Recent Progress in Lipid Nanoparticles for Cancer Theranostics: Opportunity and Challenges
Abstract
1. Introduction
2. Significance of Lipid-Based Theranostic Nanoparticles in Cancer Therapy
3. Different Types of Lipid Nanoparticles for Cancer Theranostics: An Update of Recent Studies
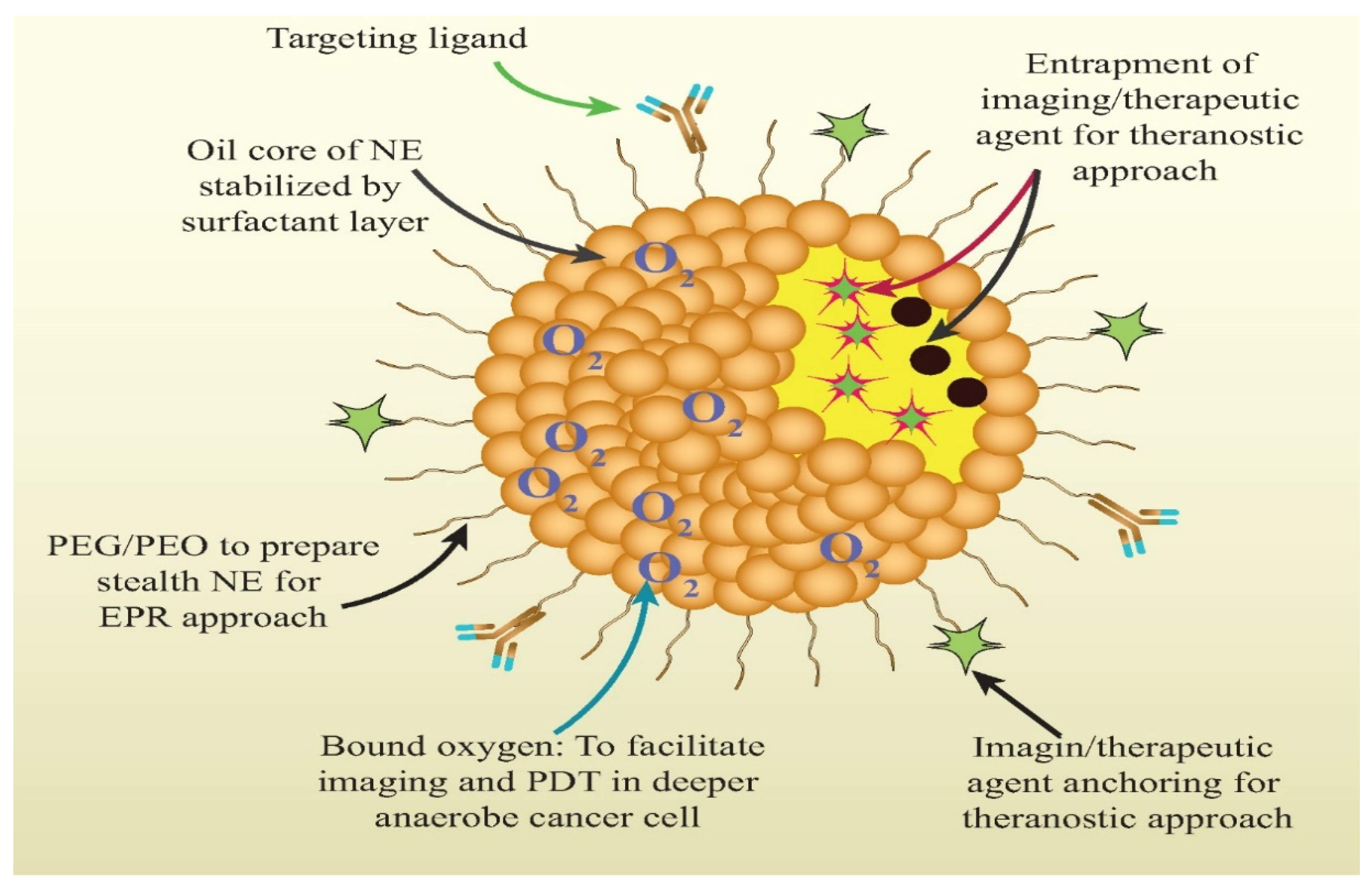
3.1. Nanoemulsion
3.2. Liposomes
3.3. Solid Lipid Nanoparticles (SLN)
3.4. Nanostructured Lipid Carriers (NLC)
3.5. Lipid Nanocapsules (LNCs)
3.6. Lipid-Based Micelles
4. Advancement in Lipid-Based Nanoparticles for Cancer Theranostics
4.1. Polymer-Lipid Hybrid System
4.2. Endogenous High-Density Lipoprotein Derived Nanoparticles
4.3. Hybrid Lipid-Inorganic Nanomaterials
4.4. Cancer Tumor Cell Targeting Theranostic Vector
5. Impact of Physicochemical Attributes of Lipid Nanoparticles in Improving In Vivo Performance of Cancer Theranostics
6. Limitation of Lipid Nanoparticles-Based Cancer Theranostics
7. Challenges in Clinical Translation of Lipid Nanoparticles for Cancer Theranostics
Approach to Overcome the Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Laversanne, M.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 15 February 2021).
- Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 15 April 2021).
- GBD Results Tool; Institute for Health Metrics, University of Washington: Seattle, WA, USA, 2020; Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 22 February 2021).
