Recent Advances in pH- or/and Photo-Responsive Nanovehicles
Abstract
:1. Introduction
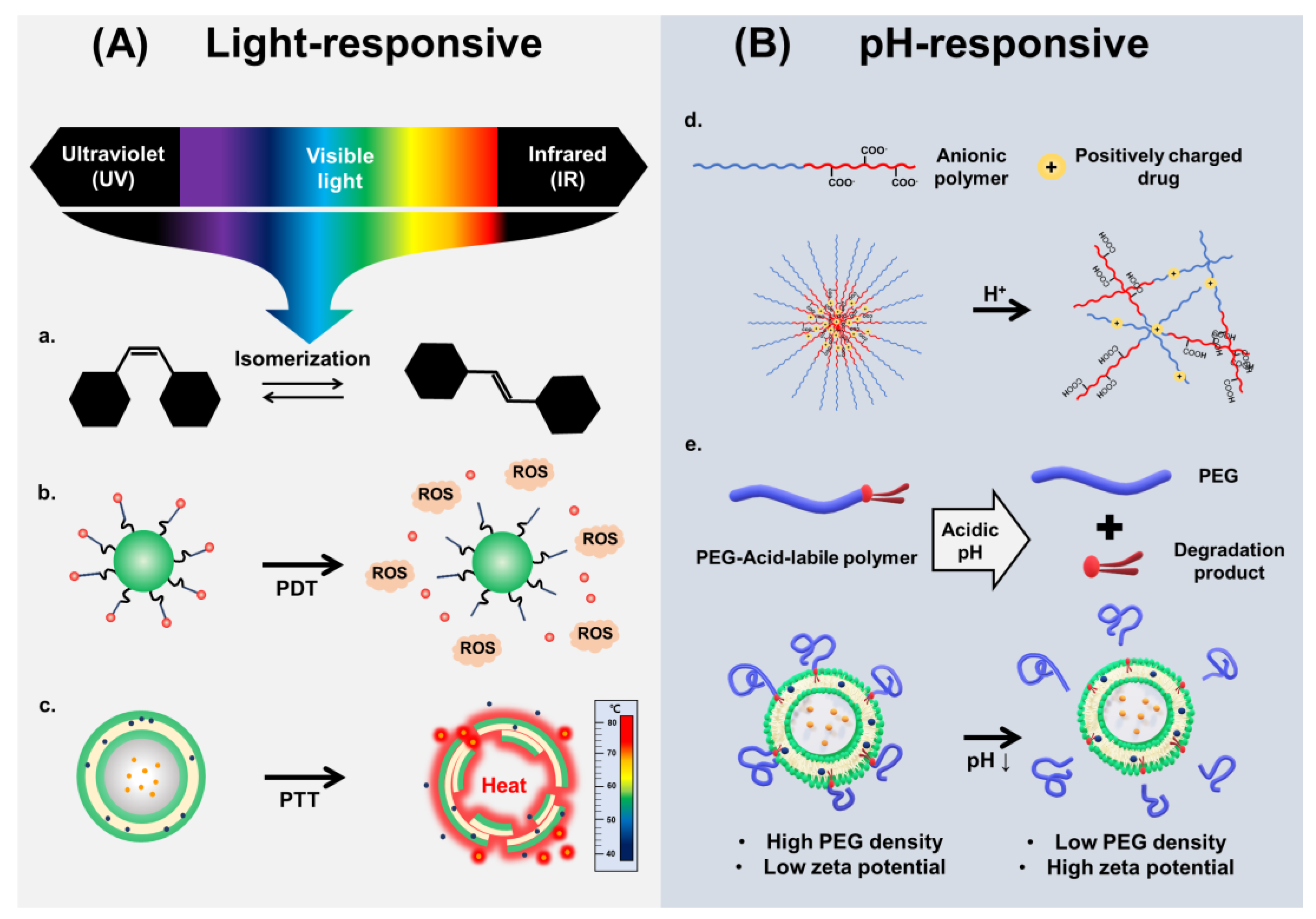
2. Photo-Responsive Nanovehicles
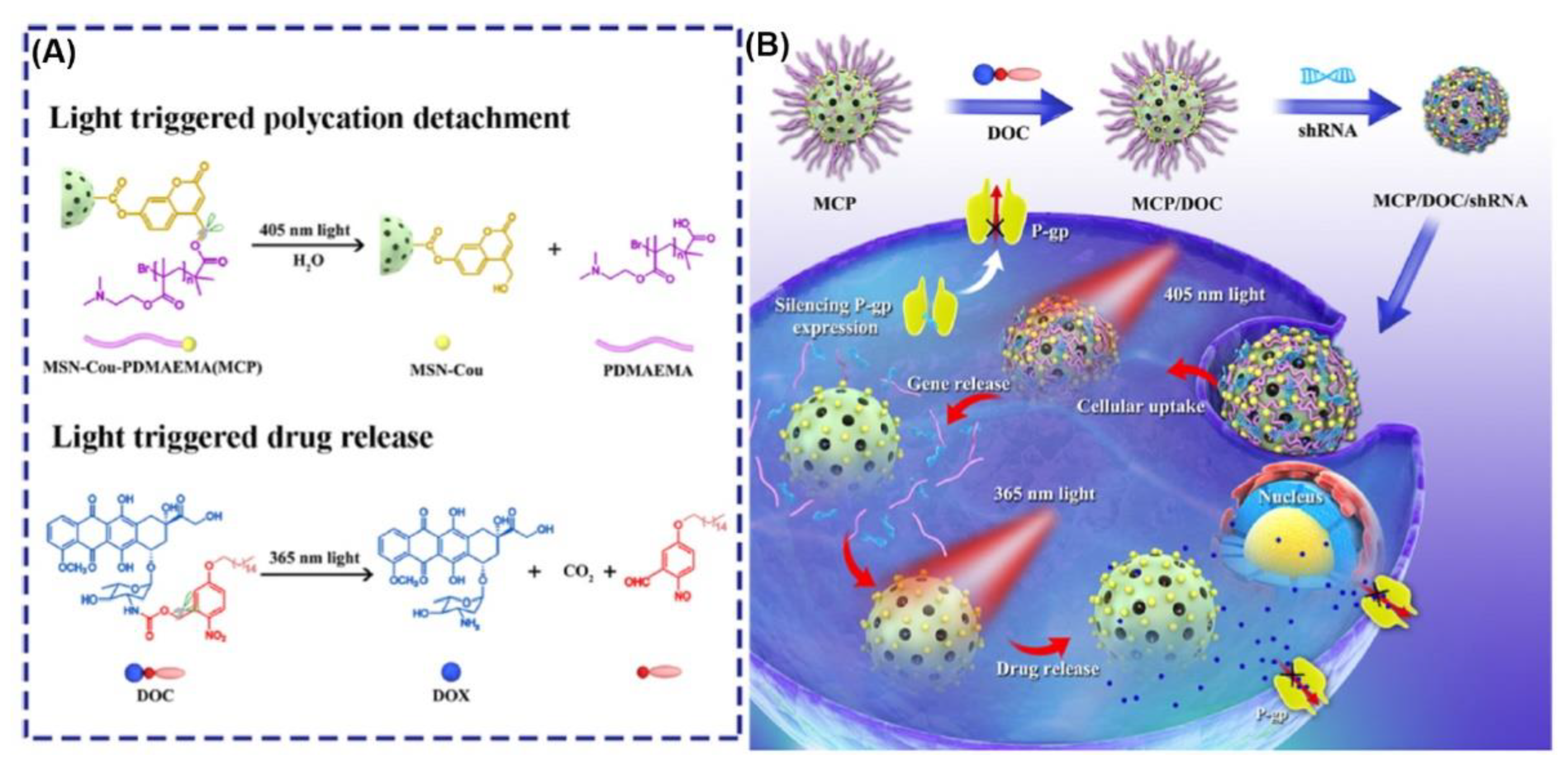
2.1. Photo-Responsive Nanovehicles Using Photo-Induced Chemical Transformation
2.2. Photo-Responsive Nanovehicles Using Photo-Mediated Materials
2.2.1. Photo-Responsive Nanovehicles Using PTT
2.2.2. Photo-Responsive Nanovehicles Using PDT
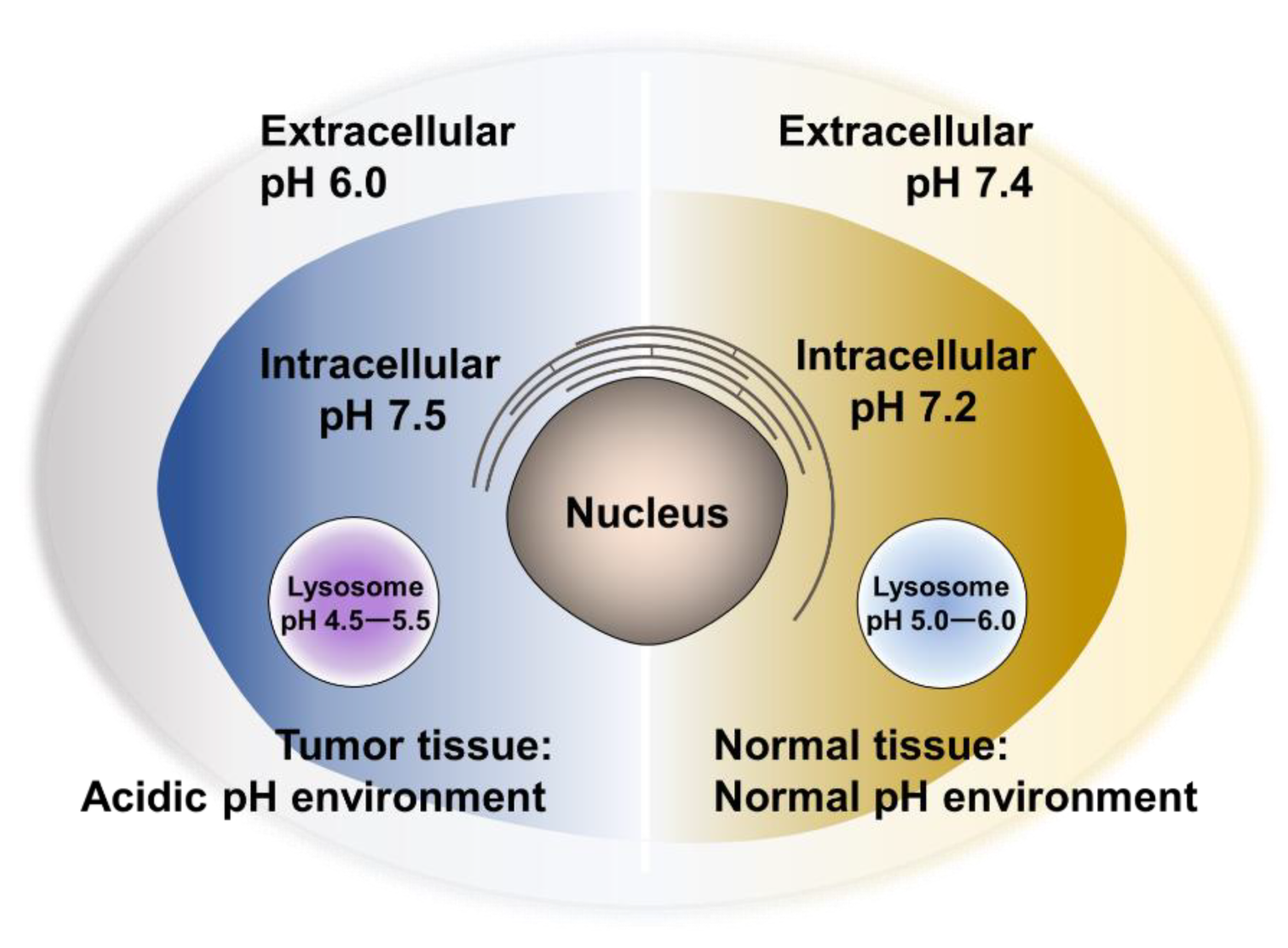
3. pH-Responsive Nanovehicles
3.1. De/Protonation-Based Nanovehicles
3.2. Acid-Labile Bond Cleavage-Based Nanovehicles
3.2.1. C=N Bonds (Carbon–Nitrogen Double-Bonds)
3.2.2. Other Acid-Labile Bonds
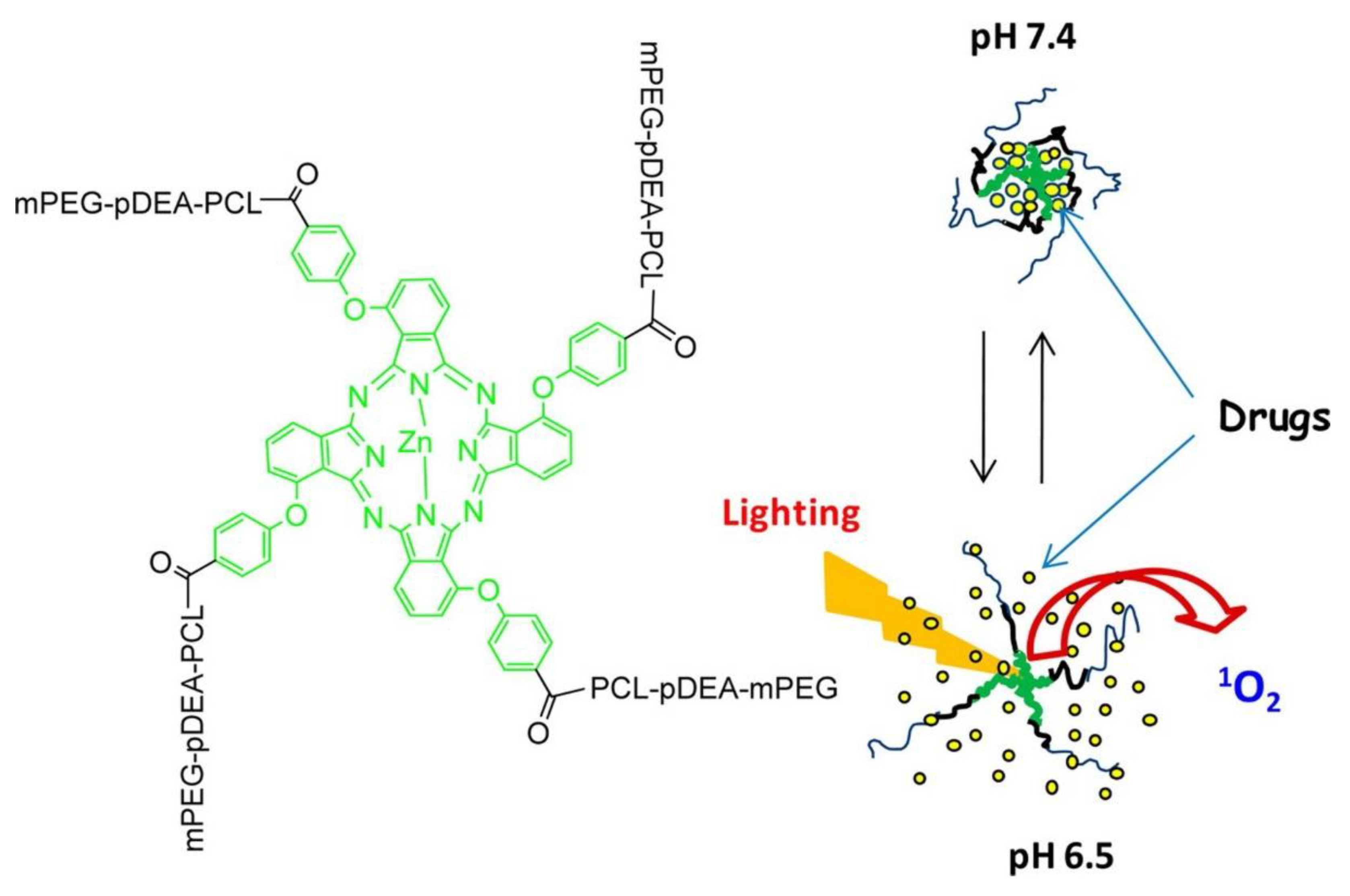
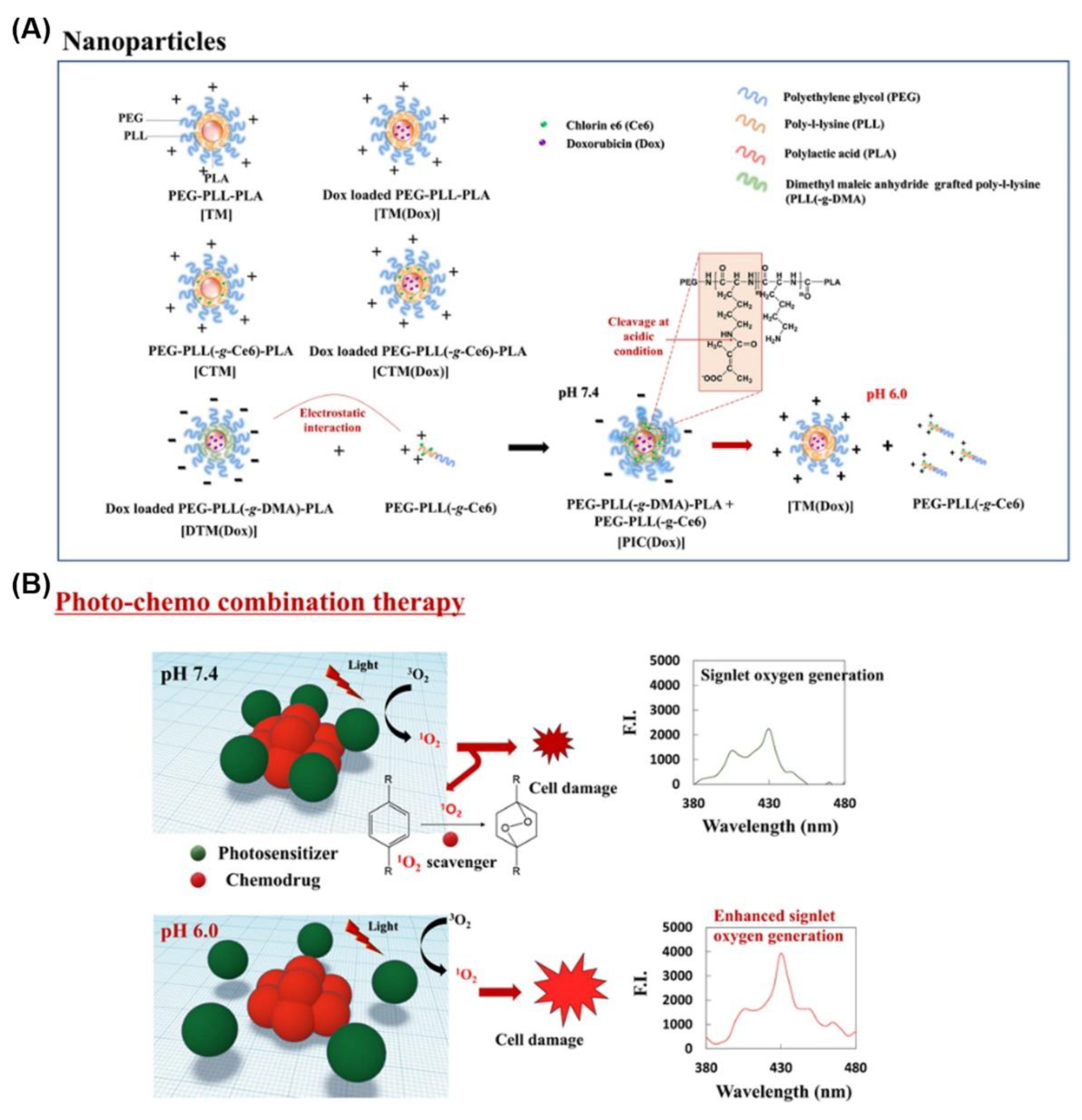
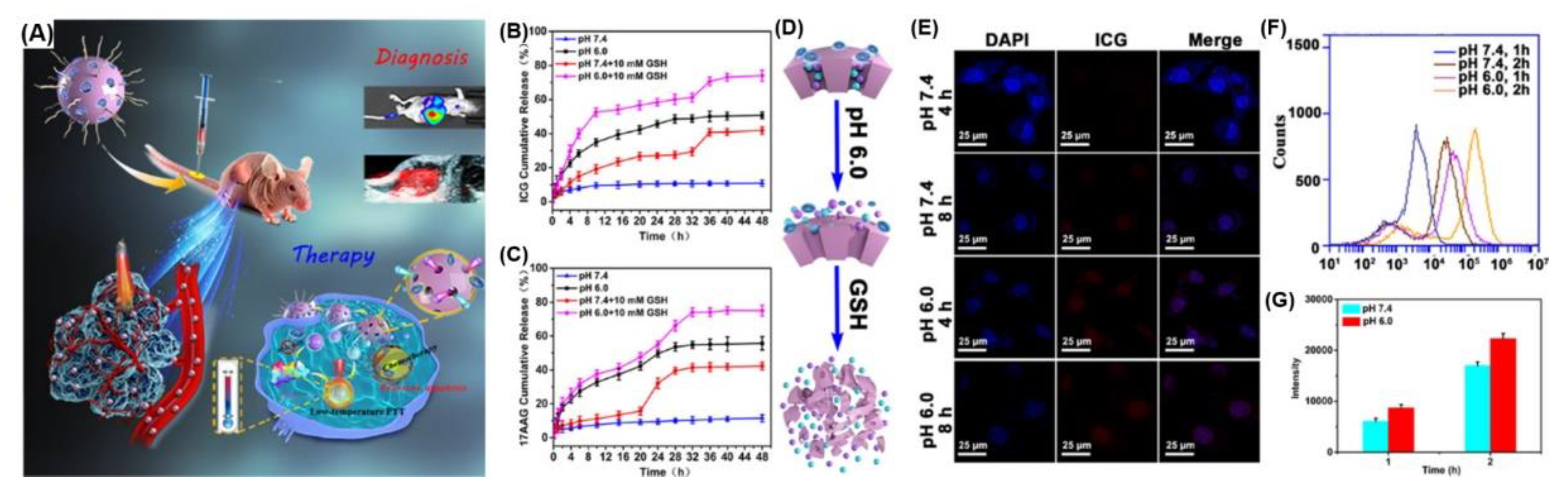
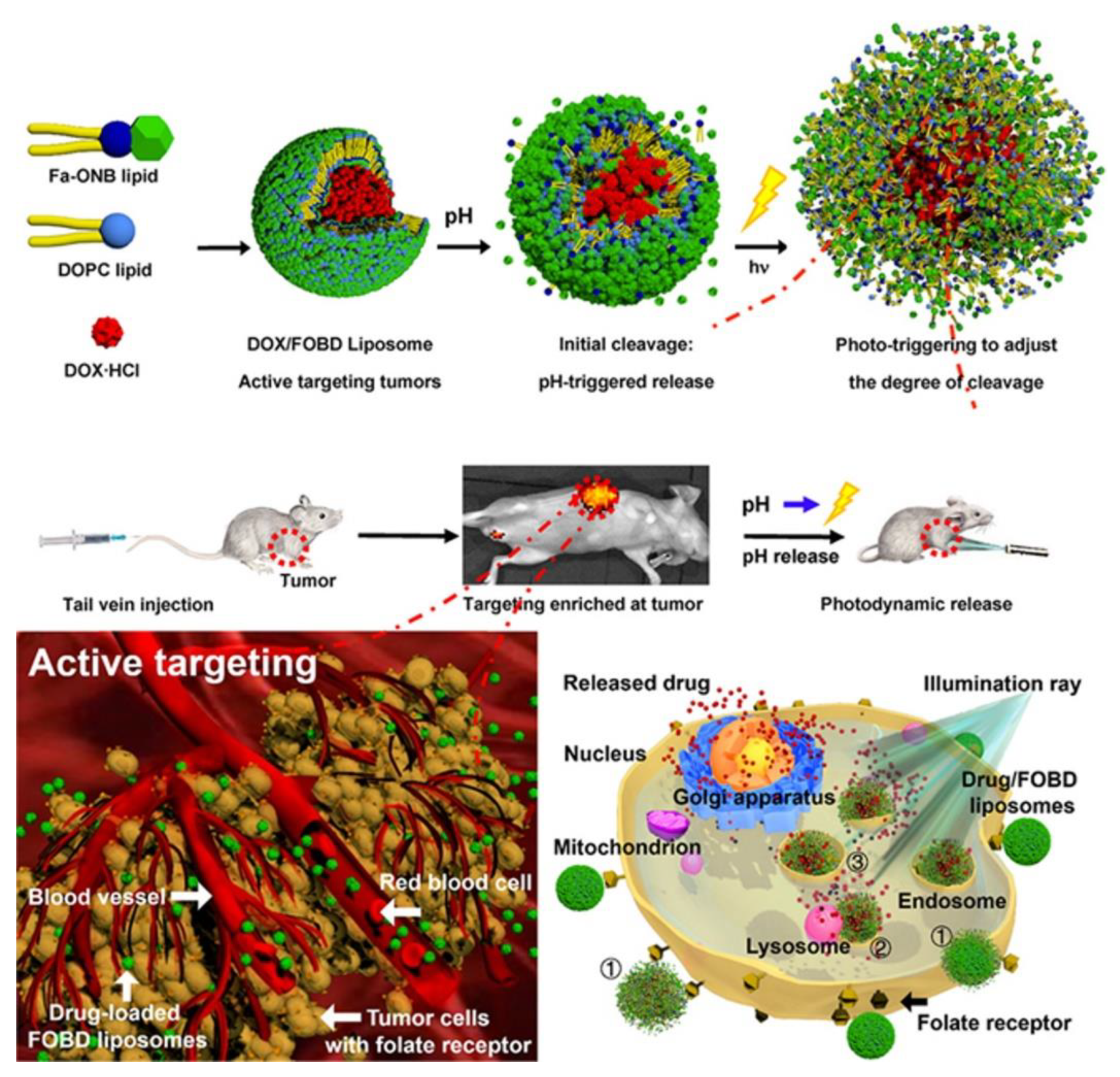
4. Photo- and pH-Dual-Responsive Nanovehicles
4.1. De/Protonation Triggered by Light- and pH-Dual-Responsive Nanovehicles
4.2. Degradation/Cleavage/Breakage Triggered by Light- and pH-Dual-Responsive Nanovehicles
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cao, Y.; DePinho, R.A.; Ernst, M.; Vousden, K. Cancer research: Past, present and future. Nat. Rev. Cancer 2011, 11, 749–754. [Google Scholar]
- Paull, R.; Wolfe, J.; Hébert, P.; Sinkula, M. Investing in nanotechnology. Nat. Biotechnol. 2003, 21, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Lee, S.-E.; Kim, D.-H.; Pyo, Y.-C.; Park, J.-S. Recent advances of nanotechnology for the delivery of anticancer drugs for breast cancer treatment. J. Pharm. Investig. 2020, 50, 261–270. [Google Scholar] [CrossRef]
- Atlihan-Gundogdu, E.; Ilem-Ozdemir, D.; Ekinci, M.; Ozgenc, E.; Demir, E.S.; Sánchez-Dengra, B.; González-Alvárez, I. Recent developments in cancer therapy and diagnosis. J. Pharm. Investig. 2020, 50, 1–13. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly (lactic acid)/poly (lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Kaasgaard, T.; Andresen, T.L. Liposomal cancer therapy: Exploiting tumor characteristics. Expert Opin. Drug Deliv. 2010, 7, 225–243. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, Z.; Liu, X.; Ni, R.; Zhu, X.; Ma, L.; Liu, J. Preparation, safety, pharmacokinetics, and pharmacodynamics of liposomes containing Brucea javanica oil. AAPS Pharmscitech 2010, 11, 878–884. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Malachowski, K.; Breger, J.; Kwag, H.R.; Wang, M.O.; Fisher, J.P.; Selaru, F.M.; Gracias, D.H.J.A.C.I.E. Stimuli-responsive theragrippers for chemomechanical controlled release. Angew. Chem. Int. Ed. 2014, 53, 8045–8049. [Google Scholar] [CrossRef] [PubMed]
- Rwei, A.Y.; Lee, J.-J.; Zhan, C.; Liu, Q.; Ok, M.T.; Shankarappa, S.A.; Langer, R.; Kohane, D.S. Repeatable and adjustable on-demand sciatic nerve block with phototriggerable liposomes. Proc. Natl. Acad. Sci. USA 2015, 112, 15719–15724. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Li, N.; Carter, K.A.; Lin, C.; Geng, J.; Shao, S.; Huang, W.C.; Qin, Y.; Atilla-Gokcumen, G.E.; Lovell, J.F. Rapid light-triggered drug release in liposomes containing small amounts of unsaturated and porphyrin–phospholipids. Small 2016, 12, 3039–3047. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, A.; Arandiyan, H.; Boyer, C.; Lim, M. Lanthanide-doped upconversion nanoparticles: Emerging intelligent light-activated drug delivery systems. Adv. Sci. 2016, 3, 1500437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [Green Version]
- Kiio, T.M.; Park, S. Physical properties of nanoparticles do matter. J. Pharm. Investig. 2020, 51, 1–17. [Google Scholar] [CrossRef]
- Chou, L.Y.; Ming, K.; Chan, W.C. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 2011, 40, 233–245. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhang, L.; Song, H.; Zhang, L. Targeted delivery system based on magnetic mesoporous silica nanocomposites with light-controlled release character. ACS Appl. Mater. Interfaces 2013, 5, 11–15. [Google Scholar] [CrossRef]
- Karimi, M.; Zangabad, P.S.; Ghasemi, A.; Hamblin, M.R. Smart Internal Stimulus-Responsive Nanocarriers for Drug and Gene Delivery; Morgan & Claypool Publishers: San Rafael, CA, USA, 2015. [Google Scholar]
- Felber, A.E.; Dufresne, M.-H.; Leroux, J.-C. pH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Adv. Drug Deliv. Rev. 2012, 64, 979–992. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Zangabad, P.S.; Rahighi, R.; Basri, S.M.M.; Mirshekari, H.; Amiri, M.; Pishabad, Z.S.; Aslani, A.; Bozorgomid, M. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torchilin, V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur. J. Pharm. Biopharm. 2009, 71, 431–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.-J. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef]
- Stubbs, M.; McSheehy, P.M.; Griffiths, J.R.; Bashford, C.L. Causes and consequences of tumour acidity and implications for treatment. Mol. Med. Today 2000, 6, 15–19. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.S.; Oh, K.T.; Kim, D.; Youn, Y.S.; Bae, Y.H. Tumor pH-responsive flower-like micelles of poly (L-lactic acid)-b-poly (ethylene glycol)-b-poly (L-histidine). J. Control. Release 2007, 123, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; He, C.; Zhang, Z.; Tan, L.; Liu, B.; Zhu, Z.; Shao, Z.; Gong, B.; Shen, Y.-M. Redox-responsive flower-like micelles of poly (L-lactic acid)-b-poly (ethylene glycol)-b-poly (L-lactic acid) for intracellular drug delivery. Polymer 2016, 90, 351–362. [Google Scholar] [CrossRef]
- Bae, Y.M.; Park, Y.I.; Nam, S.H.; Kim, J.H.; Lee, K.; Kim, H.M.; Yoo, B.; Choi, J.S.; Lee, K.T.; Hyeon, T. Endocytosis, intracellular transport, and exocytosis of lanthanide-doped upconverting nanoparticles in single living cells. Biomaterials 2012, 33, 9080–9086. [Google Scholar] [CrossRef] [PubMed]
