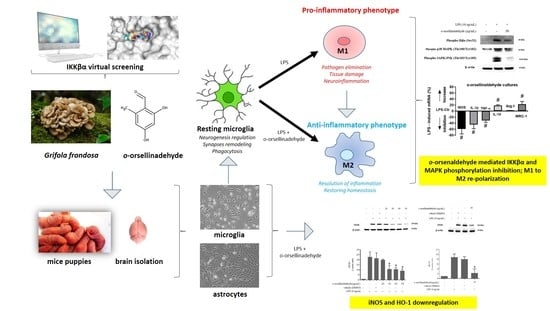
Anti-Inflammatory and Immunomodulatory Effects of the Grifola frondosa Natural Compound o-Orsellinaldehyde on LPS-Challenged Murine Primary Glial Cells. Roles of NF-κβ and MAPK
Abstract
1. Introduction
2. Materials and Methods
2.1. Prediction of Blood–Brain Barrier Permeation
2.2. Chemicals
2.3. Animals and In Vitro Cell Culture Procedure
2.3.1. Acquisition of Glial Cells
2.3.2. Isolation and Plating of Primary Microglia
2.3.3. Astrocytes Plating
2.4. Cellular Toxicity
2.5. Cell Treatments
2.5.1. Astrocytes Treatment
2.5.2. Microglia Treatment
2.6. Nitrite Determination
2.7. Western Blot Analysis
2.8. RNA Isolation and cDNA Synthesis
2.9. Real-Time RT-PCR
2.10. Statistical Analysis
3. Results
3.1. Prediction of Blood–Brain Barrier Permeability
3.2. Effects of o-Orsellinaldehyde on Cell Viability of Isolated Astrocytes and Microglia Cells
3.3. o-Orsellinaldehyde Inhibits Nitrite Production in LPS-Activated Astrocytes and Microglia Cells
3.4. o-Orsellinaldehyde Decreases iNOS Protein Expression in LPS-Challenged Astrocytes and Microglia Cells
3.5. o-Orsellinaldehyde Decreases HO-1 Protein Expression in LPS-Activated Mixed Glia and Microglia Cells
3.6. o-Orsellinaldehyde Suppressed LPS-Induced Microglial Activation Through the Nfkb and MAP Kinase Signaling Pathways
3.7. o-Orsellinaldehyde Alter Microglial M1/M2 Immunological Profile in Response to LPS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amor, S.; Puentes, F.; Baker, D.; Van Der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.S.T. Inflammation and neurodegenerative diseases. Am. J. Clin. Nutr. 2006, 83, 470S–474S. [Google Scholar] [CrossRef] [PubMed]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Hanisch, U.-K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Streit, W.J. Microglia and neuroprotection: Implications for Alzheimer’s disease. Brain Res. Rev. 2005, 48, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Zindler, E.; Zipp, F. Neuronal injury in chronic CNS inflammation. Best Pract. Res. Clin. Anaesthesiol. 2010, 24, 551–562. [Google Scholar] [CrossRef]
- Kushairi, N.; Tarmizi, N.A.K.A.; Phan, C.W.; Macreadie, I.; Sabaratnam, V.; Naidu, M.; David, P. Modulation of neuroinflammatory pathways by medicinal mushrooms, with particular relevance to Alzheimer’s disease. Trends Food Sci. Technol. 2020, 104, 153–162. [Google Scholar] [CrossRef]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Colton, C.A. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharm. 2009, 4, 399–418. [Google Scholar] [CrossRef]
- von Bartheld, C.S.; Bahney, J.; Herculano-Houzel, S. The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J. Comp. Neurol. 2016, 524, 3865–3895. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Wilhelmsson, U.; Bushong, E.A.; Price, D.L.; Smarr, B.L.; Phung, V.; Terada, M.; Ellisman, M.H.; Pekny, M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc. Natl. Acad. Sci. USA 2006, 103, 17513–17518. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef]
- Haim, L.B.; Carrillo-de Sauvage, M.A.; Ceyzériat, K.; Escartin, C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front. Cell. Neurosci. 2015, 9, 278. [Google Scholar]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dobson, P.D.; Kell, D.B. Carrier-mediated cellular uptake of pharmaceutical drugs: An exception or the rule? Nat. Rev. Drug Discov. 2008, 7, 205–220. [Google Scholar] [CrossRef]
