett, M.; Forbes, J.F.; Ford, L.; LaCroix, A.Z.; et al. Selective oestrogen receptor modulators in prevention of breast cancer: An updated meta-analysis of individual participant data. Lancet 2013, 381, 1827–1834. [Google Scholar] [CrossRef] [Green Version]
- Nazarali, S.A.; Narod, S.A. Tamoxifen for women at high risk of breast cancer. Breast Cancer 2014, 6, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Narod, S.A. Tamoxifen Chemoprevention—End of the Road? JAMA Oncol. 2015, 1, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.J. The what, why and how of aromatase inhibitors: Hormonal agents for treatment and prevention of breast cancer. Int. J. Clin. Pract. 2007, 61, 2051–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, R.; Barakat, A.; Pippen, J.; Osborne, C. Aromatase inhibitors in the treatment of breast cancer in post-menopausal female patients: An update. Breast Cancer 2011, 3, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Anastrozole Approved for HR-Positive Early Breast Cancer. Oncol. Times 2002, 24. [CrossRef]
- Cohen, M.H.; Johnson, J.R.; Li, N.; Chen, G.; Pazdur, R. Approval summary: Letrozole in the treatment of postmenopausal women with advanced breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 665–669. [Google Scholar]
- FDA Approval for Exemestane for Adjuvant Treatment for Early Breast Cancer in Postmenopausal Women. Oncol. Times 2005, 27. [CrossRef]
- Tan, S.-H.; Wolff, A.C. Luteinizing Hormone-Releasing Hormone Agonists in Premenopausal Hormone Receptor–Positive Breast Cancer. Clin. Breast Cancer 2007, 7, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Maltoni, R.; Bravaccini, S.; Donati, C.; Andreis, D. Clinical utility of fulvestrant in the treatment of breast cancer: A report on the emerging clinical evidence. Cancer Manag. Res. 2018, 10, 3083–3099. [Google Scholar] [CrossRef] [Green Version]
- Wakeling, A.E.; Dukes, M.; Bowler, J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991, 51, 3867–3873. [Google Scholar]
- Xu, H.; Yu, S.; Liu, Q.; Yuan, X.; Mani, S.; Pestell, R.G.; Wu, K. Recent advances of highly selective CDK4/6 inhibitors in breast cancer. J. Hematol. Oncol. 2017, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Xu, J.; Sun, T. Cyclin-Dependent Kinases 4/6 Inhibitors in Breast Cancer: Current Status, Resistance, and Combination Strategies. J. Cancer 2019, 10, 5504–5517. [Google Scholar] [CrossRef]
- Li, X.; Dai, D.; Chen, B.; Tang, H.; Xie, X.; Wei, W. Efficacy of PI3K/AKT/mTOR pathway inhibitors for the treatment of advanced solid cancers: A literature-based meta-analysis of 46 randomised control trials. PLoS ONE 2018, 13, e0192464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krop, I.E.; Mayer, I.A.; Ganju, V.; Dickler, M.; Johnston, S.; Morales, S.; Yardley, D.A.; Melichar, B.; Forero-Torres, A.; Lee, S.C.; et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016, 17, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Rodon, J.; Braña, I.; Siu, L.L.; De Jonge, M.J.; Homji, N.; Mills, D.; Di Tomaso, E.; Sarr, C.; Trandafir, L.; Massacesi, C.; et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2014, 32, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Folkes, A.J.; Ahmadi, K.; Alderton, W.K.; Alix, S.; Baker, S.J.; Box, G.; Chuckowree, I.S.; Clarke, P.A.; Depledge, P.; Eccles, S.A.; et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J. Med. Chem. 2008, 51, 5522–5532. [Google Scholar] [CrossRef] [PubMed]
