Multifunctional Scaffolds and Synergistic Strategies in Tissue Engineering and Regenerative Medicine
Abstract
:1. Introduction
2. Multifunctional Scaffolds
2.1. Physicochemical Cues
2.1.1. Mechanical Properties
2.1.2. Roughness and Topography
2.1.3. Wettability, Polarity and Surface Energy
2.1.4. Surface Charge
2.2. Biochemical Cues
2.2.1. Growth Factors (GFs)
2.2.2. Antibiotics and Drugs
2.2.3. Ions
2.2.4. Peptides and Proteins
3. Other Strategies in Tissue Engineering and Regenerative Medicine
3.1. Cell Therapy
3.2. Gene Therapy
3.3. Immunomodulatory Therapies
3.4. Electrical, Magnetic and Optical Stimulation
4. Multifunctional Scaffolds in Tissue Engineering
4.1. Synergistic Approaches among Physicochemical Cues
4.2. Synergistic Approaches among Biochemical Cues
4.3. Synergistic Approaches Combining Physicochemical and Biochemical Cues
5. Multifunctional Scaffolds Combined with Other Therapies
5.1. Synergistic Approaches Combining Multifunctional Scaffolds with Cell-Based Therapy
5.2. Synergistic Approaches Combining Multifunctional Scaffolds with Gene Therapy
5.3. Synergistic Approaches Combining Multifunctional Scaffolds with Immune Therapy
5.4. Synergistic Approaches Combining Multifunctional Scaffolds with Energy-Based Therapy
6. Conclusions, Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Abbreviation | Definition |
|---|---|
| AT-MSCs | Adipose tissue derived MSCs |
| ASCs | Adipose-derived stem cells |
| RGD | Arginine-glycine-aspartate |
| bFGF | Basic fibroblast growth factor |
| β-TCP | Beta-tricalcium phosphate |
| BG | Bioglass |
| BMSCs | Bone marrow stem cells |
| BMP | Bone morphogenetic proteins |
| Ca | Calcium |
| CNTs | Carbon nanotubes |
| CNCs | Cellulose nanocrystals |
| CRISPR/Cas9 | Clustered regularly interspaced short palindromic repeats -associated protein 9 |
| Col | Collagen |
| Cu | Copper |
| ECM | Extracellular matrix |
| GO | Graphene oxide |
| GFs | Growth factors |
| HDFs | Human dermal fibroblasts |
| HUVECs | Human umbilical vein endothelial cells |
| HA | Hydroxyapatite |
| nHA | Hydroxyapatite nanoparticles |
| iPCs | Induced pluripotent human stem cells |
| IGF-1 | Insulin-like growth factor–1 |
| Fe | Iron |
| Li | Lithium |
| Mg | Magnesium |
| MNPs | Magnetic nanoparticles |
| Mn | Manganese |
| MSCs | Mesenchymal stem cells |
| MAO | Microarc oxidation |
| GRGDS | Penta-peptide glycine-arginine-glycine-aspartate-serine |
| P | Phosphorus |
| pVEGF | Plasmid encoding VEGF |
| PDGF-BB | Platelet-derived growth factor–BB |
| PEDOT | Poly (3,4-ethylenedioxythiophene) |
| PGA | Poly glycolic acid |
| PLA | Poly (D, L-lactic acid) |
| PLGA | Poly (lactic-co-glycolic acid) |
| PANI | Polyaniline |
| PCL | Polycaprolactone |
| PEI | Polyethyleneimine |
| PSS | Polystyrene sulfonate |
| PVA | Polyvinyl alcohol |
| SCs | Schwann cells |
| SAMs | Self-assembled monolayers |
| Si | Silicon |
| Ag | Silver |
| SMCs | Smooth muscle cells |
| Na | Sodium |
| SDF-1α | Stromal-derived factor-1α |
| Sr | Strontium |
| TERM | Tissue engineering and regenerative medicine |
| Ti | Titanium |
| TNTs | Titanium oxide nanotubes |
| TALENs | Transcription activator-like effector nucleases |
| TGF-β1 | Transforming growth factor beta 1 |
| TGF-β3 | Transforming growth factor beta 3 |
| TNF-α | Tumor necrosis factor-alpha |
| VEGF | Vascular endothelial growth factor |
| Zn | Zinc |
| Physicochemical and Biochemical Cues | Materials | Technique | Application and Results | Ref |
|---|---|---|---|---|
| Mechanical properties and topography | Gelatin hydrogel, MNPs-decorated rod-shaped cellulose nanocrystals | Cross-linking chemistry | Cell alignment. Injectable hydrogel. | [133] |
| Surface chemistry and topography | Ti coated with Ca, P, Si and Na | MAO | Bone implant with enhanced regeneration and bone-impact contact. | [134] |
| Surface potential and topography | PCL | Electrospinning varying voltage polarity | Osteoblast proliferation, Col-like fiber formation and filopodia. | [136] |
| Surface chemistry and mechanical properties | PVA-CNT nanocomposite | Freeze drying | Osteoblast cell adhesion, proliferation, differentiation, phosphate activity, mineralization, and Col secretion. | [137] |
| Surface chemistry and electroconductivity | TNTs coated with PANI | Electrochemical oxidation and cyclic voltammetry | Enhance cell attachment, proliferation, and expression of osteogenic-related markers. | [135] |
| Surface chemistry, electroconductivity and topography | Silk coated with edged PEDOT-PSS | Electrospinning | Neuronal proliferation and differentiation. | [139] |
| Combined bioactive ions | Li and Si ions and Alginate | 3D printing | Osteoarthritis. Chondrocyte’s proliferation and maturation, and MSCs differentiation into osteogenic lineage. | [140] |
| Combined bioactive ions | Cu2+-chitosan and Sr2+-HA | Freeze drying | Bone tissue engineering. Antibacterial and osteoconductive properties. | [87] |
| Combined bioactive ions | GO coated with Cu nanoparticles and embedded into a PCL matrix | Spin coating | Bone tissue engineering. Enhance angiogenic activity, mineralization, and bactericidal effect. | [86] |
| Antibiotics and GFs | Mesoporous Titania films loaded with gentamicin and BMP-2 | EISA | Prevented Staphylococcus aureus colonization and promote preosteoblastic proliferation and differentiation. | [142] |
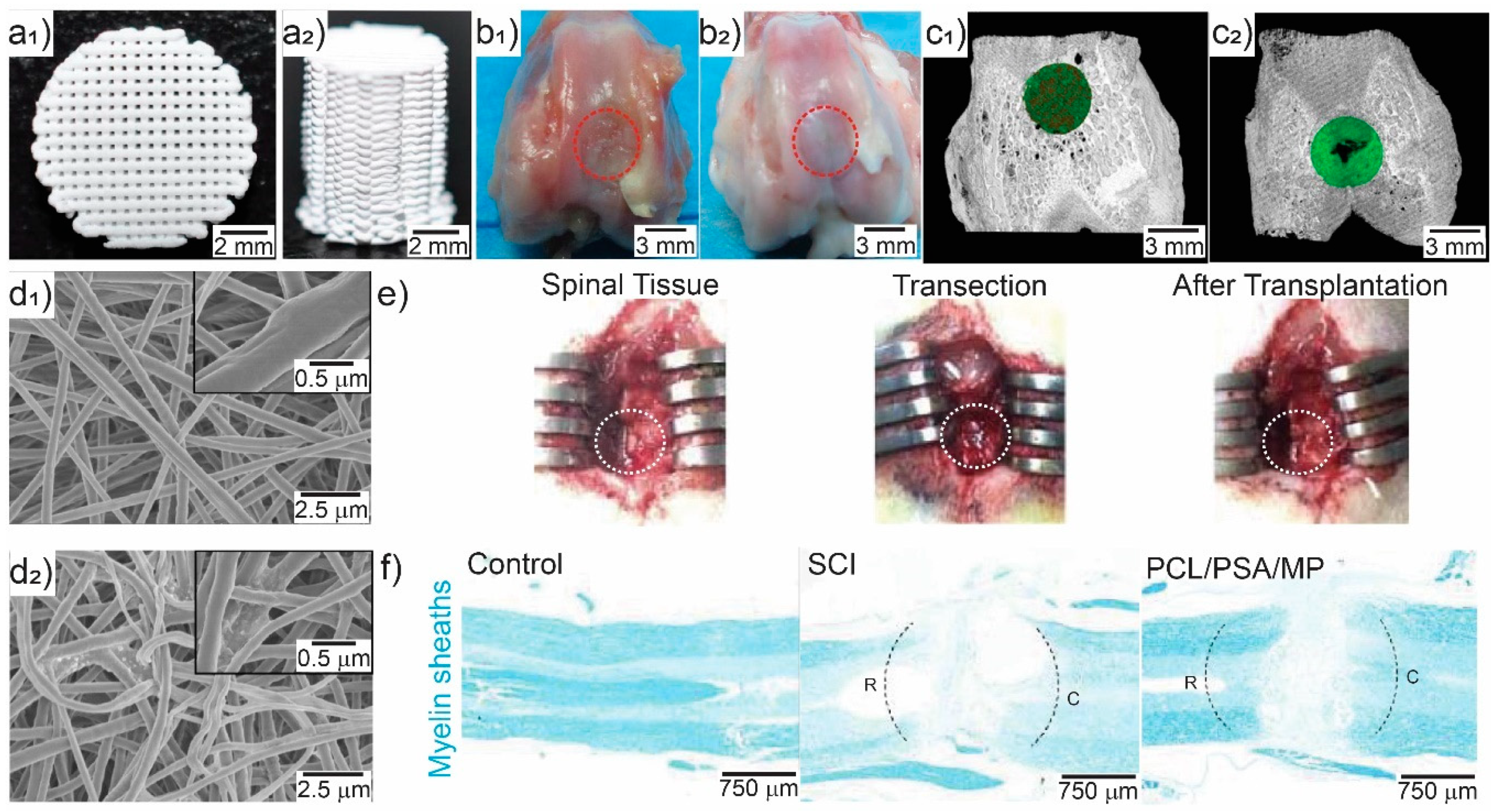
| Immunosuppressant and drug | PCL loaded with polysialic acid and methylprednisolone | Electrospinning | Spinal cord repair. Suppressed acute inflammation, apoptosis, and glia scar formation, and promoted axonal regeneration. | [141] |
| GFs and adhesive peptides | Chitosan, sodium alginate, bovine serum albumin nanoparticles, RGD, GRGDs and BMP-2 | Layer-by-Layer | BMSCs adhesion, proliferation, and differentiation into osteogenic linage. | [143] |
| GFs and adhesive peptide | Hyaluronic acid, heparin nanoparticles, RGD and VEGF | Michael addition synthesis | Neuronal repair after brain stroke. Neuronal differentiation, anti-inflammatory and angiogenic properties. | [144] |
| Mechanical properties, surface chemistry and GFs | PLGA, HA and GO loaded with bFGF and BMP-2 | Electrospinning | Bone tissue engineering. Enhanced cell adhesion, proliferation, and osteogenic differentiation. | [145] |
| Topography and bioactive proteins | Col I and IV, laminin, heparan sulfate and SMCs | Gelation at 37 °C | Muscle innervation and guided differentiation. | [146] |
| Mechanical properties and antibacterial function | GO and Ag nanoparticles loaded into PLL/PGA | Additive manufacturing | Bone tissue regeneration. Enhanced cell adhesion and proliferation. | [147] |
| Mechanical properties, surface chemistry and antibacterial | PLLA, Col, minocycline and nHA | 3D printing | Bone repair. Antibacterial properties, enhanced proliferation, and osteogenic commitment. | [148] |
| Mechanical properties and antibacterial function | Chitosan, CNCs, tetracycline | Freeze drying | Enhanced antibacterial activity, mechanical properties, osteogenic-related gene expression and mineralization. | [149] |
| Mechanical properties and bioactive proteins | Alginate/HA | 3D plotting and in situ mineralization | BMSCs improved adhesion and mineralization. | [150] |