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today. 2006, 11, 812e818. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.A. Selective tumor localization and improved therapeutic index of anthracyclines encapsulated in long-circulating liposomes. Cancer Res. 1992, 52, 891–896. [Google Scholar] [PubMed]
- Northfelt, D.W.; Dezube, B.J.; Thommes, J.A.; Miller, B.J.; Fischl, M.A.; Friedman-Kien, A.; Kaplan, L.D.; Du Mond, C.; Mamelok, R.D.; Henry, D.H. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: Results of a randomized phase III clinical trial. J. Clin. Oncol. 1998, 16, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.E.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX®/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J.; Mohammadtaghi, S.; Uster, P.S.; Glass, D.; Peters, A.M.; Vile, R.G.; Stewart, J.S. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin. Cancer Res. 2001, 7, 243–254. [Google Scholar] [PubMed]
- Hansen, A.E.; Petersen, A.L.; Henriksen, J.R.; Boerresen, B.; Rasmussen, P.; Elema, D.R.; af Rosenschöld, P.M.; Kristensen, A.T.; Kjær, A.; Andresen, T.L. Positron emission tomography based elucidation of the enhanced permeability and retention effect in dogs with cancer using Copper-64 liposomes. ACS Nano 2015, 9, 6985–6995. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. Odyssey of a cancer nanoparticle: From injection site to site of action. Nano Today 2012, 7, 606–618. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater 2016, 1, 16014. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Hrkach, J.; Von Hoff, D.; Ali, M.M.; Andrianova, E.; Auer, J.; Campbell, T.; De Witt, D.; Figa, M.; Figueiredo, M.; Horhota, A.; et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 2012, 4, 128–139. [Google Scholar] [CrossRef]
- Eliasof, S.; Lazarus, D.; Peters, C.G.; Case, R.I.; Cole, R.O.; Hwang, J.; Schluep, T.; Chao, J.; Lin, J.; Yen, Y.; et al. Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle. Proc. Natl. Acad. Sci. USA 2013, 110, 15127–15132. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.E.; Gritli, I.; Tolcher, A.; Heidel, J.D.; Lim, D.; Morgan, R.; Chmielowski, B.; Ribas, A.; Davis, M.E.; Yen, Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. USA 2014, 111, 11449–11454. [Google Scholar] [CrossRef] [PubMed]
- Meads, M.B.; Gatenby, R.A.; Dalton, W.S. Environment-mediated drug resistance: A major contributor to minimal residual disease. Nat. Rev. Cancer 2009, 9, 665–674. [Google Scholar] [CrossRef]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Langer, R.; Farokhzad, O.C. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef]
- Haider, N.; Fatima, S.; Taha, M.; Rizwanullah, M.; Firdous, J.; Ahmad, R.; Mazhar, F.; Khan, M.A. Nanomedicines in Diagnosis and Treatment of Cancer: An Update. Curr. Pharm. Des. 2020, 26, 1216–1231. [Google Scholar] [CrossRef]
- Sakr, T.M.; El-Hashash, M.A.; El-Mohty, A.A.; Essa, B.M. 99mTc-gallic-gold nanoparticles as a new imaging platform for tumor targeting. Appl. Radiat. Isot. 2020, 164, 109269. [Google Scholar] [CrossRef]
- El-Ghareb, W.I.; Swidan, M.M.; Ibrahim, I.T.; Abd El-Bary, A.; Tadros, M.I.; Sakr, T.M. 99mTc-Doxorubicin-loaded gallic acid-gold nanoparticles (99mTc-DOX-loaded GA-Au NPs) as a multifunctional theranostic agent. Int. J. Pharm. 2020, 586, 119514. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jang, J.; Cha, C. Carbon nanomaterials as versatile platforms for theranostic applications. Drug Discov. Today 2017, 22, 1430–1437. [Google Scholar] [CrossRef]
- Ding, K.; Jing, L.; Liu, C.; Hou, Y.; Gao, M. Magnetically engineered Cd-free quantum dots as dual-modality probes for fluorescence/magnetic resonance imaging of tumors. Biomaterials 2014, 35, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Aalipour, A.; Vermesh, O.; Yu, J.H.; Gambhir, S.S. Towards clinically translatable in vivo nanodiagnostics. Nat. Rev. Mater. 2017, 2, 17014. [Google Scholar] [CrossRef] [PubMed]
- Hequet, E.; Henoumont, C.; Djouana Kenfack, V.; Lemaur, V.; Lazzaroni, R.; Boutry, S.; Vander Elst, L.; Muller, R.N.; Laurent, S. Design, Characterization and Molecular Modeling of New Fluorinated Paramagnetic Contrast Agents for Dual 1H/19F MRI. Magnetochemistry 2020, 6, 8. [Google Scholar] [CrossRef]
- Ngandeu Neubi, G.M.; Opoku-Damoah, Y.; Gu, X.; Han, Y.; Zhou, J.; Ding, Y. Bio-inspired drug delivery systems: An emerging platform for targeted cancer therapy. Biomater. Sci. 2018, 6, 958–973. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef]