- Pangalos, M.N.; Schechter, L.E.; Hurko, O. Drug development for CNS disorders: Strategies for balancing risk and reducing attrition. Nat. Rev. Drug Discov. 2007, 6, 521–532. [Google Scholar] [CrossRef]
- Diederich, M. Natural products target the hallmarks of chronic diseases. Biochem. Pharm. 2020, 173, 113828. [Google Scholar] [CrossRef] [PubMed]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. Pharma Nutr. 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Trovato Salinaro, A.; Pennisi, M.; Di Paola, R.; Scuto, M.; Crupi, R.; Cambria, M.T.; Ontario, M.L.; Tomasello, M.; Uva, M.; Maiolino, L.; et al. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: Modulation by nutritional mushrooms. Immun. Ageing 2018, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Froufe, H.J.C.; Abreu, R.M.V.; Ferreira, I.C.F.R. Virtual screening of low molecular weight mushrooms compounds as potential Mdm2 inhibitors. J. Enzym. Inhib. Med. Chem. 2013, 28, 569–575. [Google Scholar] [CrossRef]
- Tomás-Hernández, S.; Garcia-Vallvé, S.; Pujadas, G.; Valls, C.; Ojeda-Montes, M.J.; Gimeno, A.; Ceretó-Massagué, A.; Roca-Martínez, J.; Suarez, M.; Arola, L.; et al. Anti-inflammatory and proapoptotic properties of the natural compound o-Orsellinaldehyde. J. Agric. Food Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 1117–1121. [Google Scholar] [CrossRef]
- Tamashiro, T.T.; Dalgard, C.L.; Byrnes, K.R. Primary microglia isolation from mixed glial cell cultures of neonatal rat brain tissue. J. Vis. Exp. 2012. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 851, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Soares, M.P.; Yamashita, K.; Bach, F.H. Heme oxygenase-1: Unleashing the protective properties of heme. Trends Immunol. 2003, 24, 449–455. [Google Scholar] [CrossRef]
- Srisook, K.; Cha, Y.N. Super-induction of HO-1 in macrophages stimulated with lipopolysaccharide by prior depletion of glutathione decreases iNOS expression and NO production. Nitric Oxide Biol. Chem. 2005, 12, 70–79. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1. [Google Scholar]
- Wang, S.; Colonna, M. Microglia in Alzheimer’s disease: A target for immunotherapy. J. Leukoc. Biol. 2019, 106, 219–227. [Google Scholar] [CrossRef]
- Czeh, M.; Gressens, P.; Kaindl, A.M. The yin and yang of microglia. Dev. Neurosci. 2011, 33, 199–209. [Google Scholar] [CrossRef]
- Khandelwal, P.J.; Herman, A.M.; Moussa, C.E.H. Inflammation in the early stages of neurodegenerative pathology. J. Neuroimmunol. 2011, 238, 1–11. [Google Scholar] [CrossRef]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and functions in brain pathologies. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Khadka, B.; Lee, J.Y.; Park, D.H.; Kim, K.T.; Bae, J.S. The role of natural compounds and their nanocarriers in the treatment of cns inflammation. Biomolecules 2020, 10, 1401. [Google Scholar] [CrossRef] [PubMed]