| Topography and GFs | PCL, PLA and TGF-β1-loaded chitosan nanoparticles | Electrospinning | SMCs differentiation. | [151] |
| Topography, bioactive ions, and proteins | BG ionic products and cell culture media | Electrospinning | Skin tissue engineering. Improved wound healing. | [152] |
| Topography, mechanical properties, and drugs | Gelatin/β-TCP, zoledronic acid | Solvent Casting | Bone defect regeneration. Enhanced new bone formation and vascularization. | [153] |
| Topography and bioactive ions | HA-coated PLGA/45S5 BG | Sponge replica and electrospinning | Enhanced proliferation, differentiation towards osteogenic lineage, and mineralization. | [154] |
| Porosity and GFs | Methacrylate gelatin, gellan gum, HA, osteogenic GFs | Photopolymerization, ice templating and freeze drying | Prevascularized 3D osteochondral tissue constructs. | [155] |
| Topography and ECM components | PCL microfibers, in vivo engineered ECM scaffolds | Melt-spinning, decellularization | Cell guidance. Oriented tissue regeneration. | [158] |
| Multifunctional Scaffolds Combined Strategies | Materials | Technique | Application and Results | Ref |
|---|---|---|---|---|
| Cell therapy | Decellularized cardiac ECM scaffolds and human umbilical cord MSCs | Chemical-based decellularization and freeze drying | Macrophage polarization towards M2 phenotype and promotion of skeletal muscle tissue regeneration. | [159] |
| Decellularized tendons from cadaveric forearms, ASCs, bFGF, IGF-1, PDGF-BB | Chemical-based decellularization | Improved ASCs proliferation and endogenous repopulation. | [160] | |
| PCL, bFGF, connective tissue growth factor, rat MSCs | Electrospinning | Abdominal wall defect repair. Improved biochemical and biomechanical properties in abdominal wall. | [162] | |
| Hyaluronic, adhesive peptide, MSCs | Crosslinking | Spinal cord transection recovery, restored locomotor functions and reduced inflammation. | [161] | |
| Gene therapy | Col/Calcium Phosphate, pVEGF | Gelation | Promote angiogenesis and bone formation in mouse intra-femoral defects. | [163] |
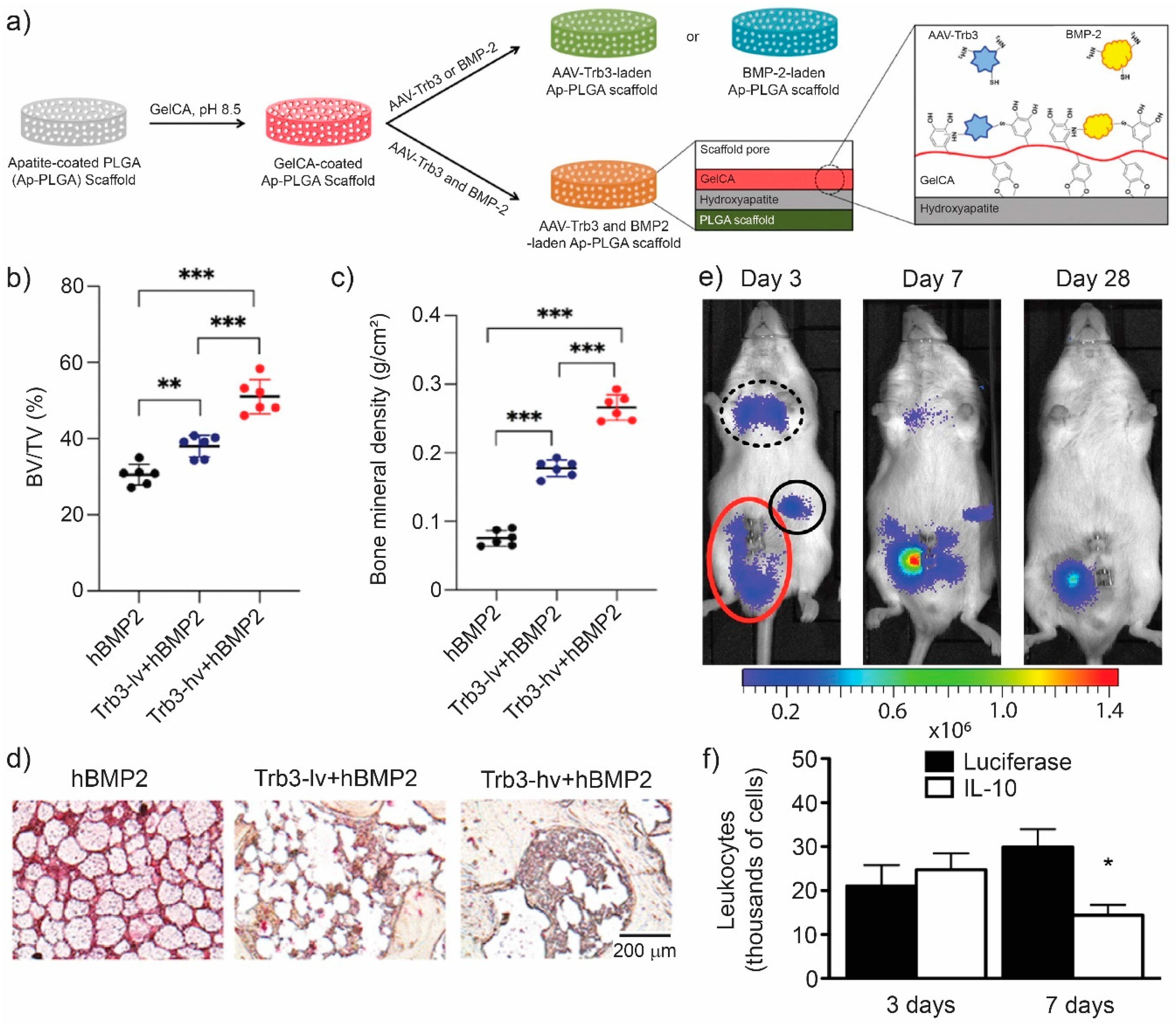
| HA-coated PLGA, Trb3 encapsulated in gelatin-conjugated caffeic acid | Solvent casting and leaching | New bone formation, inhibited fat-filled cyst formation in a non-healing mandibular defect rodent model. | [164] | |
| Col-Chondroitin sulfate, PEI, SDF-1α, proangiogenic chemokine gene | Freeze drying and cross-linking | SCs differentiation and angiogenesis. | [166] | |
| Col/nHA, BMP, pVEGF | Freeze drying and cross-linking | Bone regeneration, increased vascularization. | [167] | |
| bFGF-transfected BMSCs, nHA/polyamine 66 | Phase separation | Bone regeneration and vascularization in rat calvarial critical sized defect model. | [168] | |
| Alginate, nHA, BMSCs, plasmid BMP-2, pTGF-β3 | Ionic cross-linking | Selective differentiation of BMSCs towards cartilage or endochondral bone tissue. | [169] | |
| Immune therapy | Poly-L-lysine, hyaluronic acid, IL-4 | Layer by layer and cross-linking | Decrease immune reactions in implant rejection, improved mechanical properties, guided monocyte polarization towards anti-inflammatory and pro-healing phenotype. | [170] |
| Polydioxanone | Electrospinning | Induced M2-like profile that promotes angiogenesis. | [171] | |
| Poly (carboxybetaine methacrylate) and poly (2-hydroxyethyl methacrylate) | Photopolymerization | Prevent foreign-body reaction and capsule formation, promote healing polarized macrophages and angiogenesis. | [172] | |
| PLGA, TNF-α, MSCs | Salt fusion/solvent casting/salt leaching | Achilles’ tendon repair, M2 polarization, anti-inflammatory environment, increased type I procollagen. | [173] | |
| Decellularized MSCs | Chemical-based decellularization and freeze drying | Volumetric muscle loss, M2 polarization, skeletal muscle regeneration. | [159] | |
| Poly-L-lysine, dopamine, anti-CD40 antibody | Electrospinning | Cancer therapy. Kill tumor cells, support adhesion and proliferation of MC3T3-E1 cells. | [174] | |
| PLGA, lentivirus encoding IL-10 | Gas foaming | Reduce inflammation and leukocyte infiltration. | [165] | |
| Electrical stimulation | PLLA, PEDOT | Electrospinning | Increased MSCs growth, activity, and tissue-like formation. | [175] |
| Magnetic stimulation | Xanthan gum, chitosan, iron oxide MNPs | Self-organization | NIH3T3 fibroblast enhanced adhesion and proliferation. Hydrogel enhanced mechanical properties. | [176] |
| Photothermal stimulation | nHA/GO/Chitosan | Freeze drying and cross-linking | Treatment of osteosarcoma and tissue regeneration. | [177] |
| Photothermal stimulation | Gelatin, akermanite, CNTs, iron oxide MNPs | Freeze drying and cross-linking | Cancerous bone tumor treatment and bone tissue regeneration. | [178] |
| Cell therapy + electrical stimulation | β-TCP, AT-MSCs | Rehydration | Regeneration in large bone defects. Improved bone formation, vascularization, and less fibrous tissue. | [180] |
| Cell therapy + electro-mechanical stimulation | PLGA, polypyrrole, iPCs | Electrospinning | Cardiac tissue engineering, Improved expression of cardiac markers. | [181] |
| Cell therapy + electrical stimulation | PANI/polyestersulfone, Camphor-10-sulphonic acid, cardiovascular disease-specific iPSCs | Electrospinning | Cardiovascular diseases. Generation of cardiomyocytes. | [104] |
| GFs + electrical stimulation | PLA-AP, PLGA/HA, BMP-4, PEI coated gold nanoparticles | Freeze drying | Bone healing. Improved cell proliferation and differentiation. | [179] |
| Cellular Process | Facilitation Strategies | Ref |
|---|---|---|
| Adhesion | Stiffness and complex stiffness | [28,137,150,178] |
| Nano-micrometer surface roughness | [36] | |
| Fibronectin, Col, and adhesion promoting peptides | [93,94,143,161] | |
| Electrical stimulation | [123] | |
| Magnetic stimulation | [16,176] | |
| Surface chemistry (wettability, charge, and potential) | [51,52,53,54,56,136] | |
| Electroconductive surface | [135] | |
| Alignment, recruitment, and migration | Gradients in substrate stiffness (durotaxis) | [30] |
| Surface topographical cues (nanofibers, microridges, porous channels, etc.) | [38,39,40,133,158] | |
| Local asymmetric topographical ratchets (ratchetaxis) | [41] | |
| Growth factor, chemokines, and others chemical stimuli (chemotaxis) | [73] | |
| Electrical stimulation and electric field gradient (galvanotaxis) | [123,127] | |
| Magnetic stimulation | [16] | |
| Differentiation and polarization | Stiffness guided | [31,137,149,150] |
| Micropattern, nanotopography and porosity | [37,171] | |
| Growth factor and cytokine delivery | [72,142,143,145,170] | |
| Ion delivery | [83,140] | |
| Gene therapy (e.g., genes encoding growth factors) | [112,164,167,169] | |
| Electrical stimulation | [123,180] | |
| Magnetic stimulation | [130] | |
| Optical stimulation | [19] | |
| Electroconductive surface | [135,139] | |
| Bioactive polymers (e.g., hyaluronic acid) and ECM composition | [144,146,159] | |
| MSCs | [159] | |
| Proliferation | Growth factor and drug delivery | [142,145,153,160] |
| Ion delivery | [84,140] | |
| Electrical stimulation | [123,127] | |
| Magnetic stimulation | [16] | |