- Zhang, L.; Sheng, D.; Wang, D.; Yao, Y.; Yang, K.; Wang, Z.; Deng, L.; Chen, Y. Bioinspired multifunctional melanin-based nanoliposome for photoacoustic/magnetic resonance imaging-guided efficient photothermal ablation of cancer. Theranostics 2018, 8, 1591–1606. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Das, D.; Mishra, V.K.; Kashaw, V.; Kashaw, S.K. Nanoemulsion: A novel eon in cancer chemotherapy. Mini Rev. Med. Chem. 2017, 17, 1778–1792. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Chatterjee, B.; Mandal, U.; Sengupta, P.; Tekade, R.K. Pharmacokinetic and pharmacodynamic features of nanoemulsion following oral, intravenous, topical and nasal route. Curr. Pharm. Des. 2017, 23, 2504–2531. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Tekade, R.K.; Pandey, M.; Karmakar, S.; Pal, T.K. Safety against nephrotoxicity in paclitaxel treatment: Oral nanocarrier as an effective tool in preclinical evaluation with marked in vivo antitumor activity. Regul. Toxicol. Pharmacol. 2017, 91, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Gan, S.Y.; Haw, Y.H.; Ho, C.L.; Wong, S.; Choudhury, H. In vitro neuroprotective effects of naringenin nanoemulsion against b-amyloid toxicity through the regulation of amyloidogenesis and tau phosphorylation. Int. J. Biol. Macromol. 2018, 118, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Gorain, B.; Karmakar, S.; Biswas, E.; Dey, G.; Barik, R.; Mandal, M.; Pal, T.K. Improvement of cellular uptake, in vitro antitumor activity and sustained release profile with increased bioavailability from a nanoemulsion platform. Int. J. Pharm. 2014, 460, 131–143. [Google Scholar] [CrossRef]
- Gadhavea, D.; Gorainb, B.; Tagalpallewara, A.; Kokarea, C. Intranasal teriflunomide microemulsion: An improved chemotherapeutic approach in glioblastoma. J. Drug Deliv. Sci. Technol. 2019, 51, 276–289. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current applications of nanoemulsions in cancer therapeutics. Nanomaterials 2019, 9, E821. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, Q.; Li, Y.; Yang, Z.; Zhou, X.; Chen, S.; Jiang, Z.X. Fluorinated cryptophane-A and porphyrin-based theranostics for multimodal imaging-guided photodynamic therapy. Chem. Commun. 2020, 56, 3617–3620. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, S.; Feng, T.; Qin, X.; Wan, Y.; Jiang, S.; Li, C.; Lin, J.; Wang, T.; Zhou, X.; et al. Versatile Theranostic Nanoemulsion for Architecture-Dependent Multimodal Imaging and Dually Augmented Photodynamic Therapy. Adv Mater. 2019, 31, e1806444. [Google Scholar] [CrossRef]
- Fernandes, D.A.; Kolios, M.C. Near-infrared absorbing nanoemulsions as nonlinear ultrasound contrast agents for cancer theranostics. J. Mol. Liq. 2019, 287, 110848. [Google Scholar] [CrossRef]
- Patel, N.R.; Piroyan, A.; Ganta, S.; Morse, A.B.; Candiloro, K.M.; Solon, A.L.; Nack, A.H.; Galati, C.A.; Bora, C.; Maglaty, M.A.; et al. In Vitro and In Vivo evaluation of a novel folate-targeted theranostic nanoemulsion of docetaxel for imaging and improved anticancer activity against ovarian cancers. Cancer Biol. Ther. 2018, 19, 1555–8576. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current trends in cancer nanotheranostics: Metallic, polymeric, and lipid-based systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef]
- Petersen, A.L.; Hansen, A.E.; Gabizon, A.; Andresen, T.L. Liposome imaging agents in personalized medicine. Adv. Drug Deliv. Rev 2012, 64, 1417–1435. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Al-Jamal, K.T.; Tian, B.; Cakebread, A.; Halket, J.M.; Kostarelos, K. Tumor Targeting of Functionalized Quantum Dot– Liposome Hybrids by Intravenous Administration. Mol. Pharmaceut. 2009, 6, 520–530. [Google Scholar] [CrossRef]
- Wang, Q.; Chao, Y.M. Multifunctional quantum dots and liposome complexes in drug delivery. J. Biomed. Res. 2018, 32, 91–106. [Google Scholar]
- Lamichhane, N.; Udayakumar, T.S.; D’souza, W.D.; Simone Ii, C.B.; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image- Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef]
- Martínez-González, R.; Estelrich, J.; Busquets, M.A. Liposomes Loaded with Hydrophobic Iron Oxide Nanoparticles: Suitable T2 Contrast Agents for MRI. Int. J. Mol. Sci. 2016, 17, 1209. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Liu, D.; Zhou, G.; Li, Y.; Wang, P.; Hu, K.; Gu, N.; Ji, M. Liposomally formulated phospholipid-conjugated novel near-infrared fluorescence probe for particle size effect on cellular uptake and biodistribution in vivo. Colloids Surf. B Biointerfaces 2018, 161, 588–596. [Google Scholar] [CrossRef]
- Prasad, R.; Jain, N.K.; Yadav, A.S.; Chauhan, D.S.; Devrukhkar, J.; Kumawat, M.K.; Shinde, S.; Gorain, M.; Thakor, A.S.; Kundu, G.C.; et al. Liposomal nanotheranostics for multimode targeted in vivo bioimaging and near-infrared light mediated cancer therapy. Commun. Biol. 2020, 3, 284. [Google Scholar] [CrossRef] [PubMed]