- Leonoudakis, D.; Rane, A.; Angeli, S.; Lithgow, G.J.; Andersen, J.K.; Chinta, S.J. Anti-Inflammatory and Neuroprotective Role of Natural Product Securinine in Activated Glial Cells: Implications for Parkinson’s Disease. Mediat. Inflamm. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.; Afaq, F.; Khan, N.; Johnson, J.J.; Khusro, F.H.; Mukhtar, H. Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells. Carcinogenesis 2010, 31, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.; Behl, T.; Bungau, S.; Zengin, G.; Mehta, V.; Kumar, A.; Uddin, M.S.; Ashraf, G.M.; Abdel-Daim, M.M.; Arora, S.; et al. Nutraceuticals in neurological disorders. Int. J. Mol. Sci. 2020, 21, 4424. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Hossain, M.F.; Mamun, A.A.; Shah, M.A.; Hasana, S.; Bulbul, I.J.; Sarwar, M.S.; Mansouri, R.A.; Ashraf, G.M.; Rauf, A.; et al. Exploring the multimodal role of phytochemicals in the modulation of cellular signaling pathways to combat age-related neurodegeneration. Sci. Total Environ. 2020, 725, 138313. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.C.K. The nutritional and health benefits of mushrooms. Nutr. Bull. 2010, 35, 292–299. [Google Scholar] [CrossRef]
- Huang, Y.C.; Liu, K.C.; Chiou, Y.L.; Yang, C.H.; Chen, T.H.; Li, T.T.; Liu, L.L. Fenofibrate suppresses melanogenesis in B16-F10 melanoma cells via activation of the p38 mitogen-activated protein kinase pathway. Chem. Biol. Interact. 2013, 205, 157–164. [Google Scholar] [CrossRef]
- Liu, D.Z.; Liang, H.J.; Chen, C.H.; Su, C.H.; Lee, T.H.; Huang, C.T.; Hou, W.C.; Lin, S.Y.; Zhong, W.B.; Lin, P.J.; et al. Comparative anti-inflammatory characterization of wild fruiting body, liquid-state fermentation, and solid-state culture of Taiwanofungus camphoratus in microglia and the mechanism of its action. J. Ethnopharmacol. 2007, 113, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.M.; Jang, K.J.; Han, M.S.; Jeong, J.W.; Kim, G.Y.; Lee, J.H.; Choi, Y.H. Ganoderma lucidum ethanol extract inhibits the inflammatory response by suppressing the NF-κB and toll-like receptor pathways in lipopolysaccharide-stimulated BV2 microglial cellsC. Exp. Ther. Med. 2013, 5, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases : Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Calabrese, V.; Rizzarelli, E.; Butterfield, D.A. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity Reflex modulation View project In situ nanoparticle formation in brain View project. Nat. Rev. Neurosci. 2007, 18, 598–612. [Google Scholar] [CrossRef]
- Obara, Y.; Nakahata, N.; Kita, T.; Takaya, Y.; Kobayashi, H.; Hosoi, S.; Kiuchi, F.; Ohta, T.; Oshima, Y.; Ohizumi, Y. Stimulation of neurotrophic factor secretion from 1321N1 human astrocytoma cells by novel diterpenoids, scabronines A and G. Eur. J. Pharmacol. 1999, 370, 79–84. [Google Scholar] [CrossRef]
- Jayasuriya, W.J.A.B.N.; Handunnetti, S.M.; Wanigatunge, C.A.; Fernando, G.H.; Abeytunga, D.T.U.; Suresh, T.S. Anti-Inflammatory Activity of Pleurotus ostreatus, a Culinary Medicinal Mushroom, in Wistar Rats. Evidence-based Complement. Altern. Med. 2020, 2020. [Google Scholar] [CrossRef]