| Optical stimulation | [19] | |
| Surface chemistry (wettability, charge, and potential) | [136] | |
| Stiffness | [137] | |
| Electroconductive surface | [135,139] | |
| Adhesion promoting peptides | [143] | |
| Vascularization | VEGF delivery | [71,144] |
| Gene therapy (genes encoding VEGF, chemokines, etc.) | [112,114,163,166,167,168] | |
| Ion delivery | [86] |
References
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [Green Version]
- Frey, B.M.; Zeisberger, S.M.; Hoerstrup, S.P. Tissue Engineering and Regenerative Medicine-New Initiatives for Individual Treatment Offers. Transfus. Med. Hemotherapy 2016, 43, 318–320. [Google Scholar] [CrossRef] [Green Version]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef] [Green Version]
- Walma, D.A.C.; Yamada, K.M. The extracellular matrix in development. Development 2020, 147, dev175596. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195. [Google Scholar] [CrossRef] [Green Version]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Gubbiotti, M.A. Extracellular matrix: The driving force of mammalian diseases. Matrix Biol. 2018, 71–72, 1–9. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, M.; Göransson, S.A.; Strömblad, S. Cell to extracellular matrix interactions and their reciprocal nature in cancer. Exp. Cell Res. 2013, 319, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Lidén, Å.; Karlsen, T.V.; Guss, B.; Reed, R.K.; Rubin, K. Integrin αVβ3 can substitute for collagen-binding β1-integrins in vivo to maintain a homeostatic interstitial fluid pressure. Exp. Physiol. 2018, 103, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Wiig, H.; Swartz, M.A. Interstitial Fluid and Lymph Formation and Transport: Physiological Regulation and Roles in Inflammation and Cancer. Physiol. Rev. 2012, 92, 1005–1060. [Google Scholar] [CrossRef]
- Muzzio, N.; Azzaroni, O.; Moya, S.; Pasquale, M. Concepts for Designing Tailored Thin Film Surfaces with Potential Biological Applications, Multilayer Thin Films-Versatile Applications for Materials Engineering; Basu, S., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Hippler, M.; Lemma, E.D.; Bertels, S.; Blasco, E.; Barner-Kowollik, C.; Wegener, M.; Bastmeyer, M. 3D Scaffolds to Study Basic Cell Biology. Adv. Mater. 2019, 31, 1808110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golchin, A.; Farahany, T.Z. Biological Products: Cellular Therapy and FDA Approved Products. Stem Cell Rev. Rep. 2019, 15, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Lorden, E.R.; Levinson, H.M.; Leong, K.W. Integration of drug, protein, and gene delivery systems with regenerative medicine. Drug Deliv. Transl. Res. 2015, 5, 168–186. [Google Scholar] [CrossRef] [Green Version]
- Ross, C.L. The use of electric, magnetic, and electromagnetic field for directed cell migration and adhesion in regenerative medicine. Biotechnol. Prog. 2017, 33, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Rodrigues, M.T.; Gomes, M.E. Contributions and future perspectives on the use of magnetic nanoparticles as diagnostic and therapeutic tools in the field of regenerative medicine. Expert Rev. Mol. Diagn. 2013, 13, 553–566. [Google Scholar] [CrossRef]
- Richardson, R.T.; Ibbotson, M.R.; Thompson, A.C.; Wise, A.K.; Fallon, J.B. Optical stimulation of neural tissue. Healthc. Technol. Lett. 2020, 7, 58–65. [Google Scholar] [CrossRef]
- Moura-Netto, C.; Ferreira, L.S.; Maranduba, C.M.; Mello-Moura, A.C.V.; Marques, M.M. Low-intensity laser phototherapy enhances the proliferation of dental pulp stem cells under nutritional deficiency. Braz. Oral Res. 2016, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef]
- Gomes, M.E.; Rodrigues, M.T.; Domingues, R.M.A.; Reis, R.L. Tissue Engineering and Regenerative Medicine: New Trends and Directions—A Year in Review. Tissue Eng. Part B Rev. 2017, 23, 211–224. [Google Scholar] [CrossRef]
- Mertgen, A.-S.; Trossmann, V.T.; Guex, A.G.; Maniura-Weber, K.; Scheibel, T.; Rottmar, M. Multifunctional Biomaterials: Combining Material Modification Strategies for Engineering of Cell-Contacting Surfaces. ACS Appl. Mater. Interfaces 2020, 12, 21342–21367. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139. [Google Scholar] [CrossRef] [Green Version]
- Forces in cell biology. Nat. Cell Biol. 2017, 19, 579. [CrossRef] [PubMed] [Green Version]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Mih, J.D.; Sharif, A.S.; Liu, F.; Marinkovic, A.; Symer, M.M.; Tschumperlin, D.J. A Multiwell Platform for Studying Stiffness-Dependent Cell Biology. PLoS ONE 2011, 6, e19929. [Google Scholar] [CrossRef] [PubMed]
- Buxboim, A.; Ivanovska, I.L.; Discher, D.E. Matrix elasticity, cytoskeletal forces and physics of the nucleus: How deeply do cells ‘feel’ outside and in? J. Cell Sci. 2010, 123, 297. [Google Scholar] [CrossRef] [Green Version]
- Muzzio, N.E.; Pasquale, M.A.; Marmisollé, W.A.; von Bilderling, C.; Cortez, M.L.; Pietrasanta, L.I.; Azzaroni, O. Self-assembled phosphate-polyamine networks as biocompatible supramolecular platforms to modulate cell adhesion. Biomater. Sci. 2018, 6, 2230–2247. [Google Scholar] [CrossRef]
- Georges, P.C.; Miller, W.J.; Meaney, D.F.; Sawyer, E.S.; Janmey, P.A. Matrices with Compliance Comparable to that of Brain Tissue Select Neuronal over Glial Growth in Mixed Cortical Cultures. Biophys. J. 2006, 90, 3012–3018. [Google Scholar] [CrossRef] [Green Version]
- Whang, M.; Kim, J. Synthetic hydrogels with stiffness gradients for durotaxis study and tissue engineering scaffolds. Tissue Eng. Regen. Med. 2016, 13, 126–139. [Google Scholar] [CrossRef]
- Xing, F.; Li, L.; Zhou, C.; Long, C.; Wu, L.; Lei, H.; Kong, Q.; Fan, Y.; Xiang, Z.; Zhang, X. Regulation and Directing Stem Cell Fate by Tissue Engineering Functional Microenvironments: Scaffold Physical and Chemical Cues. Stem Cells Int. 2019, 2019, 2180925. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.N.; Jiao, A.; Hwang, N.S.; Kim, M.S.; Kang, D.H.; Kim, D.-H.; Suh, K.-Y. Nanotopography-guided tissue engineering and regenerative medicine. Adv. Drug Deliv. Rev. 2013, 65, 536–558. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.-W.; Shiwarski, D.J.; Ball, R.L.; Whitehead, K.A.; Feinberg, A.W. Engineering Aligned Skeletal Muscle Tissue Using Decellularized Plant-Derived Scaffolds. ACS Biomater. Sci. Eng. 2020, 6, 3046–3054. [Google Scholar] [CrossRef] [PubMed]
- Firkowska-Boden, I.; Zhang, X.; Jandt, K.D. Controlling Protein Adsorption through Nanostructured Polymeric Surfaces. Adv. Healthc. Mater. 2018, 7, 1700995. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, K.; Gu, X.; Leong, K.W. Biophysical Regulation of Cell Behavior—Cross Talk between Substrate Stiffness and Nanotopography. Engineering 2017, 3, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Li, Y.; Zhang, L.; Jin, L.; Yuan, F.; Tan, J.; Yuan, G.; Pei, J. Nano-micrometer surface roughness gradients reveal topographical influences on differentiating responses of vascular cells on biodegradable magnesium. Bioact. Mater. 2021, 6, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, X.; Gao, L.; Jing, L.; Zhou, Q.; Chang, J. The role of the micro-pattern and nano-topography of hydroxyapatite bioceramics on stimulating osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018, 73, 509–521. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Li, X.; Jiang, Y.-C.; Han, S.; Ma, L.; Guo, H.; Wang, Z.; Li, Q. Endothelial Cell Migration on Poly(ε-caprolactone) Nanofibers Coated with a Nanohybrid Shish-Kebab Structure Mimicking Collagen Fibrils. Biomacromolecules 2020, 21, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Muzzio, N.E.; Horowitz, C.M.; Azzaroni, O.; Moya, S.E.; Pasquale, M.A. Tilted mammalian cell colony propagation dynamics on patterned substrates. Chaos Solitons Fractals 2021, 146, 110841. [Google Scholar] [CrossRef]