- Karpuz, M.; Silindir-Gunay, M.; Ozer, A.Y.; Ozturk, S.C.; Yanik, H.; Tuncel, M.; Aydin, C.; Esendagli, G. Diagnostic and therapeutic evaluation of folate-targeted paclitaxel and vinorelbine encapsulating theranostic liposomes for non-small cell lung cancer. Eur, J. Pharm. Sci. 2021, 156, 105576. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Zhao, Y.; Kang, X.; Zhao, P.; Fu, X.; Mo, X.; Wang, Y.; Huang, Y. BBB-penetrating codelivery liposomes treat brain metastasis of non-small cell lung cancer with EGFRT790M mutation. Theranostics 2020, 10, 6122–6135. [Google Scholar] [CrossRef] [PubMed]
- Bush, N.; Healey, A.; Shah, A.; Box, G.; Kirkin, V.; Eccles, S.; Sontum, P.C.; Kotopoulis, S.; Kvåle, S.; van Wamel, A.; et al. Theranostic Attributes of Acoustic Cluster Therapy and Its Use for Enhancing the Effectiveness of Liposomal Doxorubicin Treatment of Human Triple Negative Breast Cancer in Mice. Front. Pharmacol. 2020, 11, 75. [Google Scholar] [CrossRef]
- Prabhakar, A.; Banerjee, R. Nanobubble Liposome Complexes for Diagnostic Imaging and Ultrasound-Triggered Drug Delivery in Cancers: A Theranostic Approach. ACS Omega 2019, 4, 15567–15580. [Google Scholar] [CrossRef]
- Mssi, S.V.; Torchilin, V.P. Recent trends in the use of lipidic nanoparticles as pharmaceutical carriers for cancer therapy and diagnostics. J. Mater. Chem. B 2013, 1, 5201–5209. [Google Scholar] [CrossRef]
- Lopes, R.M.; Gaspar, M.M.; Pereira, J.; Eleutério, C.V.; Carvalheiro, M.; Almeida, A.J.; Cruz, M.E.M. Liposomes versus lipid nanoparticles: Comparative study of lipid-based systems as oryzalin carriers for the treatment of leishmaniasis. J. Biomed. Nanotechnol. 2014, 10, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mader, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, K.; Cao, Y.; Chen, X.; Wang, K.; Liu, M.; Pei, R. Hydrophobic IR-780 Dye Encapsulated in cRGD-Conjugated Solid Lipid Nanoparticles for NIR Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 12217–12226. [Google Scholar] [CrossRef] [PubMed]
- Peira, E.; Marzola, P.; Podio, V.; Aime, S.; Sbarbati, A.; Gasco, M.R. In vitro and in vivo study of solid lipid nanoparticles loaded with superparamagnetic iron oxide. J. Drug Target. 2003, 11, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, E.; Seo, J.W.; Ferrara, K.; Louie, A. Novel method to label solid lipid nanoparticles with 64 Cu for positron emission tomography imaging. Bioconjug. Chem. 2011, 22, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Shuhendler, A.J.; Prasad, P.; Leung, M.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y. A novel solid lipid nanoparticle formulation for active targeting to tumor αvβ3 integrin receptors reveals cyclic RGD as a double-edged sword. Adv. Healthc. Mater. 2012, 1, 600–608. [Google Scholar] [CrossRef]
- Bae, K.H.; Lee, J.Y.; Lee, S.H.; Park, T.G.; Nam, Y.S. Optically traceable solid lipid nanoparticles loaded with siRNA and paclitaxel for synergistic chemotherapy with in situ imaging. Adv. Healthc. Mater. 2013, 2, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Bentolila, L.A.; Ebenstein, Y.; Weiss, S. Quantum dots for in vivo small-animal imaging. J. Nucl. Med. 2009, 50, 493–496. [Google Scholar] [CrossRef]
- Morel, S.; Terreno, E.; Ugazio, E.; Aime, S.; Gasco, M.R. NMR relaxometric investigations of solid lipid nanoparticles (SLN) containing gadolinium (III) complexes. Eur. J. Pharm. Biopharm. 1998, 45, 157–163. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Yang, X.; Zhou, Y.; Ping, Q.; Oupicky, D.; Sun, M. Dualfunction nanostructured lipid carriers to deliver IR780 for breast cancer treatment: Anti-metastatic and photothermal anti-tumor therapy. Acta Biomater. 2017, 53, 399–413. [Google Scholar] [CrossRef]
- Olerile, L.D.; Liu, Y.; Zhang, B.; Wang, T.; Mu, S.; Zhang, J.; Selotlegeng, L.; Zhang, N. Nearinfrared mediated quantum dots and paclitaxel co-loaded nanostructured lipid carriers for cancer theragnostic. Colloids Surf. B Biointerfaces 2017, 150, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-H.; Wen, C.-J.; Al-Suwayeh, S.A.; Huang, Y.-J.; Fang, J.-Y. Formulation design and evaluation of quantum dot-loaded nanostructured lipid carriers for integrating bioimaging and anticancer therapy. Nanomedicine 2013, 8, 1253–1269. [Google Scholar] [CrossRef] [PubMed]
- Ucar, E.; Teksoz, S.; Ichedef, C.; Kilcar, A.Y.; Medine, E.I.; Ari, K.; Parlak, Y.; Sayit Bilgin, B.E.; Unak, P. Synthesis, characterization and radiolabeling of folic acid modified nanostructured lipid carriers as a contrast agent and drug delivery system. Appl. Radiat. Isot. 2017, 119, 72–79. [Google Scholar] [CrossRef]
- Huynh, N.T.; Passirani, C.; Saulnier, P.; Benoit, J.P. Lipid nanocapsules: A new platform for nanomedicine. Int. J. Pharm. 2009, 379, 201–209. [Google Scholar] [CrossRef] [PubMed]
- AbdElhamid, A.S.; Zayed, D.G.; Helmy, M.W.; Ebrahim, S.M.; Bahey-El-Din, M.; Zein-El-Dein, E.A.; El-Gizawy, S.A.; Elzoghby, A.O. Lactoferrin-tagged quantum dots-based theranostic nanocapsules for combined COX-2 inhibitor/herbal therapy of breast cancer. Nanomedicine 2018, 13, 2637–2656. [Google Scholar] [CrossRef]