- Sarker, D.; Ang, J.E.; Baird, R.; Kristeleit, R.; Shah, K.; Moreno, V.; Clarke, P.A.; Raynaud, F.I.; Levy, G.; Ware, J.A.; et al. First-in-Human Phase I Study of Pictilisib (GDC-0941), a Potent Pan{\textendash}Class I Phosphatidylinositol-3-Kinase (PI3K) Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2015, 21, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, G.I.; Rodon, J.; Bedell, C.; Kwak, E.L.; Baselga, J.; Braña, I.; Pandya, S.S.; Scheffold, C.; Laird, A.D.; Nguyen, L.T.; et al. Phase I Safety, Pharmacokinetic, and Pharmacodynamic Study of SAR245408 (XL147), an Oral Pan-Class I PI3K Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2014, 20, 233–245. [Google Scholar] [CrossRef] [Green Version]
- Blackwell, K.; Burris, H.; Gomez, P.; Lynn Henry, N.; Isakoff, S.; Campana, F.; Gao, L.; Jiang, J.; Macé, S.; Tolaney, S.M. Phase I/II dose-escalation study of PI3K inhibitors pilaralisib or voxtalisib in combination with letrozole in patients with hormone-receptor-positive and HER2-negative metastatic breast cancer refractory to a non-steroidal aromatase inhibitor. Breast Cancer Res. Treat. 2015, 154, 287–297. [Google Scholar] [CrossRef]
- Albanell, J.; Baselga, J. Trastuzumab, a humanized anti-HER2 monoclonal antibody, for the treatment of breast cancer. Drugs Today 1999, 35, 931–946. [Google Scholar]
- Saura, C.; Bendell, J.; Jerusalem, G.; Su, S.; Ru, Q.; De Buck, S.; Mills, D.; Ruquet, S.; Bosch, A.; Urruticoechea, A.; et al. Phase Ib study of Buparlisib plus Trastuzumab in patients with HER2-positive advanced or metastatic breast cancer that has progressed on Trastuzumab-based therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 1935–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolaney, S.; Burris, H.; Gartner, E.; Mayer, I.A.; Saura, C.; Maurer, M.; Ciruelos, E.; Garcia, A.A.; Campana, F.; Wu, B.; et al. Phase I/II study of pilaralisib (SAR245408) in combination with trastuzumab or trastuzumab plus paclitaxel in trastuzumab-refractory HER2-positive metastatic breast cancer. Breast Cancer Res. Treat. 2015, 149, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Morii, N.; Yamashiro, H. Pertuzumab in the treatment of HER2-positive breast cancer: An evidence-based review of its safety, efficacy, and place in therapy. Core Evid. 2019, 14, 51–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, A.; Delaloge, S.; Holmes, F.A.; Moy, B.; Iwata, H.; Harvey, V.J.; Robert, N.J.; Silovski, T.; Gokmen, E.; von Minckwitz, G.; et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016, 17, 367–377. [Google Scholar] [CrossRef]
- Mukai, H.; Saeki, T.; Aogi, K.; Naito, Y.; Matsubara, N.; Shigekawa, T.; Ueda, S.; Takashima, S.; Hara, F.; Yamashita, T.; et al. Patritumab plus trastuzumab and paclitaxel in human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. Cancer Sci. 2016, 107, 1465–1470. [Google Scholar] [CrossRef]
- Earl, H.M.; Hiller, L.; Dunn, J.A.; Blenkinsop, C.; Grybowicz, L.; Vallier, A.-L.; Abraham, J.; Thomas, J.; Provenzano, E.; Hughes-Davies, L.; et al. Efficacy of neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin, and cyclophosphamide, for women with HER2-negative early breast cancer (ARTemis): An open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 656–666. [Google Scholar] [CrossRef] [Green Version]
- Verret, B.; Cortes, J.; Bachelot, T.; Andre, F.; Arnedos, M. Efficacy of PI3K inhibitors in advanced breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, x12–x20. [Google Scholar] [CrossRef]