- Iversen, T.-G.; Skotland, T.; Sandvig, K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today 2011, 6, 176–185. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Z.; Cai, P.; Liu, J.; Liao, L.-D.; Hong, M.; Chen, X.; Thakor, N.; Liu, B. Conjugated polymer and drug co-encapsulated nanoparticles for chemo-and photo-thermal combination therapy with two-photon regulated fast drug release. Nanoscale 2015, 7, 3067–3076. [Google Scholar] [CrossRef]
- Viger, M.L.; Grossman, M.; Fomina, N.; Almutairi, A. Low power upconverted near-IR light for efficient polymeric nanoparticle degradation and cargo release. Adv. Mater. 2013, 25, 3733–3738. [Google Scholar] [CrossRef] [PubMed]
- Seidel, Z.P.; Zhang, X.; MacMullan, M.A.; Graham, N.A.; Wang, P.; Lee, C.T., Jr. Photo-triggered delivery of siRNA and paclitaxel into breast cancer cells using catanionic vesicles. ACS Appl. Bio Mater. 2020, 3, 7388–7398. [Google Scholar] [CrossRef]
- Razavi, B.; Abdollahi, A.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Light-, temperature-, and pH-responsive micellar assemblies of spiropyran-initiated amphiphilic block copolymers: Kinetics of photochromism, responsiveness, and smart drug delivery. Mater. Sci. Eng. C 2020, 109, 110524. [Google Scholar] [CrossRef]
- Wu, M.; Lin, X.; Tan, X.; Li, J.; Wei, Z.; Zhang, D.; Zheng, Y.; Zheng, A.-x.; Zhao, B.; Zeng, Y. Photoresponsive nanovehicle for two independent wavelength light-triggered sequential release of P-GP shRNA and doxorubicin to optimize and enhance synergistic therapy of multidrug-resistant cancer. ACS Appl. Mater. Interfaces 2018, 10, 19416–19427. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, B.; Xie, X.; Zeng, F.; Wu, S. A sequential enzyme-activated and light-triggered pro-prodrug nanosystem for cancer detection and therapy. J. Mater. Chem. B 2018, 6, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Shi, J.; Zhang, Z.; Cao, Y.; Li, J.; Cao, S. PEGylated graphene oxide-capped gold nanorods/silica nanoparticles as multifunctional drug delivery platform with enhanced near-infrared responsiveness. Mater. Sci. Eng. C 2019, 104, 109889. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Luo, Y.; Nie, D.; Jin, J.; Yang, S.; Li, G.; Yang, Y.; Zhang, W. pH/photothermal dual-responsive drug delivery and synergistic chemo-photothermal therapy by novel porous carbon nanofibers. Chem. Eng. J. 2020, 397, 125402. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Y.; Di, D.; Zhang, X.; Wang, D.; Zhao, Q.; Wang, S. Gold nanoparticle-gated mesoporous silica as redox-triggered drug delivery for chemo-photothermal synergistic therapy. J. Colloid Interface Sci. 2017, 508, 323–331. [Google Scholar] [CrossRef]
- Peng, S.; He, Y.; Er, M.; Sheng, Y.; Gu, Y.; Chen, H. Biocompatible CuS-based nanoplatforms for efficient photothermal therapy and chemotherapy in vivo. Biomater. Sci. 2017, 5, 475–484. [Google Scholar] [CrossRef]
- Cano-Mejia, J.; Burga, R.A.; Sweeney, E.E.; Fisher, J.P.; Bollard, C.M.; Sandler, A.D.; Cruz, C.R.Y.; Fernandes, R. Prussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 771–781. [Google Scholar] [CrossRef]
- Yan, L.; Qiu, L. Indocyanine green targeted micelles with improved stability for near-infrared image-guided photothermal tumor therapy. Nanomedicine 2015, 10, 361–373. [Google Scholar] [CrossRef]
- Wei, X.; Liu, L.; Guo, X.; Wang, Y.; Zhao, J.; Zhou, S. Light-activated ROS-responsive nanoplatform codelivering apatinib and doxorubicin for enhanced chemo-photodynamic therapy of multidrug-resistant tumors. ACS Appl. Mater. Interfaces 2018, 10, 17672–17684. [Google Scholar] [CrossRef]
- Bio, M.; Rajaputra, P.; Lim, I.; Thapa, P.; Tienabeso, B.; Hurst, R.E.; You, Y. Efficient activation of a visible light-activatable CA4 prodrug through intermolecular photo-unclick chemistry in mitochondria. Chem. Commun. 2017, 53, 1884–1887. [Google Scholar] [CrossRef]
- Yang, B.; Wang, K.; Zhang, D.; Sun, B.; Ji, B.; Wei, L.; Li, Z.; Wang, M.; Zhang, X.; Zhang, H. Light-activatable dual-source ROS-responsive prodrug nanoplatform for synergistic chemo-photodynamic therapy. Biomater. Sci. 2018, 6, 2965–2975. [Google Scholar] [CrossRef]
- Wang, W.; Lin, L.; Ma, X.; Wang, B.; Liu, S.; Yan, X.; Li, S.; Tian, H.; Yu, X. Light-induced hypoxia-triggered living nanocarriers for synergistic cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 19398–19407. [Google Scholar] [CrossRef]
- Ge, L.; Qiao, C.; Tang, Y.; Zhang, X.; Jiang, X. Light-activated hypoxia-sensitive covalent organic framework for tandem-responsive drug delivery. Nano Lett. 2021, 21, 3218–3224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wang, P.; Li, Q.; Al-Khalaf, A.A.; Hozzein, W.N.; Zhang, F.; Li, X.; Zhao, D. Near-infrared triggered decomposition of nanocapsules with high tumor accumulation and stimuli responsive fast elimination. Angew. Chem. Int. Ed. 2018, 130, 2641–2645. [Google Scholar] [CrossRef]
- Weis, P.; Wu, S. Light-switchable azobenzene-containing macromolecules: From UV to near infrared. Macromol. Rapid Commun. 2018, 39, 1700220. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhou, Q.; Liu, H.; Liu, H. UV light induced plasticization and light activated shape memory of spiropyran doped ethylene-vinyl acetate copolymers. Soft Matter 2014, 10, 3748–3754. [Google Scholar] [CrossRef]
- Abdollahi, A.; Alinejad, Z.; Mahdavian, A.R. Facile and fast photosensing of polarity by stimuli-responsive materials based on spiropyran for reusable sensors: A physico-chemical study on the interactions. J. Mater. Chem. C 2017, 5, 6588–6600. [Google Scholar] [CrossRef]
- Tong, R.; Hemmati, H.D.; Langer, R.; Kohane, D.S. Photoswitchable nanoparticles for triggered tissue penetration and drug delivery. J. Am. Chem. Soc. 2012, 134, 8848–8855. [Google Scholar] [CrossRef]