- Ma, M.; Hao, Y.; Liu, N.; Yin, Z.; Wang, L.; Liang, X.; Zhang, X. A novel lipid-based nanomicelle of docetaxel: Evaluation of antitumor activity and biodistribution. Int. J. Nanomed. 2012, 7, 3389–3398. [Google Scholar] [CrossRef]
- Huang, H.; Dong, Y.; Zhang, Y.; Ru, D.; Wu, Z.; Zhang, J.; Shen, M.; Duan, Y.; Sun, Y. GSH-sensitive Pt(IV) prodrug-loaded phase-transitional nanoparticles with a hybrid lipid-polymer shell for precise theranostics against ovarian cancer. Theranostics 2019, 9, 1047–1065. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, G. Synthetic lipoprotein as nano-material vehicle in the targeted drug delivery. Drug Deliv. 2017, 24, 16–21. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, W.; Jin, H.; Lovell, J.F.; Yang, M.; Ding, L.; Chen, J.; Corbin, I.; Luo, Q.; Zheng, G. Biomimetic nanocarrier for direct cytosolic drug delivery. Angew. Chem. Int. Ed. Engl. 2009, 48, 9171–9175. [Google Scholar] [CrossRef]
- Yang, M.; Chen, J.; Cao, W.; Ding, L.; Ng, K.K.; Jin, H.; Zhang, Z.; Zheng, G. Attenuation of nontargeted cell-kill using a high-density lipoprotein-mimicking peptide--phospholipid nanoscaffold. Nanomedicine 2011, 6, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Jin, C.S.; Huang, H.; Ding, L.; Zhang, Z.; Chen, J.; Zheng, J. Nanoparticle-enabled, image-guided treatment planning of target specific RNAi therapeutics in an orthotopic prostate cancer model. Small 2014, 10, 3072–3082. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Delgado, T.; Rodriguez, J.A.; Helguera, G.; Penichet, M.L. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 2006, 121, 144–1458. [Google Scholar] [CrossRef] [PubMed]
- Das Gupta, A.; Shah, V.I. Correlation of transferrin receptor expression with histologic grade and immunophenotype in chronic lymphocytic leukemia and non-Hodgkin’s lymphoma. Hematol. Pathol. 1990, 4, 37–41. [Google Scholar]
- Prior, R.; Reifenberger, G.; Wechsler, W. Transferrin receptor expression in tumours of the human nervous system: Relation to tumour type, grading and tumour growth fraction. Virchows Arch. A Pathol. Anat. Histopathol. 1990, 416, 491–496. [Google Scholar] [CrossRef]
- Singh, M.; Mugler, K.; Hailoo, D.W.; Burke, S.; Nemesure, B.; Torkko, K.; Shroyer, K.R. Differential expression of transferrin receptor (TfR) in a spectrum of normal to malignant breast tissues: Implications for in situ and invasive carcinoma. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 417–423. [Google Scholar] [CrossRef]
- Callens, C.; Moura, I.C.; Lepelletier, Y.; Coulon, S.; Renand, A.; Dussiot, M.; Ghez, D.; Benhamou, M.; Monteiro, R.C.; Bazarbachi, A.; et al. Recent advances in adult T-cell leukemia therapy: Focus on a new anti-transferrin receptor monoclonal antibody. Leukemia 2008, 22, 42–48. [Google Scholar] [CrossRef]
- He, Q.; Sun, X.; Chu, C.; Jiang, Q.; Zhu, H.; He, Y.; Yue, T.; Wang, R.; Lei, P.; Shen, G. Endocytosis of a functionally enhanced GFP-tagged transferrin receptor in CHO cells. PLoS ONE 2015, 10, e0122452. [Google Scholar] [CrossRef]
- Luck, A.N.; Mason, A.B. Structure and dynamics of drug carriers and their interaction with cellular receptors: Focus on serum transferrin. Adv. Drug Deliv. Rev. 2013, 65, 1012–1019. [Google Scholar] [CrossRef]
- Tortorella, S.; Karagiannis, T.C. Transferrin receptor-mediated endocytosis: A useful target for cancer therapy. J. Membr. Biol. 2014, 247, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Tros de Ilarduya, C.; Duzgunes, N. Delivery of therapeutic nucleic acids via transferrin and transferrin receptors: Lipoplexes and other carriers. Expert Opin. Drug Deliv. 2013, 10, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; He, Y.; Ye, Q.; Zhu, H.F.; Yuan, X.M.; Liu, J.; Xing, W.; Wu, S.; Dai, W.; Shen, X.; et al. Antigen-binding characteristics of AbCD71 and its inhibitory effect on PHA-induced lymphoproliferation. Acta Pharmacol. Sin. 2007, 28, 1659–1664. [Google Scholar] [CrossRef][Green Version]
- Qing, Y.; Shuo, W.; Zhihua, W.; Huifen, Z.; Ping, L.; Lijiang, L.; Xiaorong, Z.; Liming, C.; Daiwen, X.; Yu, H.; et al. The in vitro antitumor effect and in vivo tumor-specificity distribution of human-mouse chimeric antibody against transferrin receptor. Cancer Immunol. Immunother. 2006, 55, 1111–1121. [Google Scholar] [CrossRef]
- Li, J.; Weng, X.; Liang, Z.; Zhong, M.; Chen, X.; Lu, S.; Sun, W.; Song, Y.; Wu, X.; Shen, G. Viral specific cytotoxic T cells inhibit the growth of TfR-expressing tumor cells with antibody targeted viral peptide/HLA-A2 complex. Cell Immunol. 2010, 263, 154–160. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, J.; Li, Y.; Yang, J.; Yu, Y.; Wang, M.; Xu, X.; Huang, C.; Huang, W.; Dong, J.; et al. Targeting hypoxia-inducible factor-1alpha with Tf-PEI-shRNA complex via transferrin receptor-mediated endocytosis inhibits melanoma growth. Mol. Ther. 2009, 17, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Hu, H.; Wang, Z.; Lu, T.; Hu, Z.; Zeng, X.; Zhang, S.; Liu, J.; Lei, P.; Wang, C.Y.; et al. Generation and functional characterization of the anti-transferrin receptor single-chain antibody-GAL4 (TfRscFv-GAL4) fusion protein. BMC Biotechnol. 2012, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Guo, Z.; Fu, M.; Tang, H.; Zhu, H.; Shen, G.; He, Y.; Lei, P. Establishment of a hTfR mAb-functionalized HPPS theranostic nanoplatform. Nanotheranostics 2020, 4, 119–128. [Google Scholar] [CrossRef]