- Peddi, P.F.; Hurvitz, S.A. Ado-trastuzumab emtansine (T-DM1) in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2014, 6, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Keam, B.; Hahn, S.; Ock, C.-Y.; Kim, M.; Kim, T.M.; Kim, D.-W.; Heo, D.S. First-line Pembrolizumab versus Pembrolizumab Plus Chemotherapy Versus Chemotherapy Alone in Non-small-cell Lung Cancer: A Systematic Review and Network Meta-analysis. Clin. Lung Cancer 2019, 20, 331–338.e4. [Google Scholar] [CrossRef]
- Kang, C.; Syed, Y.Y. Atezolizumab (in Combination with Nab-Paclitaxel): A Review in Advanced Triple-Negative Breast Cancer. Drugs 2020, 80, 601–607. [Google Scholar] [CrossRef]
- Huerta-Reyes, M.; Maya-Núñez, G.; Pérez-Solis, M.A.; López-Muñoz, E.; Guillén, N.; Olivo-Marin, J.-C.; Aguilar-Rojas, A. Treatment of Breast Cancer With Gonadotropin-Releasing Hormone Analogs. Front. Oncol. 2019, 9, 943. [Google Scholar] [CrossRef] [Green Version]
- Schally, A.V.; Engel, J.B.; Pinski, J.; Block, N.L. Chapter 73—LHRH Analogs. In Handbook of Biologically Active Peptides, 2nd ed.; Academic Press: Boston, MA, USA, 2013; pp. 531–540. ISBN 978-0-12-385095-9. [Google Scholar]
- Weir, H.M.; Bradbury, R.H.; Lawson, M.; Rabow, A.A.; Buttar, D.; Callis, R.J.; Curwen, J.O.; de Almeida, C.; Ballard, P.; Hulse, M.; et al. AZD9496: An oral estrogen receptor inhibitor that blocks the growth of ER-positive and ESR1-mutant breast tumors in preclinical models. Cancer Res. 2016, 76, 3307–3318. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Yu, S.; Dong, D.; Lee, L.T.O. G Protein-Coupled Estrogen Receptor: A Potential Therapeutic Target in Cancer. Front. Endocrinol. 2019, 10, 725. [Google Scholar] [CrossRef] [Green Version]
- Lykkesfeldt, A.E.; Larsen, S.S.; Briand, P. Human breast cancer cell lines resistant to pure anti-estrogens are sensitive to tamoxifen treatment. Int. J. Cancer 1995, 61, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K.; Coronado-Heinsohn, E.B.; Hilsenbeck, S.G.; McCue, B.L.; Wakeling, A.E.; McClelland, R.A.; Manning, D.L.; Nicholson, R.I. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J. Natl. Cancer Inst. 1995, 87, 746–750. [Google Scholar] [CrossRef]
- Dowsett, M.; Nicholson, R.I.; Pietras, R.J. Biological characteristics of the pure antiestrogen fulvestrant: Overcoming endocrine resistance. Breast Cancer Res. Treat. 2005, 93, S11. [Google Scholar] [CrossRef] [PubMed]
- Michaud, L.B.; Jones, K.L.; Buzdar, A.U. Combination endocrine therapy in the management of breast cancer. Oncologist 2001, 6, 538–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergh, J.; Jönsson, P.-E.; Lidbrink, E.K.; Trudeau, M.; Eiermann, W.; Brattström, D.; Lindemann, J.P.O.; Wiklund, F.; Henriksson, R. FACT: An open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 1919–1925. [Google Scholar] [CrossRef]
- Mehta, R.S.; Barlow, W.E.; Albain, K.S.; Vandenberg, T.A.; Dakhil, S.R.; Tirumali, N.R.; Lew, D.L.; Hayes, D.F.; Gralow, J.R.; Livingston, R.B.; et al. Combination Anastrozole and Fulvestrant in Metastatic Breast Cancer. N. Engl. J. Med. 2012, 367, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Johnston, S.R.; Kilburn, L.S.; Ellis, P.; Dodwell, D.; Cameron, D.; Hayward, L.; Im, Y.-H.; Braybrooke, J.P.; Brunt, A.M.; Cheung, K.-L.; et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): A composite, multicent. Lancet Oncol. 2013, 14, 989–998. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xu, D.; Li, X.; Zhang, J.; Xu, W.; Hou, J.; Zhang, W.; Tang, J. Latest Overview of the Cyclin-Dependent Kinases 4/6 Inhibitors in Breast Cancer: The Past, the Present and the Future. J. Cancer 2019, 10, 6608–6617. [Google Scholar] [CrossRef]