- Busch, L.; Avlasevich, Y.; Zwicker, P.; Thiede, G.; Landfester, K.; Keck, C.M.; Meinke, M.C.; Darvin, M.E.; Kramer, A.; Müller, G. Release of the model drug SR101 from polyurethane nanocapsules in porcine hair follicles triggered by LED-derived low dose UVA light. Int. J. Pharm. 2021, 597, 120339. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.V.; Kim, Y.; Choi, M.; Lee, D.Y.; Choi, J.; Lee, Y.; Jon, S.; Churchill, D.G. Enhanced doubly activated dual emission fluorescent probes for selective imaging of glutathione or cysteine in living systems. Anal. Chem. 2018, 90, 2648–2654. [Google Scholar] [CrossRef]
- Kumar, S.; Allard, J.-F.; Morris, D.; Dory, Y.L.; Lepage, M.; Zhao, Y. Near-infrared light sensitive polypeptide block copolymer micelles for drug delivery. J. Mater. Chem. 2012, 22, 7252–7257. [Google Scholar] [CrossRef]
- Rojas-Gutierrez, P.A.; Bhuckory, S.; Mingoes, C.; Hildebrandt, N.; DeWolf, C.; Capobianco, J.A. A route to triggered delivery via photocontrol of lipid bilayer properties using lanthanide upconversion nanoparticles. ACS Appl. Nano Mater. 2018, 1, 5345–5354. [Google Scholar] [CrossRef]
- Wang, C.; Tao, H.; Cheng, L.; Liu, Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011, 32, 6145–6154. [Google Scholar] [CrossRef] [PubMed]
- Han, R.-L.; Shi, J.-H.; Liu, Z.-J.; Hou, Y.-F.; Wang, Y. Near-infrared light-triggered hydrophobic-to-hydrophilic switch nanovalve for on-demand cancer therapy. ACS Biomater. Sci. Eng. 2018, 4, 3478–3486. [Google Scholar] [CrossRef]
- Wolpert, D.; Schade, M.; Brixner, T. Femtosecond midinfrared study of the photoinduced Wolff rearrangement of diazonaphthoquinone. J. Chem. Phys. 2008, 129, 094504. [Google Scholar] [CrossRef]
- Kim, K.N.; Oh, K.S.; Shim, J.; Schlaepfer, I.R.; Karam, S.D.; Lee, J.-J. Light-responsive polymeric micellar nanoparticles with enhanced formulation stability. Polymers 2021, 13, 377. [Google Scholar] [CrossRef]
- Li, Q.; Cao, Z.; Wang, G. Diazonaphthoquinone-based amphiphilic polymer assemblies for NIR/UV light-and pH-responsive controlled release. Polym. Chem. 2018, 9, 463–471. [Google Scholar] [CrossRef]
- Song, X.; Chen, Q.; Liu, Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2015, 8, 340–354. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Jin, Z.; Jimmy, C.Y.; Chan, K.M. An NIR-triggered and thermally responsive drug delivery platform through DNA/copper sulfide gates. Nanoscale 2015, 7, 12614–12624. [Google Scholar] [CrossRef]
- Poulose, A.C.; Veeranarayanan, S.; Mohamed, M.S.; Nagaoka, Y.; Aburto, R.R.; Mitcham, T.; Ajayan, P.M.; Bouchard, R.R.; Sakamoto, Y.; Yoshida, Y. Multi-stimuli responsive Cu 2 S nanocrystals as trimodal imaging and synergistic chemo-photothermal therapy agents. Nanoscale 2015, 7, 8378–8388. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Liang, C.; Gong, H.; Li, M.; Zheng, X.; Cheng, L.; Yang, K.; Jiang, X.; Liu, Z. Core–shell MnSe@ Bi2Se3 fabricated via a cation exchange method as novel nanotheranostics for multimodal imaging and synergistic thermoradiotherapy. Adv. Mater. 2015, 27, 6110–6117. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Detrembleur, C.; De Pauw-Gillet, M.C.; Mornet, S.; Jérôme, C.; Duguet, E. Gold nanorods coated with mesoporous silica shell as drug delivery system for remote near infrared light-activated release and potential phototherapy. Small 2015, 11, 2323–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Di, J.; Sun, Y.; Fu, J.; Wei, Z.; Matsui, H.; Alonso, A.D.C.; Zhou, S. Biocompatible PEG-chitosan@ carbon dots hybrid nanogels for two-photon fluorescence imaging, near-infrared light/pH dual-responsive drug carrier, and synergistic therapy. Adv. Funct. Mater. 2015, 25, 5537–5547. [Google Scholar] [CrossRef]
- He, S.; Krippes, K.; Ritz, S.; Chen, Z.; Best, A.; Butt, H.-J.; Mailänder, V.; Wu, S. Ultralow-intensity near-infrared light induces drug delivery by upconverting nanoparticles. Chem. Commun. 2015, 51, 431–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, W.; Guo, F.; Zhu, C.; Wang, H.; Li, H.; Huang, H.; Liu, Y.; Kang, Z. Carbon dots anchored on octahedral CoO as a stable visible-light-responsive composite photocatalyst for overall water splitting. J. Mater. Chem. A 2017, 5, 19800–19807. [Google Scholar] [CrossRef]
- Kalytchuk, S.; Poláková, K.i.; Wang, Y.; Froning, J.P.; Cepe, K.; Rogach, A.L.; Zbořil, R. Carbon dot nanothermometry: Intracellular photoluminescence lifetime thermal sensing. ACS Nano 2017, 11, 1432–1442. [Google Scholar] [CrossRef]
- Vinothini, K.; Rajendran, N.K.; Munusamy, M.A.; Alarfaj, A.A.; Rajan, M. Development of biotin molecule targeted cancer cell drug delivery of doxorubicin loaded κ-carrageenan grafted graphene oxide nanocarrier. Mater. Sci. Eng. C 2019, 100, 676–687. [Google Scholar] [CrossRef]
- Jha, R.; Singh, A.; Sharma, P.K.; Porwal, O.; Fuloria, N.K. Graphene-based nanomaterial system: A boon in the era of smart nanocarriers. J. Pharm. Investig. 2021, 51, 1–36. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Je, J.-Y.; Moorthy, M.S.; Seo, H.; Cho, W.H. pH and NIR-light-responsive magnetic iron oxide nanoparticles for mitochondria-mediated apoptotic cell death induced by chemo-photothermal therapy. Int. J. Pharm. 2017, 531, 1–13. [Google Scholar] [CrossRef]
- Wu, X.; Liu, K.; Huang, Q.; Zhang, Q.; Yang, X.; Liu, X.; Wang, R. Photothermal therapy based on CuS nanoparticles for alleviating arterial restenosis induced by mechanical injury of endovascular treatment. Front. Mater. 2021, 7, 386. [Google Scholar] [CrossRef]
- Prasad, M.; Lambe, U.P.; Brar, B.; Shah, I.; Manimegalai, J.; Ranjan, K.; Rao, R.; Kumar, S.; Mahant, S.; Khurana, S.K. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed. Pharmacother. 2018, 97, 1521–1537. [Google Scholar] [CrossRef]