- Wang, X.; Lin, M.; Kang, Y. Engineering Porous β-Tricalcium Phosphate (β-TCP) Scaffolds with Multiple Channels to Promote Cell Migration, Proliferation, and Angiogenesis. ACS Appl. Mater. Interfaces 2019, 11, 9223–9232. [Google Scholar] [CrossRef]
- Caballero, D.; Comelles, J.; Piel, M.; Voituriez, R.; Riveline, D. Ratchetaxis: Long-Range Directed Cell Migration by Local Cues. Trends Cell Biol. 2015, 25, 815–827. [Google Scholar] [CrossRef]
- Arias, C.J.; Surmaitis, R.L.; Schlenoff, J.B. Cell Adhesion and Proliferation on the “Living” Surface of a Polyelectrolyte Multilayer. Langmuir 2016, 32, 5412–5421. [Google Scholar] [CrossRef]
- Xu, J.; Mosher, D. Fibronectin and Other Adhesive Glycoproteins. In The Extracellular Matrix: An Overview; Mecham, R.P., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2011; pp. 41–75. [Google Scholar]
- Ngandu Mpoyi, E.; Cantini, M.; Reynolds, P.M.; Gadegaard, N.; Dalby, M.J.; Salmerón-Sánchez, M. Protein Adsorption as a Key Mediator in the Nanotopographical Control of Cell Behavior. ACS Nano 2016, 10, 6638–6647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Helbing, C.; Arras, M.M.L.; Jandt, K.D.; Firkowska-Boden, I. Nanocrystal Width Controls Fibrinogen Orientation and Assembly Kinetics on Poly(butene-1) Surfaces. Langmuir 2017, 33, 6563–6571. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.R.; Williams, P. Water contact angle is not a good predictor of biological responses to materials. Biointerphases 2017, 12, 02C201. [Google Scholar] [CrossRef] [Green Version]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Muzzio, N.E.; Pasquale, M.A.; Rios, X.; Azzaroni, O.; Llop, J.; Moya, S.E. Adsorption and Exchangeability of Fibronectin and Serum Albumin Protein Corona on Annealed Polyelectrolyte Multilayers and Their Consequences on Cell Adhesion. Adv. Mater. Interfaces 2019, 6, 1900008. [Google Scholar] [CrossRef]
- Sista, S.; Wen, C.E.; Hodgson, P.D.; Pande, G. The influence of surface energy of titanium-zirconium alloy on osteoblast cell functions in vitro. J. Biomed. Mater. Res. Part A 2011, 97A, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Pegueroles, M.; Aparicio, C.; Bosio, M.; Engel, E.; Gil, F.J.; Planell, J.A.; Altankov, G. Spatial organization of osteoblast fibronectin matrix on titanium surfaces: Effects of roughness, chemical heterogeneity and surface energy. Acta Biomater. 2010, 6, 291–301. [Google Scholar] [CrossRef]
- Lai, H.-C.; Zhuang, L.-F.; Liu, X.; Wieland, M.; Zhang, Z.-Y.; Zhang, Z.-Y. The influence of surface energy on early adherent events of osteoblast on titanium substrates. J. Biomed. Mater. Res. Part A 2010, 93A, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. Part A 2005, 74A, 49–58. [Google Scholar] [CrossRef]
- Comelles, J.; Estévez, M.; Martínez, E.; Samitier, J. The role of surface energy of technical polymers in serum protein adsorption and MG-63 cells adhesion. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 44–51. [Google Scholar] [CrossRef]
- Nakamura, M.; Hori, N.; Ando, H.; Namba, S.; Toyama, T.; Nishimiya, N.; Yamashita, K. Surface free energy predominates in cell adhesion to hydroxyapatite through wettability. Mater. Sci. Eng. C 2016, 62, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Schlenoff, J.B. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.-L.; Chang, H.-Y.; Lin, K.-Y.; Lee, Y.-W.; Shyue, J.-J. Effect of Surface Potential on the Adhesion Behavior of NIH3T3 Cells Revealed by Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D). J. Phys. Chem. C 2017, 121, 533–541. [Google Scholar] [CrossRef]
- Liamas, E.; Black, R.A.; Mulheran, P.A.; Tampé, R.; Wieneke, R.; Thomas, O.R.T.; Zhang, Z.J. Probing fibronectin adsorption on chemically defined surfaces by means of single molecule force microscopy. Sci. Rep. 2020, 10, 15662. [Google Scholar] [CrossRef] [PubMed]
- Baujard-Lamotte, L.; Noinville, S.; Goubard, F.; Marque, P.; Pauthe, E. Kinetics of conformational changes of fibronectin adsorbed onto model surfaces. Colloids Surf. B Biointerfaces 2008, 63, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Mezzenga, R.; Mitsi, M. The Molecular Dance of Fibronectin: Conformational Flexibility Leads to Functional Versatility. Biomacromolecules 2019, 20, 55–72. [Google Scholar] [CrossRef]
- Chen, D.; Smith, L.R.; Khandekar, G.; Patel, P.; Yu, C.K.; Zhang, K.; Chen, C.S.; Han, L.; Wells, R.G. Distinct effects of different matrix proteoglycans on collagen fibrillogenesis and cell-mediated collagen reorganization. Sci. Rep. 2020, 10, 19065. [Google Scholar] [CrossRef]
- Wolanska, K.I.; Morgan, M.R. Fibronectin remodelling: Cell-mediated regulation of the microenvironment. Biochem. Soc. Trans. 2015, 43, 122–128. [Google Scholar] [CrossRef]
- Stone, W.L.; Leavitt, L.; Varacallo, M. Physiology, Growth Factor. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Lovicu, F.J.; McAvoy, J.W.; de Iongh, R.U. Understanding the role of growth factors in embryonic development: Insights from the lens. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1204–1218. [Google Scholar] [CrossRef] [Green Version]
- Behm, B.; Babilas, P.; Landthaler, M.; Schreml, S. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 812–820. [Google Scholar] [CrossRef]
- Witsch, E.; Sela, M.; Yarden, Y. Roles for Growth Factors in Cancer Progression. Physiology 2010, 25, 85–101. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, A.C.; Briquez, P.S.; Hubbell, J.A.; Cochran, J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016, 30, 1–12. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, S.R.; Yoon, I.-S. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.-M.; Zhang, M.; Wu, Z.-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010, 31, 6279–6308. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Ou, K.-L.; Mao, C.; Hosseinkhani, H. Importance of dual delivery systems for bone tissue engineering. J. Control. Release 2016, 225, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Kempen, D.H.R.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J.A. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 2009, 30, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, C.; Zhao, Q.; Li, X.; Xu, F.; Yao, X.; Wang, M. Incorporation and release of dual growth factors for nerve tissue engineering using nanofibrous bicomponent scaffolds. Biomed. Mater. 2018, 13, 044107. [Google Scholar] [CrossRef] [Green Version]