- Tan, A.R.; Yang, X.; Berman, A.; Zhai, S.; Sparreboom, A.; Parr, A.L.; Chow, C.; Brahim, J.S.; Steinberg, S.M.; Figg, W.D.; et al. Phase I Trial of the Cyclin-Dependent Kinase Inhibitor Flavopiridol in Combination with Docetaxel in Patients with Metastatic Breast Cancer. Clin. Cancer Res. 2004, 10, 5038–5047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barroso-Sousa, R.; Shapiro, G.I.; Tolaney, S.M. Clinical Development of the CDK4/6 Inhibitors Ribociclib and Abemaciclib in Breast Cancer. Breast Care 2016, 11, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, pha. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef] [Green Version]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [Green Version]
- Beser, A.R.; Tuzlali, S.; Guzey, D.; Dolek Guler, S.; Hacihanefioglu, S.; Dalay, N. HER-2, TOP2A and chromosome 17 alterations in breast cancer. Pathol. Oncol. Res. 2007, 13, 180–185. [Google Scholar] [CrossRef]
- Yarden, Y. Biology of HER2 and its importance in breast cancer. Oncology 2001, 61, 1–13. [Google Scholar] [CrossRef]
- Witton, C.J.; Reeves, J.R.; Going, J.J.; Cooke, T.G.; Bartlett, J.M.S. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J. Pathol. 2003, 200, 290–297. [Google Scholar] [CrossRef]
- Cismowski, M. Tyrosine Kinase Inhibitors. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–4. ISBN 9780080552323. [Google Scholar]
- Xuhong, J.-C.; Qi, X.-W.; Zhang, Y.; Jiang, J. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am. J. Cancer Res. 2019, 9, 2103–2119. [Google Scholar]
- Guan, Z.; Xu, B.; DeSilvio, M.L.; Shen, Z.; Arpornwirat, W.; Tong, Z.; Lorvidhaya, V.; Jiang, Z.; Yang, J.; Makhson, A.; et al. Randomized trial of lapatinib versus placebo added to paclitaxel in the treatment of human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 1947–1953. [Google Scholar] [CrossRef]
- Chien, A.J.; Rugo, H.S. Tyrosine Kinase Inhibitors for Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer: Is Personalizing Therapy Within Reach? J. Clin. Oncol. 2017, 35, 3089–3091. [Google Scholar] [CrossRef]
- Li, X.; Yang, C.; Wan, H.; Zhang, G.; Feng, J.; Zhang, L.; Chen, X.; Zhong, D.; Lou, L.; Tao, W.; et al. Discovery and development of pyrotinib: A novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 110, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Hudis, C.A. Trastuzumab—Mechanism of Action and Use in Clinical Practice. N. Engl. J. Med. 2007, 357, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamczyk, A.; Niemiec, J.; Janecka, A.; Harazin-Lechowska, A.; Ambicka, A.; Grela-Wojewoda, A.; Domagała-Haduch, M.; Cedrych, I.; Majchrzyk, K.; Kruczak, A.; et al. Prognostic value of PIK3CA mutation status, PTEN and androgen receptor expression for metastasis-free survival in HER2-positive breast cancer patients treated with trastuzumab in adjuvant setting. Pol. J. Pathol. Off. J. Pol. Soc. Pathol. 2015, 66, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Luo, C.; Zhong, X.; Wang, Z.; Wang, Y.; Wang, Y.; He, P.; Peng, Q.; Zheng, H. Prognostic nomogram for patients with non-metastatic HER2 positive breast cancer in a prospective cohort. Int. J. Biol. Mark. 2019, 34, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, C.W.; Allison, D.E.; Flagella, K.; Presta, L.; Clarke, J.; Dybdal, N.; McKeever, K.; Sliwkowski, M.X. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol. Immunother. 2006, 55, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.C.; Carey, K.D.; Vajdos, F.F.; Leahy, D.J.; de Vos, A.M.; Sliwkowski, M.X. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004, 5, 317–328. [Google Scholar] [CrossRef] [Green Version]
- Dawson, S.J.; Provenzano, E.; Caldas, C. Triple negative breast cancers: Clinical and prognostic implications. Eur. J. Cancer 2009, 45, 27–40. [Google Scholar] [CrossRef]