- Park, Y.S.; Lee, H.S.; Won, H.; Lee, J.H.; Lee, S.Y.; Lee, H.Y. Effect of an exo-polysaccharide from the culture broth of Hericium erinaceus on enhancement of growth and differentiation of rat adrenal nerve cells. Cytotechnology 2002, 39, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T.; Liu, W.H. o-Orsellinaldehyde from the submerged culture of the edible mushroom Grifola frondosa exhibits selective cytotoxic effect against Hep 3B cells through apoptosis. J. Agric. Food Chem. 2006, 54, 7564–7569. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Park, S.Y.; Thapa, D.; Choi, M.K.; Chung, I.M.; Park, Y.J.; Yong, C.S.; Choi, H.G.; Kim, J.A. Grifola frondosa water extract alleviates intestinal inflammation by suppressing TNF-α production and its signaling. Exp. Mol. Med. 2010, 42, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Tangen, J.-M.; Mahmood, F.; Mirlashari, M.R.; Nissen-Meyer, L.S.H.; Nentwich, I.; Therkelsen, S.P.; Tjønnfjord, G.E.; Johnson, E. Antitumor, Anti-inflammatory and Antiallergic Effects of Agaricus blazei Mushroom Extract and the Related Medicinal Basidiomycetes Mushrooms, Hericium erinaceus and Grifola frondosa: A Review of Preclinical and Clinical Studies. Nutrients 2020, 12, 1339. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.S.; Thapa, D.; Park, B.C.; Kim, A.R.; Choi, M.K.; Kim, J. Grifola frondosa inhibits TNF-α-induced intestinal inflammation via suppression of MCP-1 and IL-8 expressions in HT-29 human colon epithelial cells. FASEB J. 2008, 22, 1109.1. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, L.; Chen, Y.; Chen, X.; Dong, Y.; Zheng, S.; Zhang, L.; Li, W.; Du, J.; Li, H. A Maitake (: Grifola frondosa) polysaccharide ameliorates Alzheimer’s disease-like pathology and cognitive impairments by enhancing microglial amyloid-β clearance. RSC Adv. 2019, 9, 37127–37135. [Google Scholar] [CrossRef]
- Wu, S.-J.; Chen, Y.-W.; Wang, C.-Y.; Shyu, Y.-T. Anti-inflammatory properties of high pressure-assisted extracts of Grifola frondosa in lipopolysaccharide-activated RAW 264.7 macrophages. Int. J. Food Sci. Technol. 2017, 52, 671–678. [Google Scholar] [CrossRef]
- Barañano, D.E.; Rao, M.; Ferris, C.D.; Snyder, S.H. Biliverdin reductase: A major physiologic cytoprotectant. Proc. Natl. Acad. Sci. USA 2002, 99, 16093–16098. [Google Scholar] [CrossRef] [PubMed]
- Immenschuh, S.; Ramadori, G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem. Pharm. 2000, 60, 1121–1128. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M.K. Heme oxygenase-1: Molecular mechanisms of gene expression in oxygen-related stress. Antioxid. Redox Signal. 2002, 4, 625–632. [Google Scholar] [CrossRef]
- Kapturczak, M.H.; Wasserfall, C.; Brusko, T.; Campbell-Thompson, M.; Ellis, T.M.; Atkinson, M.A.; Agarwal, A. Heme oxygenase-1 modulates early inflammatory responses: Evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 2004, 165, 1045–1053. [Google Scholar] [CrossRef]
- Matz, P.G.; Weinstein, P.R.; Sharp, F.R. Heme oxygenase-1 and heat shock protein 70 induction in glia and neurons throughout rat brain after experimental intracerebral hemorrhage. Neurosurgery 1997, 40, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.P.; Bergeron, M.; Matz, P.; Zegna, A.; Noble, L.J.; Panter, S.S.; Sharp, F.R. Heme oxygenase-1 is induced in glia throughout brain by subarachnoid hemoglobin. J. Cereb. Blood Flow Metab. 1998, 18, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Jang, S.; Johnson, R.W. Can consuming flavonoids restore old microglia to their youthful state? Nutr. Rev. 2010, 68, 719–728. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, S.M.; More, S.V.; Park, J.Y.; Kim, B.W.; In, P.J.; Yoon, S.H.; Choi, D.K. A novel synthetic HTB derivative, BECT inhibits lipopolysaccharide-mediated inflammatory response by suppressing the p38 MAPK/JNK and NF-κB activation pathways. Pharm. Rep. 2014, 66, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Jia, Y.; Huang, X. Exosomes from adipose-derived stem cells alleviate neural injury caused by microglia activation via suppressing NF-κB and MAPK pathway. J. Neuroimmunol. 2019, 334. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6. [Google Scholar] [CrossRef]
- Crain, J.M.; Nikodemova, M.; Watters, J.J. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J. Neurosci. Res. 2013, 91, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wei, Y.Z.; Wang, G.Q.; Li, D.D.; Shi, J.S.; Zhang, F. Targeting MAPK pathways by naringenin modulates microglia M1/M2 polarization in lipopolysaccharide-stimulated cultures. Front. Cell. Neurosci. 2019, 12. [Google Scholar] [CrossRef] [PubMed]







| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Accession Number | Size (bp) |
|---|---|---|---|---|
| iNOS | GCCACCTCGGATATCTCTTG | TCTGGGTCCTCTGGTCAAAC | NM_0126113 | 81 |
| IL-1β | CACCTCTCAAGCAGAGCACAG | GGGTTCCATGGTGAAGTCAAC | M98820 | 79 |
| IL-10 | GCCAAGCCTTGTCAGAAATGA | TTTCTGGGGCCATGGTTCTCT | NM_012854.2 | 73 |
| Arg-1 | ATATCTGCCAAGGACATCGTG | AGGTCTCTTCCATCACTTTGC | NO_17134 | 141 |
| MRC-1 | TGGACTAAGCCAAGGGGCAA | CAGGAGCAGGGGGAGTCTCA | NM_001106123 | 121 |
| HPRT | CTCATGGACTGATTATGGACAGGAC | GCAGGTCAGCAAAGAACTTATAGCC | S79292 | 123 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomas-Hernandez, S.; Blanco, J.; Garcia-Vallvé, S.; Pujadas, G.; Ojeda-Montes, M.J.; Gimeno, A.; Arola, L.; Minghetti, L.; Beltrán-Debón, R.; Mulero, M. Anti-Inflammatory and Immunomodulatory Effects of the Grifola frondosa Natural Compound o-Orsellinaldehyde on LPS-Challenged Murine Primary Glial Cells. Roles of NF-κβ and MAPK. Pharmaceutics 2021, 13, 806. https://doi.org/10.3390/pharmaceutics13060806
Tomas-Hernandez S, Blanco J, Garcia-Vallvé S, Pujadas G, Ojeda-Montes MJ, Gimeno A, Arola L, Minghetti L, Beltrán-Debón R, Mulero M. Anti-Inflammatory and Immunomodulatory Effects of the Grifola frondosa Natural Compound o-Orsellinaldehyde on LPS-Challenged Murine Primary Glial Cells. Roles of NF-κβ and MAPK. Pharmaceutics. 2021; 13(6):806. https://doi.org/10.3390/pharmaceutics13060806
Chicago/Turabian StyleTomas-Hernandez, Sarah, Jordi Blanco, Santiago Garcia-Vallvé, Gerard Pujadas, María José Ojeda-Montes, Aleix Gimeno, Lluís Arola, Luisa Minghetti, Raúl Beltrán-Debón, and Miquel Mulero. 2021. "Anti-Inflammatory and Immunomodulatory Effects of the Grifola frondosa Natural Compound o-Orsellinaldehyde on LPS-Challenged Murine Primary Glial Cells. Roles of NF-κβ and MAPK" Pharmaceutics 13, no. 6: 806. https://doi.org/10.3390/pharmaceutics13060806
APA StyleTomas-Hernandez, S., Blanco, J., Garcia-Vallvé, S., Pujadas, G., Ojeda-Montes, M. J., Gimeno, A., Arola, L., Minghetti, L., Beltrán-Debón, R., & Mulero, M. (2021). Anti-Inflammatory and Immunomodulatory Effects of the Grifola frondosa Natural Compound o-Orsellinaldehyde on LPS-Challenged Murine Primary Glial Cells. Roles of NF-κβ and MAPK. Pharmaceutics, 13(6), 806. https://doi.org/10.3390/pharmaceutics13060806