- Li Volsi, A.; Scialabba, C.; Vetri, V.; Cavallaro, G.; Licciardi, M.; Giammona, G. Near-infrared light responsive folate targeted gold nanorods for combined photothermal-chemotherapy of osteosarcoma. ACS Appl. Mater. Interfaces 2017, 9, 14453–14469. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, F.; Guo, Z.; Xiao, Y.; Zhang, Y.; Sun, X.; Zhe, T.; Cao, Y.; Wang, L.; Lu, Q. Silver nanoparticle-embedded hydrogel as a photothermal platform for combating bacterial infections. Chem. Eng. J. 2020, 382, 122990. [Google Scholar] [CrossRef]
- Fu, B.; Dang, M.; Tao, J.; Li, Y.; Tang, Y. Mesoporous platinum nanoparticle-based nanoplatforms for combined chemo-photothermal breast cancer therapy. J. Colloid Interface Sci. 2020, 570, 197–204. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Li, Y.; Niu, G.; Lan, M.; Jia, Q.; Liang, Q. Near-infrared organic dye-based nanoagent for the photothermal therapy of cancer. ACS Appl. Mater. Interfaces 2016, 8, 29899–29905. [Google Scholar] [CrossRef] [PubMed]
- Vipin, A.K.; Fugetsu, B.; Sakata, I.; Isogai, A.; Endo, M.; Li, M.; Dresselhaus, M.S. Cellulose nanofiber backboned Prussian blue nanoparticles as powerful adsorbents for the selective elimination of radioactive cesium. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhi, X.; Yang, M.; Zhang, J.; Lin, L.; Zhao, X.; Hou, W.; Zhang, C.; Zhang, Q.; Pan, F. Tumor-triggered drug release from calcium carbonate-encapsulated gold nanostars for near-infrared photodynamic/photothermal combination antitumor therapy. Theranostics 2017, 7, 1650. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef]
- Husni, P.; Shin, Y.; Kim, J.C.; Kang, K.; Lee, E.S.; Youn, Y.S.; Rusdiana, T.; Oh, K.T. Photo-based nanomedicines using polymeric systems in the field of cancer imaging and therapy. Biomedicines 2020, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Del Valle, A.C.; Yeh, H.-P.; He, Y.; Huang, Y.-F. Development of photo-activated ROS-responsive nanoplatform as a dual-functional drug carrier in combinational chemo-photodynamic therapy. Front. Chem. 2019, 6, 647. [Google Scholar] [CrossRef]
- Henderson, B.W.; Fingar, V.H. Relationship of tumor hypoxia and response to photodynamic treatment in an experimental mouse tumor. Cancer Res. 1987, 47, 3110–3114. [Google Scholar] [PubMed]
- Yuan, Y.; Xu, S.; Zhang, C.-J.; Liu, B. Light-responsive AIE nanoparticles with cytosolic drug release to overcome drug resistance in cancer cells. Polym. Chem. 2016, 7, 3530–3539. [Google Scholar] [CrossRef]
- Jeon, G.; Ko, Y.T. Enhanced photodyamic therapy via photosensitizer-loaded nanoparticles for cancer treatment. J. Pharm. Investig. 2019, 49, 1–8. [Google Scholar] [CrossRef]
- Yin, W.; Ke, W.; Chen, W.; Xi, L.; Zhou, Q.; Mukerabigwi, J.F.; Ge, Z. Integrated block copolymer prodrug nanoparticles for combination of tumor oxidative stress amplification and ROS-responsive drug release. Biomaterials 2019, 195, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Ballance, W.C.; Qin, E.C.; Chung, H.J.; Gillette, M.U.; Kong, H. Reactive oxygen species-responsive drug delivery systems for the treatment of neurodegenerative diseases. Biomaterials 2019, 217, 119292. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, Y.; Huang, X.; Liu, F.; Ma, C.; Feng, F.; Zhang, J.; Liu, W.; Qu, W.; Pang, H. Specific-oxygen-supply functionalized core-shell nanoparticles for smart mutual-promotion between photodynamic therapy and gambogic acid-induced chemotherapy. Biomaterials 2020, 257, 120228. [Google Scholar] [CrossRef] [PubMed]
- Uthaman, S.; Pillarisetti, S.; Mathew, A.P.; Kim, Y.; Bae, W.K.; Huh, K.M.; Park, I.-K. Long circulating photoactivable nanomicelles with tumor localized activation and ROS triggered self-accelerating drug release for enhanced locoregional chemo-photodynamic therapy. Biomaterials 2020, 232, 119702. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Ma, Y.; Sun, C.; Lu, Z.; Yao, Z.; Wang, J.; Li, D.; Yuan, Y.; Yang, X. ROS-sensitive polymeric nanocarriers with red light-activated size shrinkage for remotely controlled drug release. Chem. Mater. 2018, 30, 517–525. [Google Scholar] [CrossRef]
- Wang, C.; Huang, B.; Yang, G.; Ouyang, Y.; Tian, J.; Zhang, W. NIR-triggered multifunctional and degradable nanoplatform based on an ROS-sensitive block copolymer for imaging-guided chemo-phototherapy. Biomacromolecules 2019, 20, 4218–4229. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, G.; Kim, J.; Kim, W.J. Reactive-oxygen-species-responsive drug delivery systems: Promises and challenges. Adv. Sci. 2017, 4, 1600124. [Google Scholar] [CrossRef]
- Qian, C.; Yu, J.; Chen, Y.; Hu, Q.; Xiao, X.; Sun, W.; Wang, C.; Feng, P.; Shen, Q.D.; Gu, Z. Light-activated hypoxia-responsive nanocarriers for enhanced anticancer therapy. Adv. Mater. 2016, 28, 3313–3320. [Google Scholar] [CrossRef]
- Qian, C.; Feng, P.; Yu, J.; Chen, Y.; Hu, Q.; Sun, W.; Xiao, X.; Hu, X.; Bellotti, A.; Shen, Q.D. Anaerobe-inspired anticancer nanovesicles. Angew. Chem. 2017, 129, 2632–2637. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, M.; Liu, T.; Tan, X.; Zhang, H.; Zhang, X.; He, Z.; Sun, J. A surfactant-like chemotherapeutic agent as a nanocarrier for delivering photosensitizers against cancer: A facile drug-delivering-drug strategy. Int. J. Pharm. 2019, 562, 313–320. [Google Scholar] [CrossRef]
- Qu, J.; Peng, S.; Wang, R.; Yang, S.-T.; Zhou, Q.-H.; Lin, J. Stepwise pH-sensitive and biodegradable polypeptide hybrid micelles for enhanced cellular internalization and efficient nuclear drug delivery. Colloids Surf. B Biointerfaces 2019, 181, 315–324. [Google Scholar] [CrossRef]