- Anders, C.; Carey, L.A. Understanding and treating triple-negative breast cancer. Oncology 2008, 22, 1233–1243. [Google Scholar] [PubMed]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Vanderplas, A.; Hughes, M.E.; Theriault, R.L.; Edge, S.B.; Wong, Y.-N.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; Weeks, J.C. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012, 118, 5463–5472. [Google Scholar] [CrossRef] [Green Version]
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent Advances in the Treatment of Breast Cancer. Front. Oncol. 2018, 8, 227. [Google Scholar] [CrossRef] [Green Version]
- Tutt, A.; Tovey, H.; Cheang, M.C.U.; Kernaghan, S.; Kilburn, L.; Gazinska, P.; Owen, J.; Abraham, J.; Barrett, S.; Barrett-Lee, P.; et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT Trial. Nat. Med. 2018, 24, 628–637. [Google Scholar] [CrossRef] [Green Version]
- Bardia, A.; Mayer, I.; Vahdat, L.; Tolaney, S.; Isakoff, S.; Diamond, J.; O’Shaughnessy, J.; Moroose, R.; Santin, A.; Abramson, V.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Aschenbrenner, D.S. New Drug Approved for HER2-positive Metastatic Breast Cancer. Am. J. Nurs. 2020, 120, 23. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, S.; Reddy, R.C.J.; Maheswaran, T.; Asokan, G.S.; Dany, A.; Anand, B. Theranostics: A treasured tailor for tomorrow. J. Pharm. Bioallied Sci. 2014, 6, S6–S8. [Google Scholar] [CrossRef]
- Shah, J.V.; Gonda, A.; Pemmaraju, R.; Subash, A.; Bobadilla Mendez, C.; Berger, M.; Zhao, X.; He, S.; Riman, R.E.; Tan, M.C.; et al. Shortwave Infrared-Emitting Theranostics for Breast Cancer Therapy Response Monitoring. Front. Mol. Biosci. 2020, 7, 287. [Google Scholar] [CrossRef]
- Bartelink, I.H.; Jones, E.F.; Shahidi-Latham, S.K.; Lee, P.R.E.; Zheng, Y.; Vicini, P.; van ’t Veer, L.; Wolf, D.; Iagaru, A.; Kroetz, D.L.; et al. Tumor Drug Penetration Measurements Could Be the Neglected Piece of the Personalized Cancer Treatment Puzzle. Clin. Pharmacol. Ther. 2019, 106, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Tannock, I.F. Conventional cancer therapy: Promise broken or promise delayed? Lancet 1998, 351, SII9–SII16. [Google Scholar] [CrossRef]
- Ahn, B.-C. Personalized Medicine Based on Theranostic Radioiodine Molecular Imaging for Differentiated Thyroid Cancer. Biomed. Res. Int. 2016, 2016, 1680464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, V.; Kutty, R.V. Recent advances in nanotheranostics for triple negative breast cancer treatment. J. Exp. Clin. Cancer Res. 2019, 38, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engebraaten, O.; Vollan, H.K.M.; Børresen-Dale, A.-L. Triple-negative breast cancer and the need for new therapeutic targets. Am. J. Pathol. 2013, 183, 1064–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumer, B.; Gao, J. Theranostic nanomedicine for cancer. Nanomedicine 2008, 3, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Gregoriou, Y.; Gregoriou, G.; Yilmaz, V.; Kapnisis, K.; Prokopi, M.; Anayiotos, A.; Strati, K.; Dietis, N.; Constantinou, A.I.; Andreou, C. Resveratrol loaded polymeric micelles for theranostic targeting of breast cancer cells. Nanotheranostics 2020, 5, 113–124. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Chen, G.; Li, Y.; Xu, W.; Gong, S. Quantum-Dot-Based Theranostic Micelles Conjugated with an Anti-EGFR Nanobody for Triple-Negative Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 30297–30305. [Google Scholar] [CrossRef] [PubMed]