- Liebesny, P.H.; Byun, S.; Hung, H.-H.; Pancoast, J.R.; Mroszczyk, K.A.; Young, W.T.; Lee, R.T.; Frisbie, D.D.; Kisiday, J.D.; Grodzinsky, A.J. Growth Factor-Mediated Migration of Bone Marrow Progenitor Cells for Accelerated Scaffold Recruitment. Tissue Eng. Part A 2016, 22, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Escobar, A.; Muzzio, N.; Moya, S.E. Antibacterial Layer-by-Layer Coatings for Medical Implants. Pharmaceutics 2021, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Heras, C.; Jiménez-Holguín, J.; Doadrio, A.L.; Vallet-Regí, M.; Sánchez-Salcedo, S.; Salinas, A.J. Multifunctional antibiotic- and zinc-containing mesoporous bioactive glass scaffolds to fight bone infection. Acta Biomater. 2020, 114, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, J.S.; Blaisse, M.R.; Samuel, R.E.; Hsu, H.-P.; Harris, M.B.; Martin, S.D.; Lee, J.C.; Spector, M.; Hammond, P.T. The effectiveness of the controlled release of gentamicin from polyelectrolyte multilayers in the treatment of Staphylococcus aureus infection in a rabbit bone model. Biomaterials 2010, 31, 6019–6030. [Google Scholar] [CrossRef] [Green Version]
- Escobar, A.; Muzzio, N.E.; Andreozzi, P.; Libertone, S.; Tasca, E.; Azzaroni, O.; Grzelczak, M.; Moya, S.E. Antibacterial Layer-by-Layer Films of Poly(acrylic acid)–Gentamicin Complexes with a Combined Burst and Sustainable Release of Gentamicin. Adv. Mater. Interfaces 2019, 6, 1901373. [Google Scholar] [CrossRef]
- Aksel, H.; Mahjour, F.; Bosaid, F.; Calamak, S.; Azim, A.A. Antimicrobial Activity and Biocompatibility of Antibiotic-Loaded Chitosan Hydrogels as a Potential Scaffold in Regenerative Endodontic Treatment. J. Endod. 2020, 46, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qian, S.; Wang, L.; Zeng, J.; Miao, R.; Meng, Y.; Jin, Y.; Chen, H.; Wang, B. Reversible antibiotic loading and pH-responsive release from polymer brushes on contact lenses for therapy and prevention of corneal infections. J. Mater. Chem. B 2020, 8, 10087–10092. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Janarthanan, G.; Noh, I. Recent trends in metal ion based hydrogel biomaterials for tissue engineering and other biomedical applications. J. Mater. Sci. Technol. 2021, 63, 35–53. [Google Scholar] [CrossRef]
- Johann, D.R.; Alfred, D.; Parvis, F. Efficacy of strontium ranelate on bone mineral density in men with osteoporosis. Arzneimittelforschung 2010, 60, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhou, G.; Luk, K.D.K.; Cheung, K.M.C.; Li, Z.; Lam, W.M.; Zhou, Z.; Lu, W.W. Strontium Promotes Osteogenic Differentiation of Mesenchymal Stem Cells Through the Ras/MAPK Signaling Pathway. Cell. Physiol. Biochem. 2009, 23, 165–174. [Google Scholar] [CrossRef]
- Escobar, A.; Muzzio, N.E.; Martínez-Villacorta, Á.M.; Abarrategi, A.; Bindini, E.; Grzelczak, M.; Bordoni, A.V.; Angelomé, P.C.; Moya, S.E. Mesoporous titania coatings with carboxylated pores for complexation and slow delivery of strontium for osteogenic induction. Appl. Surf. Sci. 2020, 510, 145172. [Google Scholar] [CrossRef]
- Gregurec, D.; Politakos, N.; Yate, L.; Moya, S.E. Strontium confinement in polyacrylic acid brushes: A soft nanoarchitectonics approach for the design of titania coatings with enhanced osseointegration. Mol. Syst. Des. Eng. 2019, 4, 421–430. [Google Scholar] [CrossRef]
- Jaidev, L.R.; Kumar, S.; Chatterjee, K. Multi-biofunctional polymer graphene composite for bone tissue regeneration that elutes copper ions to impart angiogenic, osteogenic and bactericidal properties. Colloids Surf. B Biointerfaces 2017, 159, 293–302. [Google Scholar] [CrossRef]
- Gritsch, L.; Maqbool, M.; Mouriño, V.; Ciraldo, F.E.; Cresswell, M.; Jackson, P.R.; Lovell, C.; Boccaccini, A.R. Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: Copper and strontium. J. Mater. Chem. B 2019, 7, 6109–6124. [Google Scholar] [CrossRef] [Green Version]
- Hynes, R.O.; Naba, A. Overview of the Matrisome—An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [Green Version]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Coles, J.M.; Chang, D.P.; Zauscher, S. Molecular mechanisms of aqueous boundary lubrication by mucinous glycoproteins. Curr. Opin. Colloid Interface Sci. 2010, 15, 406–416. [Google Scholar] [CrossRef]
- Lin, W.; Liu, Z.; Kampf, N.; Klein, J. The Role of Hyaluronic Acid in Cartilage Boundary Lubrication. Cells 2020, 9, 1606. [Google Scholar] [CrossRef]
- Seror, J.; Merkher, Y.; Kampf, N.; Collinson, L.; Day, A.J.; Maroudas, A.; Klein, J. Articular Cartilage Proteoglycans As Boundary Lubricants: Structure and Frictional Interaction of Surface-Attached Hyaluronan and Hyaluronan–Aggrecan Complexes. Biomacromolecules 2011, 12, 3432–3443. [Google Scholar] [CrossRef]
- Huettner, N.; Dargaville, T.R.; Forget, A. Discovering Cell-Adhesion Peptides in Tissue Engineering: Beyond RGD. Trends Biotechnol. 2018, 36, 372–383. [Google Scholar] [CrossRef]
- Shachar, M.; Tsur-Gang, O.; Dvir, T.; Leor, J.; Cohen, S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 2011, 7, 152–162. [Google Scholar] [CrossRef]
- Thaker, H.D.; Som, A.; Ayaz, F.; Lui, D.; Pan, W.; Scott, R.W.; Anguita, J.; Tew, G.N. Synthetic Mimics of Antimicrobial Peptides with Immunomodulatory Responses. J. Am. Chem. Soc. 2012, 134, 11088–11091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, D.; Olívia Pereira, M. Mini-review: Antimicrobial peptides and enzymes as promising candidates to functionalize biomaterial surfaces. Biofouling 2014, 30, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, L.; Zhang, W. Control of Scaffold Degradation in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2014, 20, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cui, Z.-K.; Koo, B.; Zheng, J.; Aghaloo, T.; Lee, M. Chitosan–Lysozyme Conjugates for Enzyme-Triggered Hydrogel Degradation in Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2018, 10, 41138–41145. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Nanobiotechnology. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Wei, X.; Yang, X.; Han, Z.-P.; Qu, F.-F.; Shao, L.; Shi, Y.-F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Golchin, A.; Hosseinzadeh, S.; Roshangar, L. The role of nanomaterials in cell delivery systems. Med. Mol. Morphol. 2018, 51, 1–12. [Google Scholar] [CrossRef]
- Kelm, J.M.; Fussenegger, M. Scaffold-free cell delivery for use in regenerative medicine. Adv. Drug Deliv. Rev. 2010, 62, 753–764. [Google Scholar] [CrossRef]
- Raisin, S.; Belamie, E.; Morille, M. Non-viral gene activated matrices for mesenchymal stem cells based tissue engineering of bone and cartilage. Biomaterials 2016, 104, 223–237. [Google Scholar] [CrossRef]
- Mohammadi Amirabad, L.; Massumi, M.; Shamsara, M.; Shabani, I.; Amari, A.; Mossahebi Mohammadi, M.; Hosseinzadeh, S.; Vakilian, S.; Steinbach, S.K.; Khorramizadeh, M.R.; et al. Enhanced Cardiac Differentiation of Human Cardiovascular Disease Patient-Specific Induced Pluripotent Stem Cells by Applying Unidirectional Electrical Pulses Using Aligned Electroactive Nanofibrous Scaffolds. ACS Appl. Mater. Interfaces 2017, 9, 6849–6864. [Google Scholar] [CrossRef]
- Maeder, M.L.; Gersbach, C.A. Genome-editing Technologies for Gene and Cell Therapy. Mol. Ther. 2016, 24, 430–446. [Google Scholar] [CrossRef] [Green Version]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Blokpoel Ferreras, L.A.; Chan, S.Y.; Vazquez Reina, S.; Dixon, J.E. Rapidly Transducing and Spatially Localized Magnetofection Using Peptide-Mediated Non-Viral Gene Delivery Based on Iron Oxide Nanoparticles. ACS Appl. Nano Mater. 2021, 4, 167–181. [Google Scholar] [CrossRef]