- Lin, W.; Ma, G.; Yuan, Z.; Qian, H.; Xu, L.; Sidransky, E.; Chen, S. Development of zwitterionic polypeptide nanoformulation with high doxorubicin loading content for targeted drug delivery. Langmuir 2018, 35, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Piątkowski, M.; Janus, Ł.; Radwan-Pragłowska, J.; Bogdał, D.; Matysek, D. Biodegradable, pH-sensitive chitosan beads obtained under microwave radiation for advanced cell culture. Colloids Surf. B Biointerfaces 2018, 164, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xue, Z.; Liu, Y.; Xiao, J.; Chen, J.; Zhang, L.; Guo, J.; Lin, W. Delivery of anticancer drug using pH-sensitive micelles from triblock copolymer MPEG-b-PBAE-b-PLA. Mater. Sci. Eng. C 2018, 84, 254–262. [Google Scholar] [CrossRef]
- Xu, Z.; Xue, P.; Gao, Y.-E.; Liu, S.; Shi, X.; Hou, M.; Kang, Y. pH-responsive polymeric micelles based on poly (ethyleneglycol)-b-poly (2-(diisopropylamino) ethyl methacrylate) block copolymer for enhanced intracellular release of anticancer drugs. J. Colloid Interface Sci. 2017, 490, 511–519. [Google Scholar] [CrossRef]
- Zhou, X.X.; Jin, L.; Qi, R.Q.; Ma, T. pH-responsive polymeric micelles self-assembled from amphiphilic copolymer modified with lipid used as doxorubicin delivery carriers. R. Soc. Open Sci. 2018, 5, 171654. [Google Scholar] [CrossRef] [Green Version]
- Bai, T.; Shao, D.; Chen, J.; Li, Y.; Xu, B.B.; Kong, J. pH-responsive dithiomaleimide-amphiphilic block copolymer for drug delivery and cellular imaging. J. Colloid Interface Sci. 2019, 552, 439–447. [Google Scholar] [CrossRef]
- Mishra, A.K.; Lim, J.; Lee, J.; Park, S.; Seo, Y.; Hwang, H.; Kim, J.K. Control drug release behavior by highly stable and pH sensitive poly (N-vinylpyrrolidone)-block-poly (4-vinylpyridine) copolymer micelles. Polymer 2021, 213, 123329. [Google Scholar] [CrossRef]
- Lo, Y.-L.; Huang, X.-S.; Chen, H.-Y.; Huang, Y.-C.; Liao, Z.-X.; Wang, L.-F. ROP and ATRP fabricated redox sensitive micelles based on PCL-SS-PMAA diblock copolymers to co-deliver PTX and CDDP for lung cancer therapy. Colloids Surf. B Biointerfaces 2021, 198, 111443. [Google Scholar] [CrossRef]
- Rasib, S.; Ahmad, Z.; Khan, A.; Akil, H.; Othman, M.; Hamid, Z.; Ullah, F. Synthesis and evaluation on pH-and temperature-responsive chitosan-p (MAA-co-NIPAM) hydrogels. Int. J. Biol. Macromol. 2018, 108, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, E.; Haddadi-Asl, V.; Roghani-Mamaqani, H. Synthesis of dual thermo-and pH-sensitive poly (N-isopropylacrylamide-co-acrylic acid)-grafted cellulose nanocrystals by reversible addition-fragmentation chain transfer polymerization. J. Biomed. Mater. Res. Part A 2018, 106, 231–243. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Don, T.-M.; Lin, Y.-T.; Hsu, S.-C.; Chiu, W.-Y. Synthesis of pH-sensitive sulfonamide-based hydrogels with controllable crosslinking density by post thermo-curing. J. Polym. Res. 2019, 26, 18. [Google Scholar] [CrossRef]
- Chang, G.; Li, C.; Lu, W.; Ding, J. N-Boc-Histidine-Capped PLGA-PEG-PLGA as a smart polymer for drug delivery sensitive to tumor extracellular pH. Macromol. Biosci. 2010, 10, 1248–1256. [Google Scholar] [CrossRef]
- Hong, W.; Chen, D.; Jia, L.; Gu, J.; Hu, H.; Zhao, X.; Qiao, M. Thermo-and pH-responsive copolymers based on PLGA-PEG-PLGA and poly (L-histidine): Synthesis and in vitro characterization of copolymer micelles. Acta Biomater. 2014, 10, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Miura, S.; Na, K.; Bae, Y.H. pH-responsive and charge shielded cationic micelle of poly (L-histidine)-block-short branched PEI for acidic cancer treatment. J. Control. Release 2013, 172, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.P.; Jeong, Y.I.; John, J.V.; Chung, C.-W.; Kang, D.H.; Selvaraj, M.; Suh, H.; Kim, I. Dual stimuli-responsive poly (N-isopropylacrylamide)-b-poly (L-histidine) chimeric materials for the controlled delivery of doxorubicin into liver carcinoma. Biomacromolecules 2013, 14, 1434–1443. [Google Scholar] [CrossRef]
- Lee, E.S.; Shin, H.J.; Na, K.; Bae, Y.H. Poly (l-histidine)–PEG block copolymer micelles and pH-induced destabilization. J. Control. Release 2003, 90, 363–374. [Google Scholar] [CrossRef]
- Na, K.; Lee, E.S.; Bae, Y.H. Self-organized nanogels responding to tumor extracellular pH: pH-dependent drug release and in vitro cytotoxicity against MCF-7 cells. Bioconjugate Chem. 2007, 18, 1568–1574. [Google Scholar] [CrossRef]
- Wang, C.Y.; Huang, L. Polyhistidine mediates an acid-dependent fusion of negatively charged liposomes. Biochemistry 1984, 23, 4409–4416. [Google Scholar] [CrossRef]
- Yang, S.R.; Lee, H.J.; Kim, J.-D. Histidine-conjugated poly (amino acid) derivatives for the novel endosomolytic delivery carrier of doxorubicin. J. Control. Release 2006, 114, 60–68. [Google Scholar] [CrossRef]
- Yin, H.; Lee, E.S.; Kim, D.; Lee, K.H.; Oh, K.T.; Bae, Y.H. Physicochemical characteristics of pH-sensitive poly (L-histidine)-b-poly (ethylene glycol)/poly (L-lactide)-b-poly (ethylene glycol) mixed micelles. J. Control. Release 2008, 126, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Sim, T.; Han, S.M.; Lim, C.; Won, W.R.; Lee, E.S.; Youn, Y.S.; Oh, K.T. A pH-sensitive polymer for cancer targeting prepared by one-step modulation of functional side groups. Macromol. Res. 2019, 27, 795–802. [Google Scholar] [CrossRef]
- Yi, M.; Lu, Q.; Zhao, Y.; Cheng, C.; Zhang, S. Synthesis and self-assembly of the pH-responsive anionic copolymers for enhanced doxorubicin-loading capacity. Langmuir 2018, 34, 7877–7886. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, L.; Zhou, Z.; Sun, W.; Huang, Y. Dual-pH responsive micelle platform for co-delivery of axitinib and doxorubicin. Int. J. Pharm. 2016, 507, 50–60. [Google Scholar] [CrossRef]
- Kanamala, M.; Palmer, B.D.; Jamieson, S.M.; Wilson, W.R.; Wu, Z. Dual pH-sensitive liposomes with low pH-triggered sheddable PEG for enhanced tumor-targeted drug delivery. Nanomedicine 2019, 14, 1971–1989. [Google Scholar] [CrossRef]