- Parhi, P.; Sahoo, S.K. Trastuzumab guided nanotheranostics: A lipid based multifunctional nanoformulation for targeted drug delivery and imaging in breast cancer therapy. J. Colloid Interface Sci. 2015, 451, 198–211. [Google Scholar] [CrossRef]
- Hafner, S.; Raabe, M.; Wu, Y.; Wang, T.; Zuo, Z.; Rasche, V.; Syrovets, T.; Weil, T.; Simmet, T. High-Contrast Magnetic Resonance Imaging and Efficient Delivery of an Albumin Nanotheranostic in Triple-Negative Breast Cancer Xenografts. Adv. Ther. 2019, 2, 1900084. [Google Scholar] [CrossRef]
- Li, J.; Cai, P.; Shalviri, A.; Henderson, J.T.; He, C.; Foltz, W.D.; Prasad, P.; Brodersen, P.M.; Chen, Y.; DaCosta, R.; et al. A Multifunctional Polymeric Nanotheranostic System Delivers Doxorubicin and Imaging Agents across the Blood–Brain Barrier Targeting Brain Metastases of Breast Cancer. ACS Nano 2014, 8, 9925–9940. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fu, J.; Wang, X.; Chen, Q.; Zhang, W.; Cao, Y.; Ran, H. Biomimetic “Nanoplatelets” as a Targeted Drug Delivery Platform for Breast Cancer Theranostics. ACS Appl. Mater. Interfaces 2021, 13, 3605–3621. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Yang, H.; Wan, C.; Zheng, D.; Zhou, Z.; Xie, S.; Xu, L.; Du, J.; Li, F. Her2-Functionalized Gold-Nanoshelled Magnetic Hybrid Nanoparticles: A Theranostic Agent for Dual-Modal Imaging and Photothermal Therapy of Breast Cancer. Nanoscale Res. Lett. 2019, 14, 235. [Google Scholar] [CrossRef] [Green Version]
- Brabec, V.; Nováková, O. DNA binding mode of ruthenium complexes and relationship to tumor cell toxicity. Coordination Chem. Rev. 2006, 9, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Shen, J.; Liu, J.; Zeng, L.; Jin, L.; Weng, C. Spectral characteristics, DNA-binding and cytotoxicity of two functional Ru (II) mixed-ligand complexes. Dalton Trans. 2012, 41, 4575–4587. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Kim, H.-C.; Wolfram, J.; Mu, C.; Zhang, W.; Liu, H.; Xie, Y.; Mai, J.; Zhang, H.; Li, Z.; et al. A Liposome Encapsulated Ruthenium Polypyridine Complex as a Theranostic Platform for Triple-Negative Breast Cancer. Nano Lett. 2017, 17, 2913–2920. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.; Wan, C.; Yang, H.; Xu, L.; Dong, Q.; Du, C.; Du, J.; Li, F. Her2-Targeted Multifunctional Nano-Theranostic Platform Mediates Tumor Microenvironment Remodeling and Immune Activation for Breast Cancer Treatment. Int. J. Nanomed. 2020, 15, 10007–10028. [Google Scholar] [CrossRef]
- Tang, L.; Yang, X.; Yin, Q.; Cai, K.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J.A.; et al. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Xiao, W.; Hu, C.; Xie, R.; Gao, H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J. Control. Release Off. J. Control. Release Soc. 2018, 278, 127–139. [Google Scholar] [CrossRef]
- Liu, R.; Hu, C.; Yang, Y.; Zhang, J.; Gao, H. Theranostic nanoparticles with tumor-specific enzyme-triggered size reduction and drug release to perform photothermal therapy for breast cancer treatment. Acta Pharm. Sin. B 2019, 9, 410–420. [Google Scholar] [CrossRef]
- Burke, A.R.; Singh, R.N.; Carroll, D.L.; Wood, J.C.S.; D’Agostino, R.B.; Ajayan, P.M.; Torti, F.M.; Torti, S.V. The resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapy. Biomaterials 2012, 33, 2961–2970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Wang, H.; Gao, F.; Xu, Z.; Dai, F.; Liu, W. An Injectable Supramolecular Polymer Nanocomposite Hydrogel for Prevention of Breast Cancer Recurrence with Theranostic and Mammoplastic Functions. Adv. Funct. Mater. 2018, 28, 1801000. [Google Scholar] [CrossRef]