- Rodgers, T.; Muzzio, N.; Watson, C.; Romero, G. Stabilization of Poly (β-Amino Ester) Nanoparticles for the Efficient Intracellular Delivery of PiggyBac Transposon. Bioengineering 2021, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhou, Y.; Chen, J.; Huang, N.; Wang, Z.; Cheng, Y. Gene Therapy for Drug-Resistant Glioblastoma via Lipid-Polymer Hybrid Nanoparticles Combined with Focused Ultrasound. Int. J. Nanomed. 2021, 16, 185–199. [Google Scholar] [CrossRef]
- San Juan, A.M.T.; Rodgers, T.; Bedolla, C.; Noriega, F.; Romero, G. Layer by layer surface engineering of poly(lactide-co-glycolide) nanoparticles for plasmid DNA delivery. J. Appl. Polym. Sci. 2020, 137, 49377. [Google Scholar] [CrossRef]
- Kelly, D.C.; Raftery, R.M.; Curtin, C.M.; O’Driscoll, C.M.; O’Brien, F.J. Scaffold-Based Delivery of Nucleic Acid Therapeutics for Enhanced Bone and Cartilage Repair. J. Orthop. Res. 2019, 37, 1671–1680. [Google Scholar] [CrossRef]
- Raftery, R.M.; Mencía Castaño, I.; Chen, G.; Cavanagh, B.; Quinn, B.; Curtin, C.M.; Cryan, S.A.; O’Brien, F.J. Translating the role of osteogenic-angiogenic coupling in bone formation: Highly efficient chitosan-pDNA activated scaffolds can accelerate bone regeneration in critical-sized bone defects. Biomaterials 2017, 149, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Gao, M.; Lin, J.; Wu, W.; Wang, J.; Chew, S.Y. Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Sci. Rep. 2017, 7, 42212. [Google Scholar] [CrossRef] [Green Version]
- Laiva, A.L.; Raftery, R.M.; Keogh, M.B.; O’Brien, F.J. Pro-angiogenic impact of SDF-1α gene-activated collagen-based scaffolds in stem cell driven angiogenesis. Int. J. Pharm. 2018, 544, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.M.; Maestas, D.R.; Comeau, H.Y.; Elisseeff, J.H. The Immune System and Its Contribution to Variability in Regenerative Medicine. Tissue Eng. Part B Rev. 2020, 27, 39–47. [Google Scholar] [CrossRef]
- Sattler, S.; Fairchild, P.; Watt, F.M.; Rosenthal, N.; Harding, S.E. The adaptive immune response to cardiac injury—the true roadblock to effective regenerative therapies? NPJ Regen. Med. 2017, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Pino, C.J.; Westover, A.J.; Johnston, K.A.; Buffington, D.A.; Humes, H.D. Regenerative Medicine and Immunomodulatory Therapy: Insights From the Kidney, Heart, Brain, and Lung. Kidney Int. Rep. 2018, 3, 771–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakoli, S.; Ghaderi Jafarbeigloo, H.R.; Shariati, A.; Jahangiryan, A.; Jadidi, F.; Jadidi Kouhbanani, M.A.; Hassanzadeh, A.; Zamani, M.; Javidi, K.; Naimi, A. Mesenchymal stromal cells; a new horizon in regenerative medicine. J. Cell. Physiol. 2020, 235, 9185–9210. [Google Scholar] [CrossRef] [PubMed]
- Sane, M.S.; Misra, N.; Mousa, O.M.; Czop, S.; Tang, H.; Khoo, L.T.; Jones, C.D.; Mustafi, S.B. Cytokines in umbilical cord blood-derived cellular product: A mechanistic insight into bone repair. Regen. Med. 2018, 13, 881–898. [Google Scholar] [CrossRef] [Green Version]
- de Araújo Farias, V.; Carrillo-Gálvez, A.B.; Martín, F.; Anderson, P. TGF-β and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor Rev. 2018, 43, 25–37. [Google Scholar] [CrossRef]
- Andorko, J.I.; Jewell, C.M. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng. Transl. Med. 2017, 2, 139–155. [Google Scholar] [CrossRef] [Green Version]
- Thrivikraman, G.; Boda, S.K.; Basu, B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 2018, 150, 60–86. [Google Scholar] [CrossRef]
- Leppik, L.; Oliveira, K.M.C.; Bhavsar, M.B.; Barker, J.H. Electrical stimulation in bone tissue engineering treatments. Eur. J. Trauma Emerg. Surg. 2020, 46, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Cheng, Y.; Cai, J.; Zhao, X.; Ouyang, Y.; Yuan, W.-E.; Fan, C. Advances in electrical and magnetic stimulation on nerve regeneration. Regen. Med. 2019, 14, 969–979. [Google Scholar] [CrossRef]
- Bhavsar, M.B.; Han, Z.; DeCoster, T.; Leppik, L.; Costa Oliveira, K.M.; Barker, J.H. Electrical stimulation-based bone fracture treatment, if it works so well why do not more surgeons use it? Eur. J. Trauma Emerg. Surg. 2020, 46, 245–264. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical Stimulation: A Novel Tool for Tissue Engineering. Tissue Eng. Part B Rev. 2012, 19, 48–57. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Y.; Ding, S.; Zhang, K.; Mao, H.-q.; Yang, Y. Application of conductive PPy/SF composite scaffold and electrical stimulation for neural tissue engineering. Biomaterials 2020, 255, 120164. [Google Scholar] [CrossRef]
- Chen, R.; Romero, G.; Christiansen, M.G.; Mohr, A.; Anikeeva, P. Wireless magnetothermal deep brain stimulation. Science 2015, 347, 1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, G.; Christiansen, M.G.; Stocche Barbosa, L.; Garcia, F.; Anikeeva, P. Localized Excitation of Neural Activity via Rapid Magnetothermal Drug Release. Adv. Funct. Mater. 2016, 26, 6471–6478. [Google Scholar] [CrossRef]
- Lima, J.; Gonçalves, A.I.; Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. The effect of magnetic stimulation on the osteogenic and chondrogenic differentiation of human stem cells derived from the adipose tissue (hASCs). J. Magn. Magn. Mater. 2015, 393, 526–536. [Google Scholar] [CrossRef] [Green Version]
- Pardo, A.; Gómez-Florit, M.; Barbosa, S.; Taboada, P.; Domingues, R.M.A.; Gomes, M.E. Magnetic Nanocomposite Hydrogels for Tissue Engineering: Design Concepts and Remote Actuation Strategies to Control Cell Fate. ACS Nano 2021, 15, 175–209. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, L.-Y.; Chen, X.; Chen, Z.; Wu, F.-G. Hydrogel-based phototherapy for fighting cancer and bacterial infection. Sci. China Mater. 2017, 60, 487–503. [Google Scholar] [CrossRef]
- Araújo-Custódio, S.; Gomez-Florit, M.; Tomás, A.R.; Mendes, B.B.; Babo, P.S.; Mithieux, S.M.; Weiss, A.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Injectable and Magnetic Responsive Hydrogels with Bioinspired Ordered Structures. ACS Biomater. Sci. Eng. 2019, 5, 1392–1404. [Google Scholar] [CrossRef]
- Zhou, R.; Wei, D.; Cao, J.; Feng, W.; Cheng, S.; Du, Q.; Li, B.; Wang, Y.; Jia, D.; Zhou, Y. Synergistic Effects of Surface Chemistry and Topologic Structure from Modified Microarc Oxidation Coatings on Ti Implants for Improving Osseointegration. ACS Appl. Mater. Interfaces 2015, 7, 8932–8941. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Shrestha, S.; Shrestha, B.K.; Park, C.H.; Kim, C.S. A controlled surface geometry of polyaniline doped titania nanotubes biointerface for accelerating MC3T3-E1 cells growth in bone tissue engineering. Chem. Eng. J. 2018, 350, 57–68. [Google Scholar] [CrossRef]
- Metwally, S.; Karbowniczek, J.E.; Szewczyk, P.K.; Marzec, M.M.; Gruszczyński, A.; Bernasik, A.; Stachewicz, U. Single-Step Approach to Tailor Surface Chemistry and Potential on Electrospun PCL Fibers for Tissue Engineering Application. Adv. Mater. Interfaces 2019, 6, 1801211. [Google Scholar] [CrossRef]