- Gu, J.; Cheng, W.-P.; Liu, J.; Lo, S.-Y.; Smith, D.; Qu, X.; Yang, Z. pH-triggered reversible “stealth” polycationic micelles. Biomacromolecules 2008, 9, 255–262. [Google Scholar] [CrossRef]
- Ding, C.; Gu, J.; Qu, X.; Yang, Z. Preparation of multifunctional drug carrier for tumor-specific uptake and enhanced intracellular delivery through the conjugation of weak acid labile linker. Bioconjugate Chem. 2009, 20, 1163–1170. [Google Scholar] [CrossRef]
- Orbán, E.; Mező, G.; Schlage, P.; Csík, G.; Kulić, Ž.; Ansorge, P.; Fellinger, E.; Möller, H.M.; Manea, M. In vitro degradation and antitumor activity of oxime bond-linked daunorubicin–GnRH-III bioconjugates and DNA-binding properties of daunorubicin–amino acid metabolites. Amino Acids 2011, 41, 469–483. [Google Scholar] [CrossRef]
- Vrettos, E.I.; Karampelas, T.; Sayyad, N.; Kougioumtzi, A.; Syed, N.; Crook, T.; Murphy, C.; Tamvakopoulos, C.; Tzakos, A.G. Development of programmable gemcitabine-GnRH pro-drugs bearing linker controllable “click” oxime bond tethers and preclinical evaluation against prostate cancer. Eur. J. Med. Chem. 2021, 211, 113018. [Google Scholar] [CrossRef]
- Gillies, E.R.; Goodwin, A.P.; Fréchet, J.M. Acetals as pH-sensitive linkages for drug delivery. Bioconjugate Chem. 2004, 15, 1254–1263. [Google Scholar] [CrossRef]
- Heffernan, M.J.; Murthy, N. Polyketal nanoparticles: A new pH-sensitive biodegradable drug delivery vehicle. Bioconjugate Chem. 2005, 16, 1340–1342. [Google Scholar] [CrossRef]
- Bawa, K.K.; Jazani, A.M.; Shetty, C.; Oh, J.K. PLA-based triblock copolymer micelles exhibiting dual acidic ph/reduction responses at dual core and core/corona interface locations. Macromol. Rapid Commun. 2018, 39, 1800477. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Liu, J.; Zhao, X.; Zhang, Y.; Liu, J.; Xu, S.; Deng, L.; Dong, A.; Zhang, J. PEG-b-PCL copolymer micelles with the ability of pH-controlled negative-to-positive charge reversal for intracellular delivery of doxorubicin. Biomacromolecules 2014, 15, 4281–4292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Hong, M.; Tang, G.; Qian, L.; Lin, J.; Jiang, Y.; Pei, Y. Partly PEGylated polyamidoamine dendrimer for tumor-selective targeting of doxorubicin: The effects of PEGylation degree and drug conjugation style. Biomaterials 2010, 31, 1360–1371. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, Q.; Hong, C.-Y.; Pan, C.-Y. Unimolecular micelles of camptothecin-bonded hyperbranched star copolymers via β-thiopropionate linkage: Synthesis and drug delivery. J. Mater. Chem. B 2016, 4, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Dan, K.; Pan, R.; Ghosh, S. Aggregation and pH responsive disassembly of a new acid-labile surfactant synthesized by thiol− acrylate michael addition reaction. Langmuir 2011, 27, 612–617. [Google Scholar] [CrossRef]
- Zhang, Z.; He, C.; Chen, X. Hydrogels based on pH-responsive reversible carbon–nitrogen double-bond linkages for biomedical applications. Mater. Chem. Front. 2018, 2, 1765–1778. [Google Scholar] [CrossRef]
- Gurav, D.D.; Kulkarni, A.S.; Khan, A.; Shinde, V.S. pH-responsive targeted and controlled doxorubicin delivery using hyaluronic acid nanocarriers. Colloids Surf. B Biointerfaces 2016, 143, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, A.-K.; Lucas, H.; Schindler, L.; Chytil, P.; Etrych, T.; Mäder, K.; Mueller, T. Improved tumor-specific drug accumulation by polymer therapeutics with pH-sensitive drug release overcomes chemotherapy resistance. Mol. Cancer Ther. 2016, 15, 998–1007. [Google Scholar] [CrossRef] [Green Version]
- She, W.; Pan, D.; Luo, K.; He, B.; Cheng, G.; Zhang, C.; Gu, Z. PEGylated dendrimer-doxorubicin cojugates as pH-sensitive drug delivery systems: Synthesis and in vitro characterization. J. Biomed. Nanotechnol. 2015, 11, 964–978. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.-B.; Gu, W.-X.; Pang, C.; Ma, J.; Gao, H. Construction of coumarin-based cross-linked micelles with pH responsive hydrazone bond and tumor targeting moiety. J. Mater. Chem. B 2016, 4, 1480–1488. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, D.; Wang, G.-L.; Yang, H.-G.; Xu, H.-F.; Chen, J.-H.; Xie, Y.; Wang, Z.-Q. An efficient PEGylated liposomal nanocarrier containing cell-penetrating peptide and pH-sensitive hydrazone bond for enhancing tumor-targeted drug delivery. Int. J. Nanomed. 2015, 10, 6199. [Google Scholar]
- Jain, N.K.; Tare, M.S.; Mishra, V.; Tripathi, P.K. The development, characterization and in vivo anti-ovarian cancer activity of poly (propylene imine)(PPI)-antibody conjugates containing encapsulated paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, Q.; Guo, Z.; Li, M.; Yang, X.; Wan, G.; Chen, H.; Zhang, Q.; Wang, Y. An intelligent biomimetic nanoplatform for holistic treatment of metastatic triple-negative breast cancer via photothermal ablation and immune remodeling. ACS Nano 2020, 14, 15161–15181. [Google Scholar] [CrossRef]
- Kalia, J.; Raines, R.T. Hydrolytic stability of hydrazones and oximes. Angew. Chem. Int. Ed. 2008, 47, 7523–7526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulrich, S.; Boturyn, D.; Marra, A.; Renaudet, O.; Dumy, P. Oxime ligation: A chemoselective click-type reaction for accessing multifunctional biomolecular constructs. Chem. A Eur. J. 2014, 20, 34–41. [Google Scholar] [CrossRef]
- Chen, D.; Wang, H. Novel pH-sensitive biodegradable polymeric drug delivery systems based on ketal polymers. J. Nanosci. Nanotechnol. 2014, 14, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Du, F.; Wang, Y.; Ji, S.; Liang, D.; Yu, L.; Li, Z. An acid-labile block copolymer of PDMAEMA and PEG as potential carrier for intelligent gene delivery systems. Biomacromolecules 2008, 9, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Du, J.-Z.; Li, H.-J.; Wang, J. Tumor-acidity-cleavable maleic acid amide (TACMAA): A powerful tool for designing smart nanoparticles to overcome delivery barriers in cancer nanomedicine. Acc. Chem. Res. 2018, 51, 2848–2856. [Google Scholar] [CrossRef]