| Imaging Modality | Principle | Diagnostic Accuracy | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Mammography (First-line tool for breast screening) | Low-dose ionizing x-ray creates detailed images of the breast. | Sensitivity: 75–90% Specificity: 90–95% Spatial Resolution: 50 µm | Most cost-effective. Good response with high specificity and sensitivity. Portable device. | Uses ionizing radiation. Sensitivity decreases with increasing breast density. Accuracy is low in young women. High false-positive results in young women due to dense breasts. Poor contrast compared to MRI. | [9,10,11,12] |
| Magnetic Resonance Imaging | Uses low-energy radio waves and strong magnets to obtain detailed images of structures within the breast | Sensitivity: 75–100% Specificity: 83–98.4% Spatial Resolution: 25–100 µm | Ability to detect breast malignancies that often escape from clinical, mammograms, and ultrasound detection. | Expensive, inability to standardize the test. Unnecessary breast biopsies due to inability to distinguish between malignant and benign lesions. | [13,14] |
| Magnetic Resonance Spectroscopy | Employs magnetic field on body fluids and tissue samples to obtain chemical information of that region | Sensitivity: 93% Specificity: 70% Spatial Resolution: up to 0.25 cm3 | Overcomes limitations of mammography. Radiation-free imaging technology. Excellent Sensitivity. Excellent spatial resolution. All imaging planes possible. | Expensive and time-consuming. Low specificity. Not portable. False-positive results in some benign tumors. | [15,16,17,18] |
| Dynamic Contrast Enhanced MRI (DCE-MRI) | Multiple MRI scans taken post i.v. injection of contrast agent | Sensitivity: 89–99% Specificity: 37–86% Spatial Resolution: 25–100 µm | Exhibits good performance in monitoring response post therapy. | False-negative results observed due to artifacts based on bleeding and tumor structure. | [14,19,20,21] |
| Diffusion-Weighted Imaging | Employs diffusion of water molecules to generate contrast. | Sensitivity: 83% Specificity: 84% Spatial Resolution: 25–100 µm | Non-radioactive imaging technique | Failure to detect high water content malignant lesions due to high apparent diffusion coefficients. | [14,22] |
| MR Elastography (MRE) | Dynamic elasticity imaging technique that combines MRI imaging with low frequency. Employs mechanical waves to create an elastogram to assess tissue stiffness. | Sensitivity: 90–100% Specificity: 37–80% Spatial Resolution: 25–100 µm | Non-invasive, non-ionizing and cross-sectional imaging modality | Lacking in spatial resolution and detection of small focal lesions. | [14,23,24] |
| Positron Emission Tomography conjugated with computed Tomography (PET-CT) | Combines nuclear medicine technique and computed tomography resulting in high detailed images. | Sensitivity: 90–100% Specificity: 75–90% Spatial Resolution: 2–10 mm | Non-invasive. Provides twice the diagnostic benefits (Elevated activity within the body detected by PET scan and intricate images of tissues and organs by CT scan). | High-cost. Unable to detect tumors less than 8 mm. | [14,23] |
| Sentinel lymph node biopsy (SLNB) | Surgical procedure to detect spreading of cancer in lymphatic system. | Sensitivity: 90.5% Specificity: 85.7% Spatial Resolution: Not Applicable | Significantly reduces post-operative complications | Not useful for patients with locally advanced cancers and inflammatory breast cancer. | [24,25] |
| Breast Specific Gamma Imaging | Employs use of a radiotracer. Image captured using a special camera. | Sensitivity: 90–96% Specificity: 71–80% Spatial Resolution: ≥7 mm | Able to identify smaller lesions (<1 cm) | High radiation dose. Not suited for routine tumor screening. | [26,27,28] |