- Kaur, T.; Thirugnanam, A. Tailoring in vitro biological and mechanical properties of polyvinyl alcohol reinforced with threshold carbon nanotube concentration for improved cellular response. RSC Adv. 2016, 6, 39982–39992. [Google Scholar] [CrossRef]
- Sikorski, P. Electroconductive scaffolds for tissue engineering applications. Biomater. Sci. 2020, 8, 5583–5588. [Google Scholar] [CrossRef]
- Magaz, A.; Spencer, B.F.; Hardy, J.G.; Li, X.; Gough, J.E.; Blaker, J.J. Modulation of Neuronal Cell Affinity on PEDOT–PSS Nonwoven Silk Scaffolds for Neural Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 6906–6916. [Google Scholar] [CrossRef]
- Deng, C.; Yang, Q.; Sun, X.; Chen, L.; Feng, C.; Chang, J.; Wu, C. Bioactive scaffolds with Li and Si ions-synergistic effects for osteochondral defects regeneration. Appl. Mater. Today 2018, 10, 203–216. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.-J.; Li, W.-S.; Xu, X.-L.; Hu, J.-B.; Kang, X.-Q.; Qi, J.; Ying, X.-Y.; You, J.; Du, Y.-Z. Polycaprolactone/polysialic acid hybrid, multifunctional nanofiber scaffolds for treatment of spinal cord injury. Acta Biomater. 2018, 77, 15–27. [Google Scholar] [CrossRef]
- Escobar, A.; Muzzio, N.; Coy, E.; Liu, H.; Bindini, E.; Andreozzi, P.; Wang, G.; Angelomé, P.; Delcea, M.; Grzelczak, M.; et al. Antibacterial Mesoporous Titania Films with Embedded Gentamicin and Surface Modified with Bone Morphogenetic Protein 2 to Promote Osseointegration in Bone Implants. Adv. Mater. Interfaces 2019, 6, 1801648. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, L.; Han, L.; Wang, K.; Lu, X.; Fang, L.; Qu, S.; Chan, C.W. Self-assembled Biodegradable Nanoparticles and Polysaccharides as Biomimetic ECM Nanostructures for the Synergistic effect of RGD and BMP-2 on Bone Formation. Sci. Rep. 2016, 6, 25090. [Google Scholar] [CrossRef] [Green Version]
- Nih, L.R.; Gojgini, S.; Carmichael, S.T.; Segura, T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat. Mater. 2018, 17, 642–651. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Q.; Zheng, S.; Zhu, J.; Qi, Z.; Fu, C.; Yang, X.; Zhao, Y. Synergistic delivery of bFGF and BMP-2 from poly(l-lactic-co-glycolic acid)/graphene oxide/hydroxyapatite nanofibre scaffolds for bone tissue engineering applications. RSC Adv. 2018, 8, 31911–31923. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, S.; Bitar, K.N. The influence of extracellular matrix composition on the differentiation of neuronal subtypes in tissue engineered innervated intestinal smooth muscle sheets. Biomaterials 2014, 35, 7429–7440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuai, C.; Guo, W.; Wu, P.; Yang, W.; Hu, S.; Xia, Y.; Feng, P. A graphene oxide-Ag co-dispersing nanosystem: Dual synergistic effects on antibacterial activities and mechanical properties of polymer scaffolds. Chem. Eng. J. 2018, 347, 322–333. [Google Scholar] [CrossRef]
- Martin, V.; Ribeiro, I.A.; Alves, M.M.; Gonçalves, L.; Claudio, R.A.; Grenho, L.; Fernandes, M.H.; Gomes, P.; Santos, C.F.; Bettencourt, A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. C 2019, 101, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Dutta, S.D.; Ganguly, K.; Lim, K.-T. Multifunctional bioactive chitosan/cellulose nanocrystal scaffolds eradicate bacterial growth and sustain drug delivery. Int. J. Biol. Macromol. 2021, 170, 178–188. [Google Scholar] [CrossRef]
- Luo, Y.; Lode, A.; Wu, C.; Chang, J.; Gelinsky, M. Alginate/Nanohydroxyapatite Scaffolds with Designed Core/Shell Structures Fabricated by 3D Plotting and in Situ Mineralization for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 6541–6549. [Google Scholar] [CrossRef]
- Moghadasi Boroujeni, S.; Mashayekhan, S.; Vakilian, S.; Ardeshirylajimi, A.; Soleimani, M. The synergistic effect of surface topography and sustained release of TGF-β1 on myogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2016, 104, 1610–1621. [Google Scholar] [CrossRef]
- Xu, Y.; Peng, J.; Dong, X.; Xu, Y.; Li, H.; Chang, J. Combined chemical and structural signals of biomaterials synergistically activate cell-cell communications for improving tissue regeneration. Acta Biomater. 2017, 55, 249–261. [Google Scholar] [CrossRef]
- Rahmanian, M.; Seyfoori, A.; Dehghan, M.M.; Eini, L.; Naghib, S.M.; Gholami, H.; Farzad Mohajeri, S.; Mamaghani, K.R.; Majidzadeh, A.K. Multifunctional gelatin–tricalcium phosphate porous nanocomposite scaffolds for tissue engineering and local drug delivery: In vitro and in vivo studies. J. Taiwan Inst. Chem. Eng. 2019, 101, 214–220. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Kwon, J.-S.; Kim, K.-M.; Hong, H.J.; Koh, W.-G.; Lee, J.; Lee, H.-J.; Choi, H.-J.; Yi, S.; Shin, H.; et al. Synergistic Effect of Porous Hydroxyapatite Scaffolds Combined with Bioactive Glass/Poly(lactic-co-glycolic acid) Composite Fibers Promotes Osteogenic Activity and Bioactivity. ACS Omega 2019, 4, 2302–2310. [Google Scholar] [CrossRef] [Green Version]
- Canadas, R.F.; Ren, T.; Marques, A.P.; Oliveira, J.M.; Reis, R.L.; Demirci, U. Biochemical Gradients to Generate 3D Heterotypic-Like Tissues with Isotropic and Anisotropic Architectures. Adv. Funct. Mater. 2018, 28, 1804148. [Google Scholar] [CrossRef]
- Rajab, T.K.; O’Malley, T.J.; Tchantchaleishvili, V. Decellularized scaffolds for tissue engineering: Current status and future perspective. Artif. Organs 2020, 44, 1031–1043. [Google Scholar] [CrossRef]
- Sackett, S.D.; Tremmel, D.M.; Ma, F.; Feeney, A.K.; Maguire, R.M.; Brown, M.E.; Zhou, Y.; Li, X.; O’Brien, C.; Li, L.; et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci. Rep. 2018, 8, 10452. [Google Scholar] [CrossRef]
- Zhu, M.; Li, W.; Dong, X.; Yuan, X.; Midgley, A.C.; Chang, H.; Wang, Y.; Wang, H.; Wang, K.; Ma, P.X.; et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 2019, 10, 4620. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Liu, S.; Zhang, H.; Zhu, B.; Su, Y.; Zheng, C.; Tian, R.; Wang, M.; Kuang, H.; Zhao, X.; et al. Mesenchymal stem cells and extracellular matrix scaffold promote muscle regeneration by synergistically regulating macrophage polarization toward the M2 phenotype. Stem Cell Res. Ther. 2018, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Farnebo, S.; Farnebo, L.; Kim, M.; Woon, C.; Pham, H.; Chang, J. Optimized Repopulation of Tendon Hydrogel: Synergistic Effects of Growth Factor Combinations and Adipose-Derived Stem Cells. HAND 2016, 12, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-M.; Han, M.; Jiang, X.-C.; Yin, X.-Z.; Chen, F.; Zhang, T.-Y.; Ren, H.; Zhang, J.-W.; Hou, T.-J.; Chen, Z.; et al. Peptide-Tethered Hydrogel Scaffold Promotes Recovery from Spinal Cord Transection via Synergism with Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2017, 9, 3330–3342. [Google Scholar] [CrossRef]
- Hansen, S.G.; Taskin, M.B.; Chen, M.; Wogensen, L.; Vinge Nygaard, J.; Axelsen, S.M. Electrospun nanofiber mesh with fibroblast growth factor and stem cells for pelvic floor repair. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Keeney, M.; van den Beucken, J.J.J.P.; van der Kraan, P.M.; Jansen, J.A.; Pandit, A. The ability of a collagen/calcium phosphate scaffold to act as its own vector for gene delivery and to promote bone formation via transfection with VEGF165. Biomaterials 2010, 31, 2893–2902. [Google Scholar] [CrossRef]