- Shen, W.-C.; Ryser, H.J.-P. cis-Aconityl spacer between daunomycin and macromolecular carriers: A model of pH-sensitive linkage releasing drug from a lysosomotropic conjugate. Biochem. Biophys. Res. Commun. 1981, 102, 1048–1054. [Google Scholar] [CrossRef]
- Zloh, M.; Dinand, E.; Brocchini, S. Aconityl-derived polymers for biomedical applications. Modeling study of cis–trans isomerisation. Theor. Chem. Acc. 2003, 109, 206–212. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, N.; Qiu, Z.; Lu, X.; Fang, M.; Chen, X.; Ren, L.; Wang, G.; Ouyang, P. Superior anti-neoplastic activities of triacontanol-PEG conjugate: Synthesis, characterization and biological evaluations. Drug Deliv. 2018, 25, 1546–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Tang, Z.; Zhang, D.; Deng, M.; Chen, X. pH and redox dual-sensitive polysaccharide nanoparticles for the efficient delivery of doxorubicin. Biomater. Sci. 2017, 5, 2169–2178. [Google Scholar] [CrossRef]
- Qiu, L.; Li, J.-W.; Hong, C.-Y.; Pan, C.-Y. Silver nanoparticles covered with pH-sensitive camptothecin-loaded polymer prodrugs: Switchable fluorescence “off” or “on” and drug delivery dynamics in living cells. ACS Appl. Mater. Interfaces 2017, 9, 40887–40897. [Google Scholar] [CrossRef]
- Guragain, S.; Bastakoti, B.P.; Malgras, V.; Nakashima, K.; Yamauchi, Y. Multi-stimuli-responsive polymeric materials. Chemistry 2015, 21, 13164–13174. [Google Scholar] [CrossRef]
- Pethe, A.M.; Yadav, K.S. Polymers, responsiveness and cancer therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Yuan, W.; Gao, X.; Pei, E.; Li, Z. Light- and pH-dually responsive dendrimer-star copolymer containing spiropyran groups: Synthesis, self-assembly and controlled drug release. Polym. Chem. 2018, 9, 3651–3661. [Google Scholar] [CrossRef]
- Wu, M.X.; Gao, J.; Wang, F.; Yang, J.; Song, N.; Jin, X.; Mi, P.; Tian, J.; Luo, J.; Liang, F.; et al. Multistimuli responsive core-shell nanoplatform constructed from Fe3O4 @MOF equipped with Pillar[6]arene Nanovalves. Small 2018, 14, e1704440. [Google Scholar] [CrossRef]
- Han, H.; Valdeperez, D.; Jin, Q.; Yang, B.; Li, Z.; Wu, Y.; Pelaz, B.; Parak, W.J.; Ji, J. Dual enzymatic reaction-assisted gemcitabine delivery systems for programmed pancreatic cancer therapy. ACS Nano 2017, 11, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; He, H.; Liu, Y.; Cao, D.; Yan, J.; Duan, S.; Chen, Y.; Yin, L. Cancer-selective bioreductive chemotherapy mediated by dual hypoxia-responsive nanomedicine upon photodynamic therapy-induced hypoxia aggravation. Biomacromolecules 2019, 20, 2649–2656. [Google Scholar] [CrossRef]
- Du, J.Z.; Du, X.J.; Mao, C.Q.; Wang, J. Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. J. Am. Chem. Soc. 2011, 133, 17560–17563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, C.X.; Chen, L.G.; Yan, X.P. Dual-stimuli responsive and reversibly activatable theranostic nanoprobe for precision tumor-targeting and fluorescence-guided photothermal therapy. Nat. Commun. 2017, 8, 14998. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.N.; Chen, Q.W.; Ding, X.C.; Wen, J.; Zhang, Y.H.; Li, H.J.; Xu, Y.Q.; Liu, F.Y.; Chen, S.S.; Sun, S.G. BSA modified, disulfide-bridged mesoporous silica with low biotoxicity for dual-responsive drug delivery. Micropor. Mesopor. Mat. 2019, 278, 257–266. [Google Scholar] [CrossRef]
- Man, F.; Lammers, T.; de Rosales, R.T. Imaging nanomedicine-based drug delivery: A review of clinical studies. Mol. Imaging Biol. 2018, 20, 683–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schattling, P.; Jochum, F.D.; Theato, P. Multi-stimuli responsive polymers—The all-in-one talents. Polym. Chem. 2014, 5, 25–36. [Google Scholar] [CrossRef]
- Sim, T.; Lim, C.; Hoang, N.H.; Shin, Y.; Kim, J.C.; Park, J.Y.; Her, J.; Lee, E.S.; Youn, Y.S.; Oh, K.T. An on-demand pH-sensitive nanocluster for cancer treatment by combining photothermal therapy and chemotherapy. Pharmaceutics 2020, 12, 839. [Google Scholar] [CrossRef]
- Sim, T.; Lim, C.; Hoang, N.H.; Kim, J.E.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Synergistic photodynamic therapeutic effect of indole-3-acetic acid using a pH sensitive nano-carrier based on poly (aspartic acid-graft-imidazole)-poly(ethylene glycol). J. Mater. Chem. B 2017, 5, 8498–8505. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Sun, X.; Zhang, B.; Kang, H.; Zhang, F.; Jin, Y. Doxorubicin-loaded photosensitizer-core pH-responsive copolymer nanocarriers for combining photodynamic therapy and chemotherapy. ACS Biomater. Sci. Eng. 2017, 3, 1008–1016. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Zhang, X.; Luo, L.; Li, L.; Xing, S.; He, Y.; Cao, W.; Zhu, R.; Gao, D. Gold nanoshell coated thermo-pH dual responsive liposomes for resveratrol delivery and chemo-photothermal synergistic cancer therapy. J. Mater. Chem. B 2017, 5, 2161–2171. [Google Scholar] [CrossRef]
- Lim, C.; Moon, J.; Sim, T.; Won, W.R.; Lee, E.S.; Youn, Y.S.; Oh, K.T. A nano-complex system to overcome antagonistic photo-chemo combination cancer therapy. J. Control. Release 2019, 295, 164–173. [Google Scholar] [CrossRef]