| Ultrasound | Employs sound waves to image breast tissues | Sensitivity: 80–89% Specificity: 34–88% Spatial Resolution: 50–500 µm | Accessible, real-time lesion visualization, cost-effective, patient compliant. | Failure to detect microcalcifications, possibility of false-positives. | [14,29,30] |
| Drug | Drug Class | Subtype of Breast Cancer Treated | Status | References | |
|---|---|---|---|---|---|
| 1 | Tamoxifen (Brand name: Nolvadex) | Anti-estrogen | ER-positive breast cancer | Approved | [111,112,113] |
| 2 | Aminoglutethimide, Fadrozole and Vorozole | First- and second-generation AIs | ER-positive breast cancer | Approved | [116] |
| 3 | Anastrozole (Brand name: Arimidex) | Third generation AIs | ER-positive breast cancer | Approved | [118,119,120] |
| 4 | Letrozole (Brand name: Femara) | ||||
| 5 | Exemestane (Brand name: Aromasin) | ||||
| 6 | Goserelin and Leuprolide | - | LH-RH sensitive breast cancer | Approved | [121] |
| 7 | Fulvestrant | SERD Degrader | Breast cancer | Approved | [122,123] |
| 8 | Ribociclib (LEE011) | CDK4/6 inhibitor | Epidermal growth factor receptor 2-negative advanced or metastatic breast cancer | Approved | [124,125,126] |
| 9 | Palbociclib (PD0332991) | Approved | |||
| 10 | Abemaciclib (LY2835219) | Approved | |||
| 11 | Buparlisib | pan-PI3Ki | (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer | Approved | [127,128] |
| 12 | Pictilisib | PI3K inhibitor | HR+/HER− advanced breast cancer | Phase I clinical trial | [129,130] |
| 13 | Pilaralisib (XL147) | Phase I/II dose-escalation study | [131,132] | ||
| 14 | Voxtalisib | Phase I/II dose-escalation study | [132] | ||
| 15 | Trastuzumab (Herceptin) | mAb | HER2-overexpressing breast cancer | Approved | [133,134,135] |
| 16 | Pertuzumab | Approved | [136] | ||
| 17 | Neratinib | TKI | advanced or metastatic HER2+ breast cancer | Approved | [137] |
| 18 | Patritumab | anti-HER3 mAb | HER2+ advanced breast cancer | Preclinical models | [138] |
| 19 | Bevacizumab | anti-GF mAb | TNBC patients with germline mutations/ HER2-negative breast cancer | Approved | [139] |
| 20 | Sacituzumab govitecan-hziy | Antibody–drug conjugate | Relapsed or refractory metastatic TNBC | Approved | [140] |
| 21 | T-DM1 (Kadcyla) | Antibody–drug conjugate | HER-2 metastatic prescription adjuvant treatment when the patient has taken neoadjuvant treatment with trastuzumab (Herceptin) and a taxane | Approved | [141] |
| 22 | Enhertu | Antibody–drug conjugate | HER-2 metastatic that has resurged and cannot be removed surgically | Approved | [142] |
| 23 | Pembrolizumab (Brand name: Keytruda) | IgG4-ĸ mAb | metastatic TNBC or TNBC that has resurged and cannot be surgically removed | Approved | [143] |
| 24 | Atezolizumab combination with nab-paclitaxel | mAb | PD-L1+ TNBC | Approved | [144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhushan, A.; Gonsalves, A.; Menon, J.U. Current State of Breast Cancer Diagnosis, Treatment, and Theranostics. Pharmaceutics 2021, 13, 723. https://doi.org/10.3390/pharmaceutics13050723
Bhushan A, Gonsalves A, Menon JU. Current State of Breast Cancer Diagnosis, Treatment, and Theranostics. Pharmaceutics. 2021; 13(5):723. https://doi.org/10.3390/pharmaceutics13050723
Chicago/Turabian StyleBhushan, Arya, Andrea Gonsalves, and Jyothi U. Menon. 2021. "Current State of Breast Cancer Diagnosis, Treatment, and Theranostics" Pharmaceutics 13, no. 5: 723. https://doi.org/10.3390/pharmaceutics13050723