- Chen, W.; Jarzyna, P.A.; van Tilborg, G.A.F.; Nguyen, V.A.; Cormode, D.P.; Klink, A.; Griffioen, A.W.; Randolph, G.J.; Fisher, E.A.; Mulder, W.J.M.; et al. RGD peptide functionalized and reconstituted high-density lipoprotein nanoparticles as a versatile and multimodal tumor targeting molecular imaging probe. FASEB J. 2010, 24, 1689–1699. [Google Scholar] [CrossRef]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, E.I.; Vulesevic, B.; Argawal, A.; Ross, A.; Bejjani, P.; Podrebara, J.; Ravichandran, R.; Phopase, J.; Suuronen, E.J.; Griffith, M. Coloured cornea replacements with anti-infective properties: Expanding the safe use of silver nanoparticles in regenerative medicine. Nanoscale 2016, 8, 6484–6489. [Google Scholar] [CrossRef]
- Chandra, S.; Barick, K.C.; Bahadur, D. Oxide and hybrid nanostructures for therapeutic applications. Adv. Drug Deliv. Rev. 2011, 63, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Ko, Y.T. Lipid-coated gold nanocomposites for enhanced cancer therapy. Int. J. Nanomed. 2015, 10, 33–45. [Google Scholar]
- Bae, P.K.; Chung, B.H. Multiplexed detection of various breast cancer cells by perfluorocarbon/quantum dot nanoemulsions conjugated with antibodies. Nano Converg. 2014, 1, 23. [Google Scholar] [CrossRef]
- Allijn, I.E.; Leong, W.; Tang, J.; Gianella, A.; Mieszawska, A.J.; Fay, F.; Ma, G.; Russell, S.; Callo, C.B.; Gordon, R.E.; et al. Gold Nanocrystal Labeling Allows Low-Density Lipoprotein Imaging from the Subcellular to Macroscopic Level. ACS Nano 2013, 7, 9761–9770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, H.; Liu, A.Y.; Shen, J.J.; Shah, V.; Zhang, C.; Hong, J.; Ding, Y. Gold conjugate-based liposomes with hybrid cluster bomb structure for liver cancer therapy. Biomaterials 2016, 74, 280–291. [Google Scholar] [CrossRef]
- Huang, X.; Tang, S.; Mu, X.; Dai, Y.; Chen, G.; Zhou, Z.; Ruan, F.; Yang, Z.; Zheng, N. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat. Nanotechnol. 2011, 6, 28–32. [Google Scholar] [CrossRef]
- Adams, C.P.; Walker, K.A.; Obare, S.O.; Docherty, K.M. Size-Dependent Antimicrobial Effects of Novel Palladium Nanoparticles. PLoS ONE 2014, 9, e85981. [Google Scholar] [CrossRef] [PubMed]
- Miesen, T.J.; Engstrom, A.M.; Miesen, T.J.; Miesen, T.J.; Frost, D.C.; Ajjarapu, R.; Lira, C.N.; Mackiewicz, M.R. A hybrid lipid membrane coating “shape-locks” silver nanoparticles to prevent surface oxidation and silver ion dissolution. RCS Adv. 2020, 10, 15677–15693. [Google Scholar]
- Bhattacharyya, K.; Goldschmidt, B.S.; Hannink, M.; Alexander, S.; Jurkevic, A.; Viator, J.A. Gold nanoparticle-mediated detection of circulating cancer cells. Clin. Lab. Med. 2012, 32, 89–101. [Google Scholar] [CrossRef]
- Tahmasbi Rad, A.; Chen, C.W.; Aresh, W.; Xia, Y.; Lai, P.S.; Nieh, M.P. Combinational Effects of Active Targeting, Shape, and Enhanced Permeability and Retention for Cancer Theranostic Nanocarriers. ACS Appl. Mater Interfaces 2019, 11, 10505–10519. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F. To Exploit the Tumor Microenvironment: Since the Epr Effect Fails in the Clinic, What Is the Future of Nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Singh, V.; Jurney, P.; Shi, L.; Sreenivasan, S.; Roy, K. Mammalian Cells Preferentially Internalize Hydrogel Nanodiscs over Nanorods and Use Shape-Specific Uptake Mechanisms. Proc. Natl. Acad. Sci. USA. 2013, 110, 17247–17252. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The Importance of Nanoparticle Shape in Cancer Drug Delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef]
- Nagahama, K.; Kawano, D.; Oyama, N.; Takemoto, A.; Kumano, T.; Kawakami, J. Self-Assembling Polymer Micelle/Clay Nanodisk/ Doxorubicin Hybrid Injectable Gels for Safe and Efficient Focal Treatment of Cancer. Biomacromolecules 2015, 16, 880–889. [Google Scholar] [CrossRef]
- Sun, W.; Parowatkin, M.; Steffen, W.; Butt, H.J.; Mailänder, V.; Wu, S. Ruthenium-Containing Block Copolymer Assemblies: Red- Light-Responsive Metallopolymers with Tunable Nanostructures for Enhanced Cellular Uptake and Anticancer Phototherapy. Adv. Healthc. Mater. 2016, 5, 467–473. [Google Scholar] [CrossRef]