- Fan, J.; Lee, C.-S.; Kim, S.; Zhang, X.; Pi-Anfruns, J.; Guo, M.; Chen, C.; Rahnama, M.; Li, J.; Wu, B.M.; et al. Trb3 controls mesenchymal stem cell lineage fate and enhances bone regeneration by scaffold-mediated local gene delivery. Biomaterials 2021, 264, 120445. [Google Scholar] [CrossRef] [PubMed]
- Gower, R.M.; Boehler, R.M.; Azarin, S.M.; Ricci, C.F.; Leonard, J.N.; Shea, L.D. Modulation of leukocyte infiltration and phenotype in microporous tissue engineering scaffolds via vector induced IL-10 expression. Biomaterials 2014, 35, 2024–2031. [Google Scholar] [CrossRef] [Green Version]
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. SDF-1α gene-activated collagen scaffold drives functional differentiation of human Schwann cells for wound healing applications. Biotechnol. Bioeng. 2021, 118, 725–736. [Google Scholar] [CrossRef]
- Curtin, C.M.; Tierney, E.G.; McSorley, K.; Cryan, S.-A.; Duffy, G.P.; O’Brien, F.J. Combinatorial Gene Therapy Accelerates Bone Regeneration: Non-Viral Dual Delivery of VEGF and BMP2 in a Collagen-Nanohydroxyapatite Scaffold. Adv. Healthc. Mater. 2015, 4, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Li, J.; Li, Y.; Gao, Y.; Zuo, Y.; Hsu, Y.; Hu, J. Angiogenesis and osteogenesis enhanced by bFGF ex vivo gene therapy for bone tissue engineering in reconstruction of calvarial defects. J. Biomed. Mater. Res. Part A 2011, 96A, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, T.; Tierney, E.G.; Cunniffe, G.M.; O’Brien, F.J.; Kelly, D.J. Gene Delivery of TGF-β3 and BMP2 in an MSC-Laden Alginate Hydrogel for Articular Cartilage and Endochondral Bone Tissue Engineering. Tissue Eng. Part A 2016, 22, 776–787. [Google Scholar] [CrossRef]
- Knopf-Marques, H.; Singh, S.; Htwe, S.S.; Wolfova, L.; Buffa, R.; Bacharouche, J.; Francius, G.; Voegel, J.-C.; Schaaf, P.; Ghaemmaghami, A.M.; et al. Immunomodulation with Self-Crosslinked Polyelectrolyte Multilayer-Based Coatings. Biomacromolecules 2016, 17, 2189–2198. [Google Scholar] [CrossRef] [Green Version]
- Garg, K.; Pullen, N.A.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Cao, Z.; Bai, T.; Carr, L.; Ella-Menye, J.-R.; Irvin, C.; Ratner, B.D.; Jiang, S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013, 31, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Aktas, E.; Chamberlain, C.S.; Saether, E.E.; Duenwald-Kuehl, S.E.; Kondratko-Mittnacht, J.; Stitgen, M.; Lee, J.S.; Clements, A.E.; Murphy, W.L.; Vanderby, R. Immune modulation with primed mesenchymal stem cells delivered via biodegradable scaffold to repair an Achilles tendon segmental defect. J. Orthop. Res. 2017, 35, 269–280. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Cheng, R.; Gu, Y.; Yin, Y.; Sun, Z.; Pan, G.; Deng, Z.; Yang, H.; Deng, L.; et al. An immunological electrospun scaffold for tumor cell killing and healthy tissue regeneration. Mater. Horiz. 2018, 5, 1082–1091. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Hu, B.; Li, Z.; Li, J.; Gao, Y.; Wang, Z.; Hao, J. Synergistic Effects of Electrical Stimulation and Aligned Nanofibrous Microenvironment on Growth Behavior of Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 18543–18550. [Google Scholar] [CrossRef]
- Rao, K.M.; Kumar, A.; Han, S.S. Polysaccharide-based magnetically responsive polyelectrolyte hydrogels for tissue engineering applications. J. Mater. Sci. Technol. 2018, 34, 1371–1377. [Google Scholar] [CrossRef]
- Ma, L.; Feng, X.; Liang, H.; Wang, K.; Song, Y.; Tan, L.; Wang, B.; Luo, R.; Liao, Z.; Li, G.; et al. A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration. Mater. Today 2020, 36, 48–62. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Mohammadi-Aghdam, M.; Saber-Samandari, S. A novel magnetic bifunctional nanocomposite scaffold for photothermal therapy and tissue engineering. Int. J. Biol. Macromol. 2019, 138, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhang, J.; Zou, J.; Yang, X.; Guo, H.; Tian, H.; Zhang, P.; Wang, Y.; Zhang, N.; Zhuang, X.; et al. Electroactive composite scaffold with locally expressed osteoinductive factor for synergistic bone repair upon electrical stimulation. Biomaterials 2020, 230, 119617. [Google Scholar] [CrossRef]
- Leppik, L.; Zhihua, H.; Mobini, S.; Thottakkattumana Parameswaran, V.; Eischen-Loges, M.; Slavici, A.; Helbing, J.; Pindur, L.; Oliveira, K.M.C.; Bhavsar, M.B.; et al. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci. Rep. 2018, 8, 6307. [Google Scholar] [CrossRef] [PubMed]
- Gelmi, A.; Cieslar-Pobuda, A.; de Muinck, E.; Los, M.; Rafat, M.; Jager, E.W.H. Direct Mechanical Stimulation of Stem Cells: A Beating Electromechanically Active Scaffold for Cardiac Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 1471–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Correia, C.R.; Bjørge, I.M.; Nadine, S.; Mano, J.F. Minimalist Tissue Engineering Approaches Using Low Material-Based Bioengineered Systems. Adv. Healthc. Mater. 2021, 2002110. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.B.; Mauck, R.L. Tissue Engineering and Regenerative Medicine: Recent Innovations and the Transition to Translation. Tissue Eng. Part B Rev. 2012, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [Green Version]
- Jabbari, E. Challenges for Natural Hydrogels in Tissue Engineering. Gels 2019, 5, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radke, D.; Jia, W.; Sharma, D.; Fena, K.; Wang, G.; Goldman, J.; Zhao, F. Tissue Engineering at the Blood-Contacting Surface: A Review of Challenges and Strategies in Vascular Graft Development. Adv. Healthc. Mater. 2018, 7, 1701461. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Challenges With the Development of Biomaterials for Sustainable Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, B.T.; Ives, C.J.; Mohiuddin, O.A.; Bunnell, B.A. Beyond the Present Constraints That Prevent a Wide Spread of Tissue Engineering and Regenerative Medicine Approaches. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.F. The Biomaterials Conundrum in Tissue Engineering. Tissue Eng. Part A 2014, 20, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Sahakyants, T.; Vacanti, J.P. Tissue engineering: From the bedside to the bench and back to the bedside. Pediatric Surg. Int. 2020, 36, 1123–1133. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzzio, N.; Moya, S.; Romero, G. Multifunctional Scaffolds and Synergistic Strategies in Tissue Engineering and Regenerative Medicine. Pharmaceutics 2021, 13, 792. https://doi.org/10.3390/pharmaceutics13060792
Muzzio N, Moya S, Romero G. Multifunctional Scaffolds and Synergistic Strategies in Tissue Engineering and Regenerative Medicine. Pharmaceutics. 2021; 13(6):792. https://doi.org/10.3390/pharmaceutics13060792
Chicago/Turabian StyleMuzzio, Nicolas, Sergio Moya, and Gabriela Romero. 2021. "Multifunctional Scaffolds and Synergistic Strategies in Tissue Engineering and Regenerative Medicine" Pharmaceutics 13, no. 6: 792. https://doi.org/10.3390/pharmaceutics13060792
APA StyleMuzzio, N., Moya, S., & Romero, G. (2021). Multifunctional Scaffolds and Synergistic Strategies in Tissue Engineering and Regenerative Medicine. Pharmaceutics, 13(6), 792. https://doi.org/10.3390/pharmaceutics13060792