- Wu, J.; Bremner, D.H.; Niu, S.; Shi, M.; Wang, H.; Tang, R.; Zhu, L.-M. Chemodrug-gated biodegradable hollow mesoporous organosilica nanotheranostics for multimodal imaging-guided low-temperature photothermal therapy/chemotherapy of cancer. ACS Appl. Mater. Interfaces 2018, 10, 42115–42126. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chang, C.; Liu, Z.; Zhou, Y.; Xu, Q.; Li, C.; Huang, Z.; Xu, H.; Xu, P.; Lu, B. Hyaluronic acid targeted and pH-responsive nanocarriers based on hollow mesoporous silica nanoparticles for chemo-photodynamic combination therapy. Colloids Surf. B Biointerfaces 2020, 194, 111166. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Pandit, A.H.; Wang, L.F.; Rattan, S. Strategy to design a smart photocleavable and pH sensitive chitosan based hydrogel through a novel crosslinker: A potential vehicle for controlled drug delivery. RSC Adv. 2020, 10, 14694–14704. [Google Scholar] [CrossRef] [Green Version]
- Knezevic, N.Z.; Trewyn, B.G.; Lin, V.S. Light- and pH-responsive release of doxorubicin from a mesoporous silica-based nanocarrier. Chemistry 2011, 17, 3338–3342. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhou, Y.; Zhang, Y.; Zhang, C.; Deng, X.; Dong, C.; Shuang, S. Facile fabrication route of janus gold-mesoporous silica nanocarriers with dual-drug delivery for tumor therapy. ACS Biomater. Sci. Eng. 2020, 6, 1573–1581. [Google Scholar] [CrossRef]
- Zhou, Y.; Chang, C.; Liu, Z.; Zhao, Q.; Xu, Q.; Li, C.; Chen, Y.; Zhang, Y.; Lu, B. Hyaluronic acid-functionalized hollow mesoporous silica nanoparticles as pH-sensitive nanocarriers for cancer chemo-photodynamic therapy. Langmuir 2021, 37, 2619–2628. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Y.; Wang, Q.; Liu, T.; Sun, J.; Zhang, R. Remote light-responsive nanocarriers for controlled drug delivery: Advances and perspectives. Small 2019, 15, 1903060. [Google Scholar] [CrossRef]
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2019, 17, 849–865. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Pan, Y.-J.; Chen, Y.-Y.; Wang, D.-R.; Wei, C.; Guo, J.; Lu, D.-R.; Chu, C.-C.; Wang, C.-C. Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials 2012, 33, 6570–6579. [Google Scholar] [CrossRef] [PubMed]




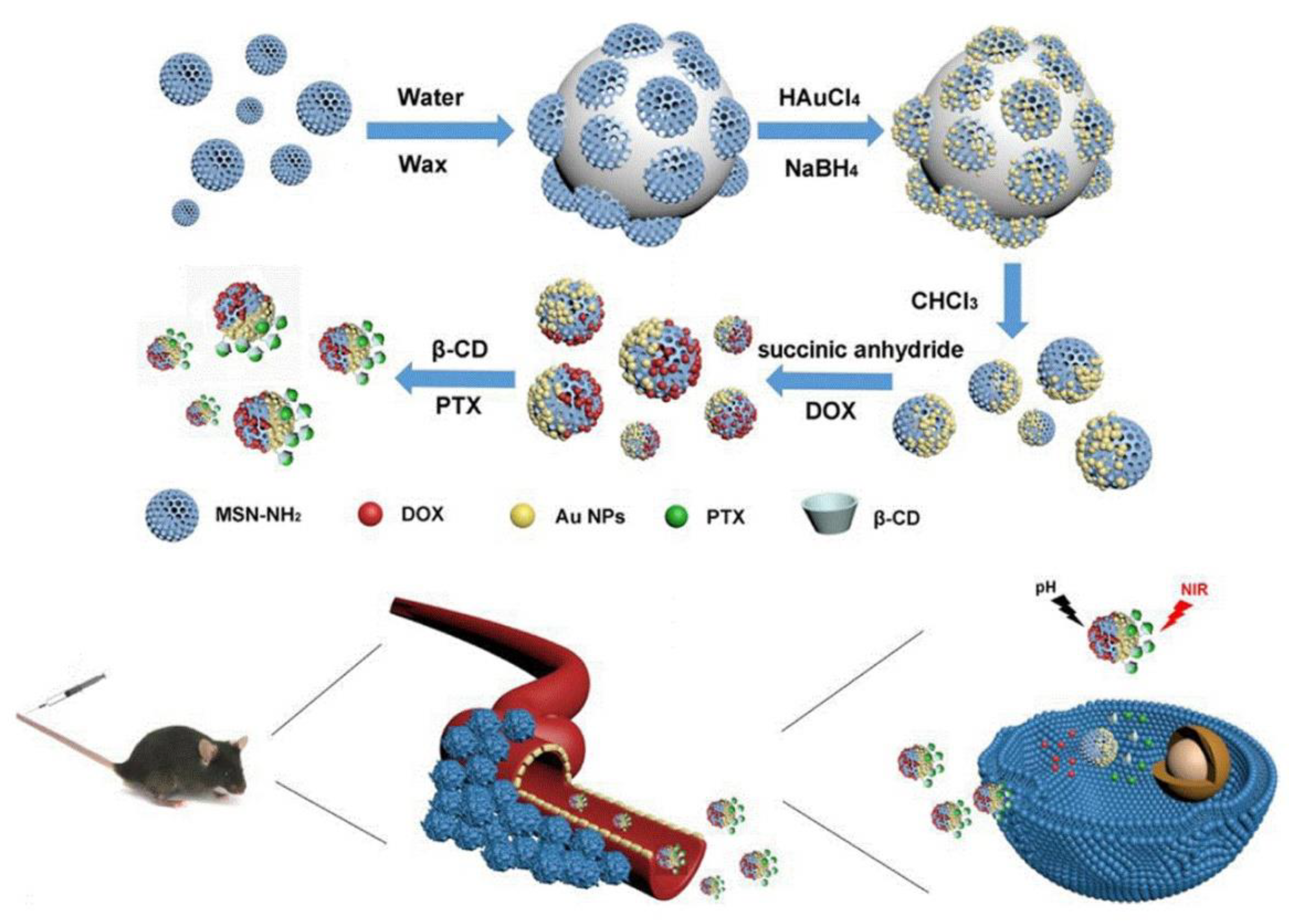
| Classification | DDS | Photosensitive Material | Wave Length | Tumor Model | Ref. |
|---|---|---|---|---|---|
| Photo-Induced Chemical Transformation | |||||
| Photo- Isomerization | Photo-responsive cationic vesicle | Azobenzene | 350 nm, 434 nm | MDA-MB-231 | [32] |
| Micelle based on SP-(PDMAEMA-block-PMMA), SP-(PMMA-block-PDMAEMA) blocks | Spiropyran | 365 nm | HeLa | [33] | |
| Photo-Induced Cleavage | MCP/DOC/shRNA | Coumarinyl ester | 405 nm, 365 nm | HepG2/ADR | [34] |
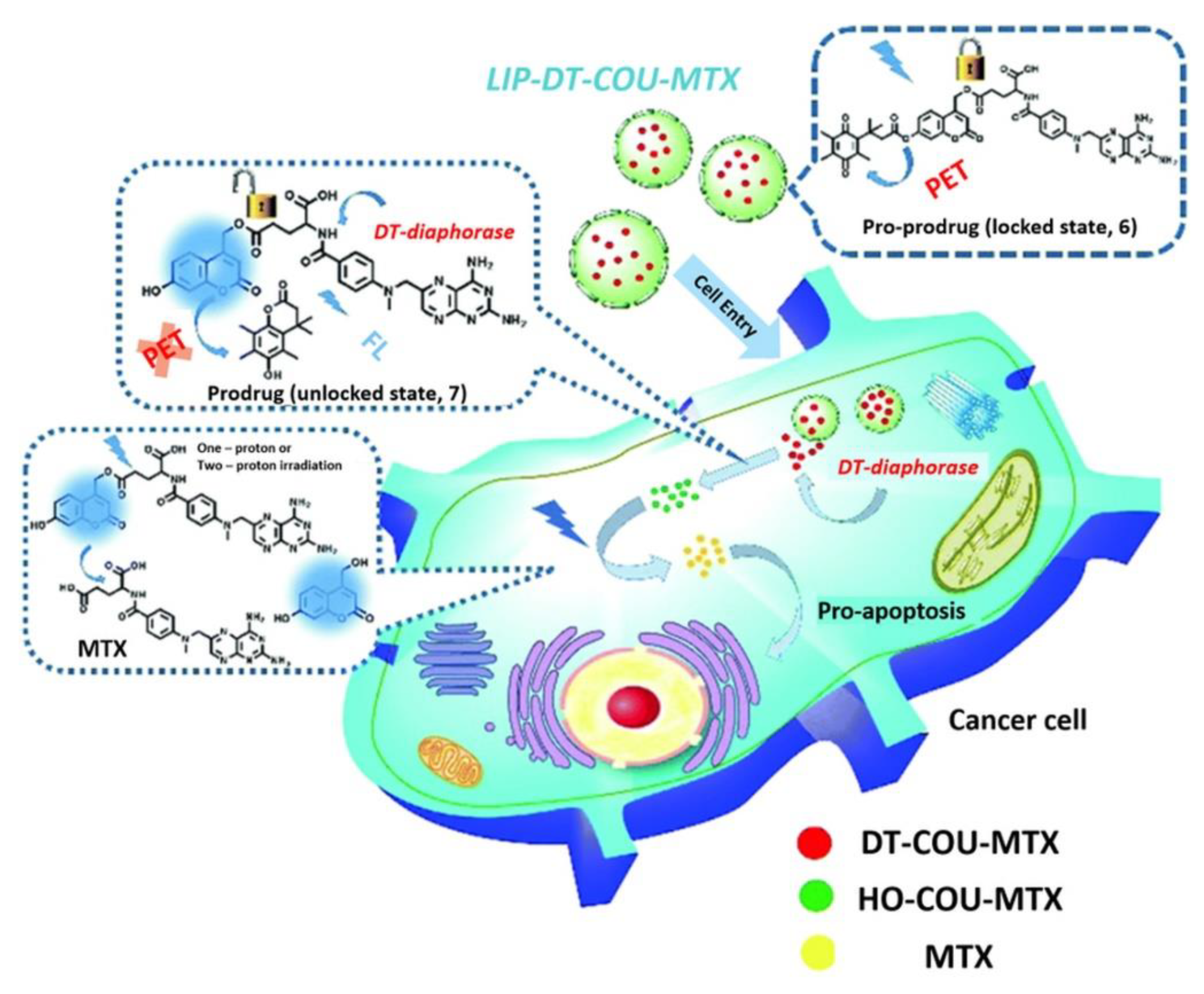
| LIP-DT-COU-MTX | Coumarin | 800 nm | HeLa, A549 | [35] | |
| Photo-mediated materials | |||||
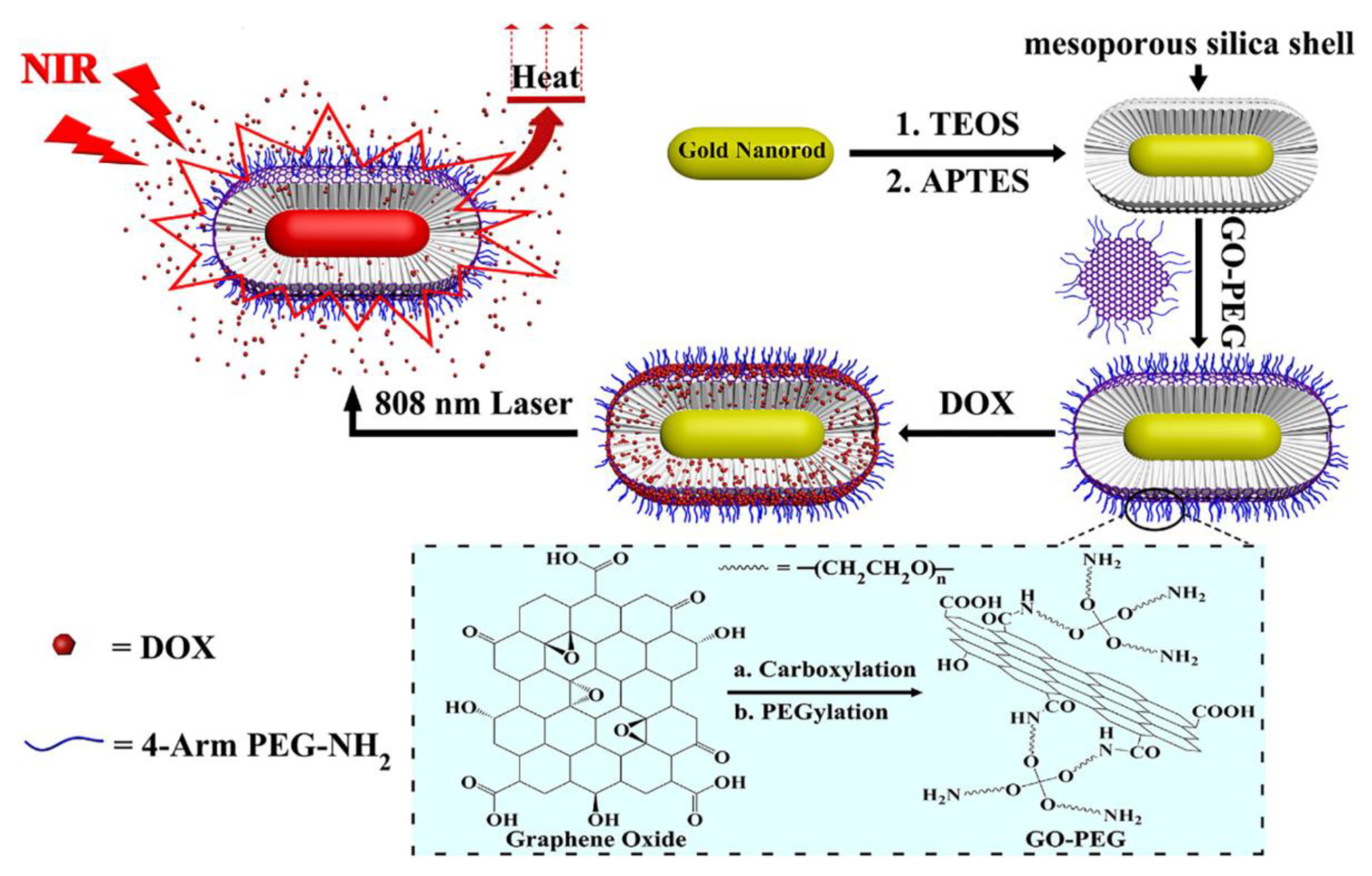
| Photothermal Therapy (PTT) | GNRs/SiO2/GO-PEG | Graphene oxide | 808 nm | MCF-7 | [36] |
| DOX@PCNFs | Carbon nanotubes | 808 nm | Mg-63 | [37] | |
| DOX/MSN–Au | Au | 808 nm | A549 | [38] | |
| CuS@MPS-DOX | Cupric sulfide | 808 nm | U87MG | [39] | |
| PBNP | Prussian blue | 808 nm | Neuro2a | [40] | |
| FM | Indocyanine green | 808 nm | KB | [41] | |
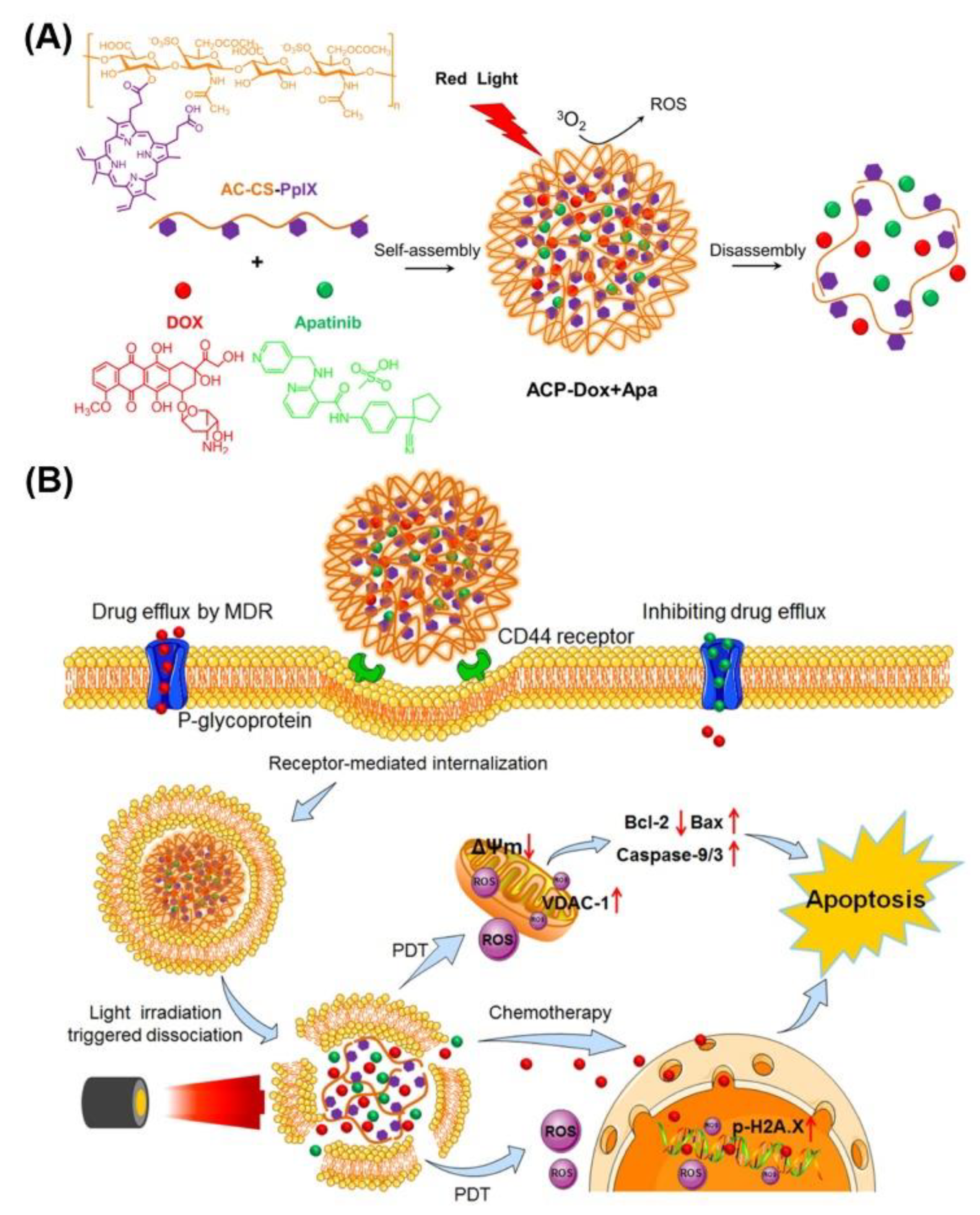
| Photodynamic Therapy (PDT) | ACP-DOX + Apa | Protoporphyrin IX | 635 nm | MCF-7/ADR | [42] |
| Rh-L-CA4 | Protoporphyrin IX | 531 nm | AY-27 | [43] | |
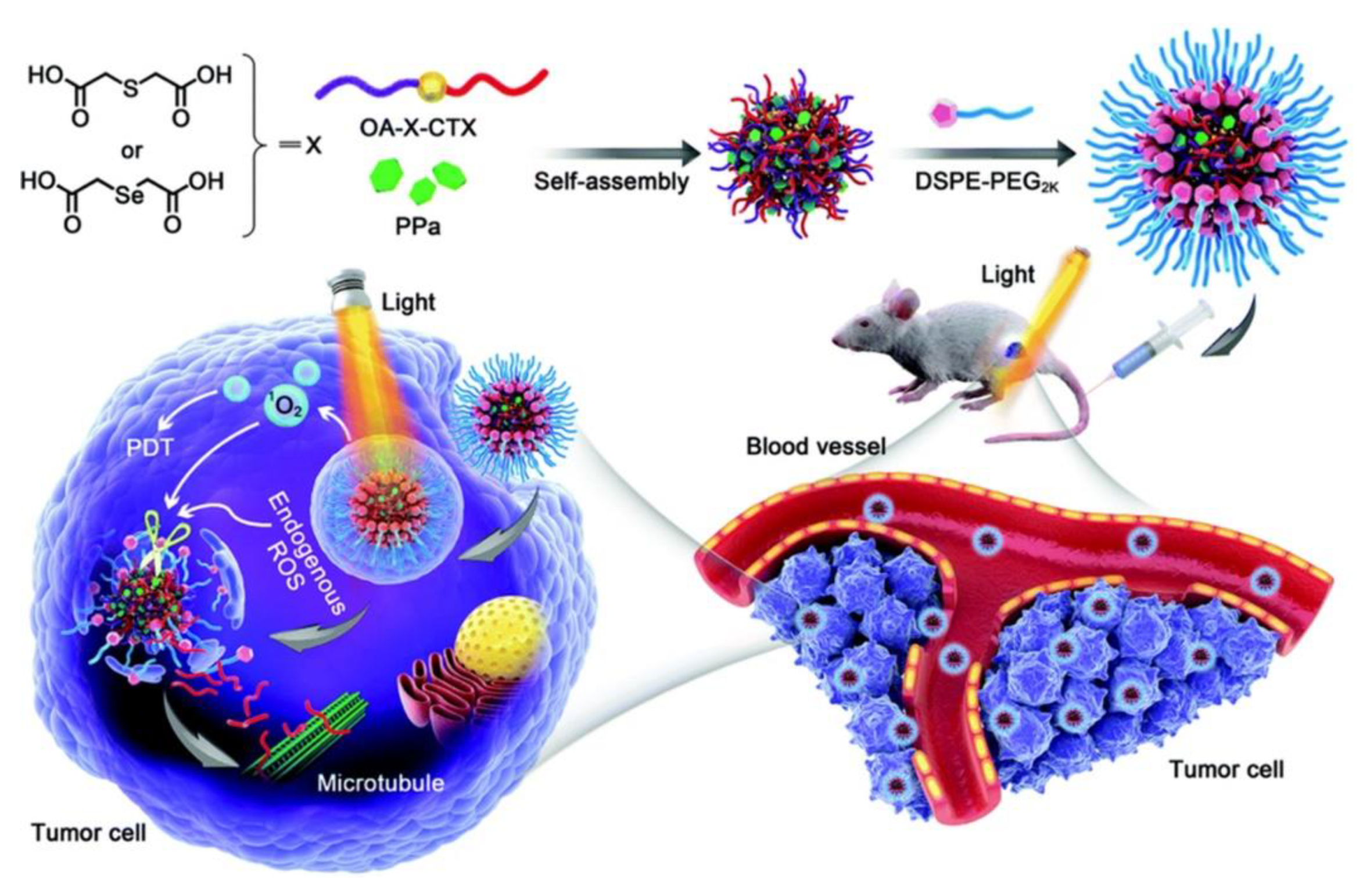
| PPa@prodrug NPs | Pyropheophorbide a | 660 nm | 4T1 | [44] | |
| Ce6-PEG-Azo-PCL | Chlorine e6 | 671 nm | HeLa | [45] | |
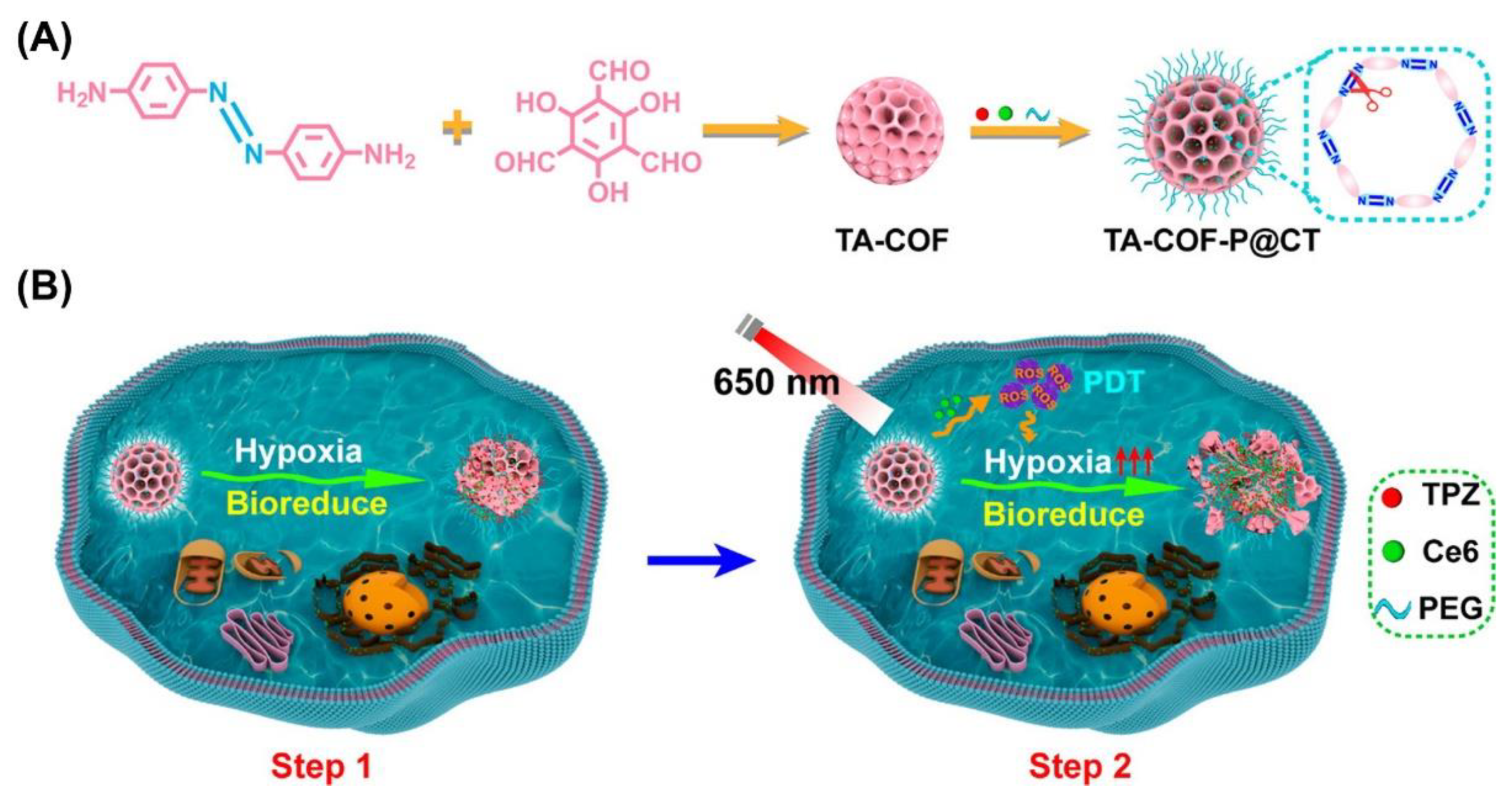
| TA-COF-P@CT | Chlorine e6 | 650 nm | 4T1 | [46] | |
| Type | Polymer | Chemical Structure | pKa |
|---|---|---|---|
| Cationic Polymers | Poly(β-amino ester) |  | 6.50 |
| Poly(2-(diisopropylamino) ethyl methacrylate) | 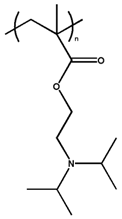 | 6.20 | |
| Poly(histidine) | 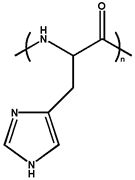 | ~7.0 | |
| Poly(aspartic acid-graft-imidazole) |  | 6.50 | |
| Poly(4-vinylpyridine) |  | 5.62 | |
| Anionic Polymers | Poly(aspartic acid) |  | 4.88 |
| Poly(acrylic acid) |  | 4.75 | |
| Poly(methacrylic acid) | 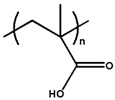 | 6.30 | |
| Poly-sulfonamides |  | 6.80 |
| Acid-Labile Bond | Chemical Structure | Degradation Products | pH Range | Ref. | ||
|---|---|---|---|---|---|---|
| 1 | C=N bond | Hydrazone |  |  | ~5.0 | [124,125] |
| Imine |  |  | ~6.8 | [126,127] | ||
| Oxime |  |  | ~5.0 | [128,129] | ||
| 2 | Acetal and Ketal bond | 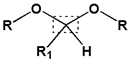 |  | 5.0~5.4 | [130,131,132] | |
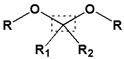 | 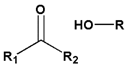 | |||||
| 3 | Amide bond | Beta- carboxyl amide | 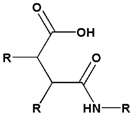 | 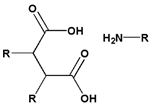 | 4.5~6.0 | [133,134] |
| Cis- aconityl amide |  | 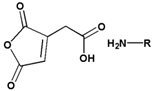 | ||||
| 4 | Ester bond | Ester |  | 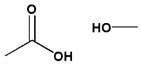 | ~6.0 | [135,136] |
| Succinic ester |  | 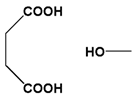 | ||||
| β-Thiopropionate |  | 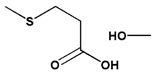 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.; Husni, P.; Kang, K.; Lee, D.; Lee, S.; Lee, E.; Youn, Y.; Oh, K. Recent Advances in pH- or/and Photo-Responsive Nanovehicles. Pharmaceutics 2021, 13, 725. https://doi.org/10.3390/pharmaceutics13050725
Shin Y, Husni P, Kang K, Lee D, Lee S, Lee E, Youn Y, Oh K. Recent Advances in pH- or/and Photo-Responsive Nanovehicles. Pharmaceutics. 2021; 13(5):725. https://doi.org/10.3390/pharmaceutics13050725
Chicago/Turabian StyleShin, Yuseon, Patihul Husni, Kioh Kang, Dayoon Lee, Sehwa Lee, Eunseong Lee, Yuseok Youn, and Kyungtaek Oh. 2021. "Recent Advances in pH- or/and Photo-Responsive Nanovehicles" Pharmaceutics 13, no. 5: 725. https://doi.org/10.3390/pharmaceutics13050725
APA StyleShin, Y., Husni, P., Kang, K., Lee, D., Lee, S., Lee, E., Youn, Y., & Oh, K. (2021). Recent Advances in pH- or/and Photo-Responsive Nanovehicles. Pharmaceutics, 13(5), 725. https://doi.org/10.3390/pharmaceutics13050725