- Sun, W.; Wen, Y.; Thiramanas, R.; Chen, M.; Han, J.; Gong, N.; Wagner, M.; Jiang, S.; Meijer, M.S.; Bonnet, S.; et al. Red-Light- Controlled Release of Drug−Ru Complex Conjugates from Metallopolymer Micelles for Phototherapy in Hypoxic Tumor Environments. Adv. Funct. Mater. 2018, 28, 1804227. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.D. Factors Controlling the Pharmacokinetics, Biodistribution and Intratumoral Penetration of Nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef]
- Tan, J.; Shah, S.; Thomas, A.; Ou-Yang, H.D.; Liu, Y. The Influence of Size, Shape and Vessel Geometry on Nanoparticle Distribution. Microfluid. Nanofluid. 2013, 14, 77–87. [Google Scholar] [CrossRef]
- Carregal-Romero, S.; Plaza-García, S.; Piñol, R.; Murillo, J.L.; Ruiz-Cabello, J.; Padro, D.; Millán, A.; Ramos-Cabrer, P. MRI Study of the Influence of Surface Coating Aging on the In Vivo Biodistribution of Iron Oxide Nanoparticles. Biosensors 2018, 8, 127. [Google Scholar] [CrossRef]
- Gómez-Vallejo, V.; Puigivila, M.; Plaza-García, S.; Szczupak, B.; Piñol, R.; Murillo, J.L.; Sorribas, V.; Lou, G.; Veintemillas, S.; Ramos-Cabrer, P.; et al. PEG-copolymer-coated iron oxide nanoparticles that avoid the reticuloendothelial system and act as kidney MRI contrast agents. Nanoscale 2018, 10, 14153–14164. [Google Scholar] [CrossRef]
- Majumder, B.; Baraneedharan, U.; Thiyagarajan, S.; Radhakrishnan, P.; Narasimhan, H.; Dhandapani, M.; Brijwani, N.; Pinto, D.D.; Prasath, A.; Shanthappa, B.U.; et al. Predicting clinical response to anticancer drugs using an ex vivo platform that captures tumour heterogeneity. Nat. Commun. 2015, 6, 6169. [Google Scholar] [CrossRef]
- Dilnawaz, F.; Acharya, S.; Sahoo, S.K. Recent trends of nanomedicinal approaches in clinics. Int. J. Pharm. 2018, 538, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine—Challenge and perspectives. Angew. Chem. Int. Ed. Engl. 2009, 48, 872–897. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Jackman, J.A.; Meszaros, T.; Fulop, T.; Urbanics, R.; Szebeni, J.; Cho, N.J. Comparison of complement activation-related pseudoallergy in miniature and domestic pigs: Foundation of a validatable immune toxicity model. Nanomedicine 2016, 12, 933–943. [Google Scholar] [CrossRef]
- Szebeni, J.; Storm, G. Complement activation as a bioequivalence issue relevant to the development of generic liposomes and other nanoparticulate drugs. Biochem. Biophys. Res. Commun. 2015, 468, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: Pathways for translational development and commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Dreifuss, T.; Betzer, O.; Shilo, M.; Popovtzer, A.; Motiei, M.; Popovtzer, R. A challenge for theranostics: Is the optimal particle for therapy also optimal for diagnostics? Nanoscale 2015, 7, 15175–15184. [Google Scholar] [CrossRef]
- Sainz, V.; Conniot, J.; Matos, A.I.; Peres, C.; Zupancic, E.; Moura, L.; Silva, L.C.; Florindo, H.F.; Gaspar, R.S. Regulatory aspects on nanomedicines. Biochem. Biophys. Res. Commun. 2015, 468, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R. Regulatory issues surrounding nanomedicines: Setting the scene for the next generation of nanopharmaceuticals. Nanomedicine 2007, 2, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Tinkle, S.; McNeil, S.E.; Muhlebach, S.; Bawa, R.; Borchard, G.; Barenholz, Y.; Tamarkin, L.; Desai, N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014, 1313, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Accomasso, L.; Cristallini, C.; Giachino, C. Risk assessment and risk minimization in nanomedicine: A need for predictive, alternative, and 3Rs strategies. Front. Pharmacol. 2018, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Ragelle, H.; Danhier, F.; Preat, V.; Langer, R.; Anderson, D.G. Nanoparticle-based drug delivery systems: A commercial and regulatory outlook as the field matures. Expert Opin. Drug Deliv. 2017, 14, 851–864. [Google Scholar] [CrossRef]




| Lipidic Nanocarrier | Chemotherapeutic Agent | Diagnostic Agent/Modality | Experimental Model | Theranostic Outcome | Ref. |
|---|---|---|---|---|---|
| Nanoemulsion | PDT | fluorinated cryptophane-A and porphyrin self-assembled onto the surface of fluorinated nanoemulsions-19F MRI and fluorescence imaging | Xenograft A549 tumor mice. | A high therapeutic efficacy; low toxicity; high tumor accumulation of nanoemulsion | [38] |
| PDT | Fluorescence probe/photoacoustic/19F magnetic resonance multimodal | A375 melanoma xenograft model | The remarkable efficiency of PDT on hypoxic solid tumors via a single injection of the drug; outstanding diagnostic ability | [39] | |
| Doxorubicin and Paclitaxel | Perfluorohexane (PFH) vaporized bubbles as an Ultrasound contrast agent | MCF-7 cells | Markedly enhanced PFH-NEs targeting and lodging in tumor region with simultaneous treatment monitoring. | [40] | |
| Paclitaxel and PDT | Porphyrin NE shell-based photoacoustic imaging and fluorescence imaging; CT contrast | Mice bearing tumors | multimodal cancer imaging, highly efficient phototherapy and image-guided drug delivery | [41] | |
| Liposomes | Doxorubicin HCl | gold nanoparticles (AuNPs) and emissive graphene quantum dots (GQDs) | Breast tumor-bearing mice models | specific and enhanced cellular uptake, prolonged internalization in tumor and substantial contrasting and therapeutic efficacy | [49] |
| Paclitaxel and vinorelbine | Tc-99m radiolabeled | NSCLC tumor-bearing C57BL/6 mice | Effectively inhibited tumor growth completely restricted lung metastasis | [50] | |
| Gefitinib and simvastatin | Fluorescence imaging | Brain Metastasis (BM) mouse model developed by intracranial transplant of the H1975 NSCLC cells | Efficient permeation across the blood–brain barrier and high capability of reversing drug resistance. | [51] | |
| Doxorubicin | Acoustic cluster therapy (ACT); Ultrasound insonition | orthotopic human tumor xenografts in athymic mice | Substantial increase therapeutic efficacy of Doxil® when combined with ACT | [52] | |
| Paclitaxel and ultrasound responsive drug delivery | Ultrasound imaging | MiaPaCa-2, Panc-1, MDA-MB-231, and AW-8507 cell lines | 300-fold higher anticancer activity in contrast to ABRAXANE. | [53] | |
| SLN | Paclitaxel and siRNA | Quantum dots | A549 cancer cells | Efficient in situ visualization of intracellular translocation of SLNs into cancer cells. | [54,62] |
| 64Cu, PET imaging, and ex vivo gamma counting | Mice | 64Cu-radiolabelled SLN and their biodistribution was efficiently quantitatively evaluated | [59] | ||
| NLC | Paclitaxel | Quantum dots | HepG2 cells/Female Kunming mice | Imaging established splendid capability of the co-loaded NLC to specifically target and detect the H22 tumor. | [65] |
| IR 780 and Photothermal therapy | fluorescent probe coumarin 6 | 4T1-luc cell line in BALB/c female mice | Notably enhanced photothermal anti-tumor effect as well as anti-metastatic efficacy in vivo | [64] | |
| Camptothecin | Quantum dots | Melanoma cells | camptothecin accumulation in melanomas increased by 6.4-fold | [66] | |
| Paclitaxel | 99mTc(CO)3+ | Wistar Albino rats. | Substantially high cellular uptake and concurrent imaging | [67] | |
| Lipid nanocapsule | Celecoxib and honokiol | fluorescent mercaptopropionic acid-capped cadmium telluride was coupled with quantum dots as an imaging probe | human breast cancer cells: MCF-7 and MDA-MB-231; EAT model | Highly improved and superior anticancer efficacy; Efficiently traceable LNC internalization | [69] |
| Lipid-Polymer Hybrid | Platinum (IV) (Pt(IV)) prodrug | (glutathione (GSH)-sensitive platinum (IV) for Ultrasound imaging | αvβ3- and αvβ5-positive SKOV3 human ovarian tumor cells and αvβ3- and αvβ5-negative A2780 human ovarian tumor cells | Significant therapeutic efficacy and limited side effect | [71] |
| Lipidic Nanocarrier | Attributes | Cancer Type | Sponsors | Clinical Trial ID/Phase |
|---|---|---|---|---|
| Liposomes | Evaluating Immunogenic Chemotherapy Combined With Ipilimumab and Nivolumab in Patients With Metastatic Luminal B Breast Cancer | Breast Cancer | Oslo University Hospital | NCT03409198, Phase 2B |
| Liposomes | To study the distribution profile and radiation dosimetry of 188Re-BMEDAliposomes. | Tumors | Nuclear Energy Research Institute of Taiwan. | NCT02271516 Phase 1 |
| Liposomes | To study the MTD of EphA2 siRNA –encapsulated liposomes, evaluate efficacy in the tumor cell, which we cannot be cured by treatment. | Solid Tumors | M.D. Anderson Cancer Center National Cancer Institute (NCI) | NCT02191878 Phase 3 |
| Lipid-based Nanoparticles | To study proposes targeted delivery cytotoxic drugs, via formulated LTSL activated by using focused ultrasound (FUS). | Liver Tumor | University of Oxford | NCT02181075 Phase 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukhari, S.I.; Imam, S.S.; Ahmad, M.Z.; Vuddanda, P.R.; Alshehri, S.; Mahdi, W.A.; Ahmad, J. Recent Progress in Lipid Nanoparticles for Cancer Theranostics: Opportunity and Challenges. Pharmaceutics 2021, 13, 840. https://doi.org/10.3390/pharmaceutics13060840
Bukhari SI, Imam SS, Ahmad MZ, Vuddanda PR, Alshehri S, Mahdi WA, Ahmad J. Recent Progress in Lipid Nanoparticles for Cancer Theranostics: Opportunity and Challenges. Pharmaceutics. 2021; 13(6):840. https://doi.org/10.3390/pharmaceutics13060840
Chicago/Turabian StyleBukhari, Sarah I., Syed Sarim Imam, Mohammad Zaki Ahmad, Parameswara Rao Vuddanda, Sultan Alshehri, Wael A. Mahdi, and Javed Ahmad. 2021. "Recent Progress in Lipid Nanoparticles for Cancer Theranostics: Opportunity and Challenges" Pharmaceutics 13, no. 6: 840. https://doi.org/10.3390/pharmaceutics13060840
APA StyleBukhari, S. I., Imam, S. S., Ahmad, M. Z., Vuddanda, P. R., Alshehri, S., Mahdi, W. A., & Ahmad, J. (2021). Recent Progress in Lipid Nanoparticles for Cancer Theranostics: Opportunity and Challenges. Pharmaceutics, 13(6), 840. https://doi.org/10.3390/pharmaceutics13060840









