A Setup for Microscopic Studies of Ultrasounds Effects on Microliters Scale Samples: Analytical, Numerical and Experimental Characterization
Abstract
:1. Introduction
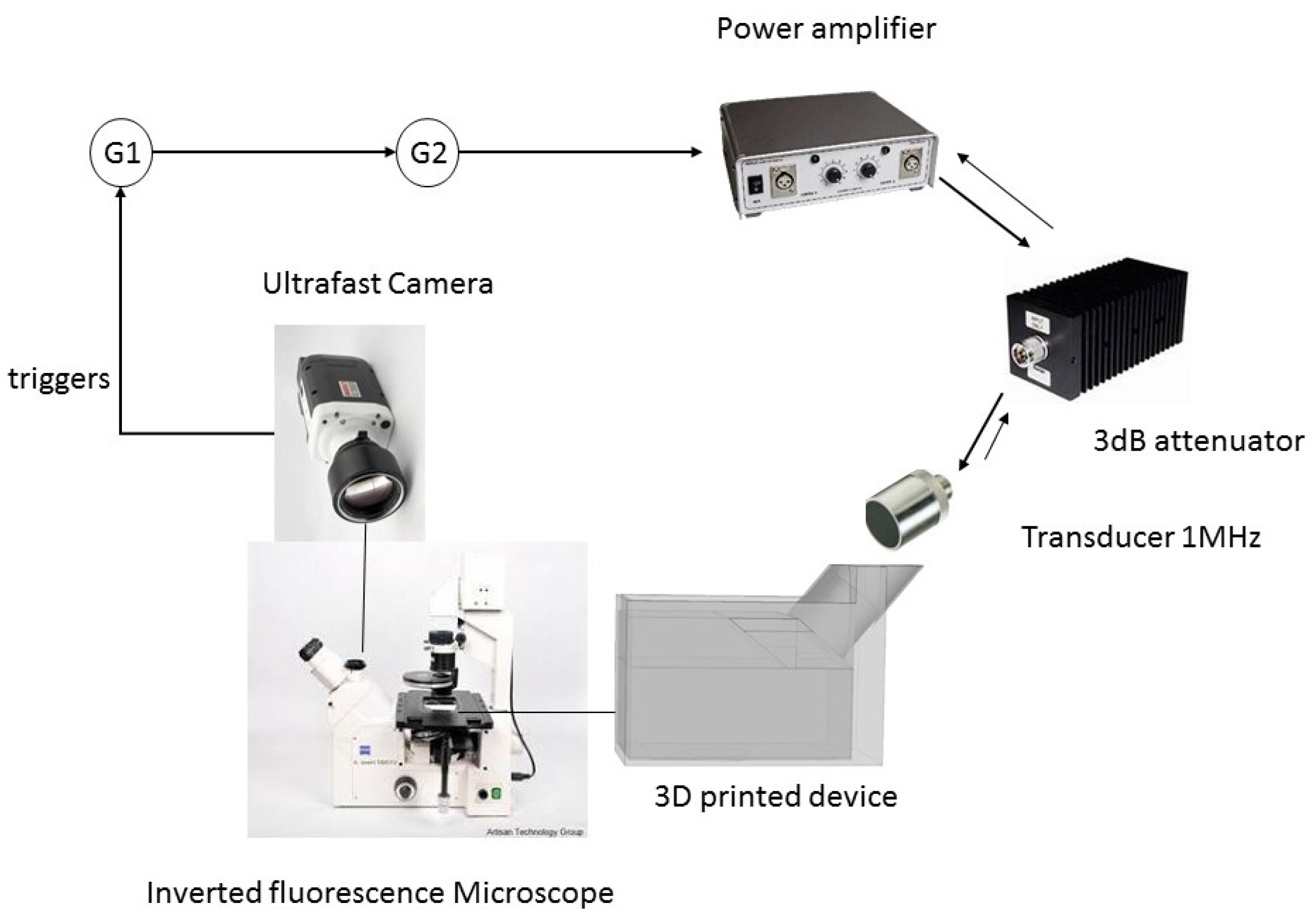
2. Materials and Methods
3. Results
3.1. Development of a Model to Predict the Ultrasonic Field in a Small Medium Volume Encompassed by Rigid Walls
3.2. Practical Design of the New Applicator
3.2.1. Transducer Type
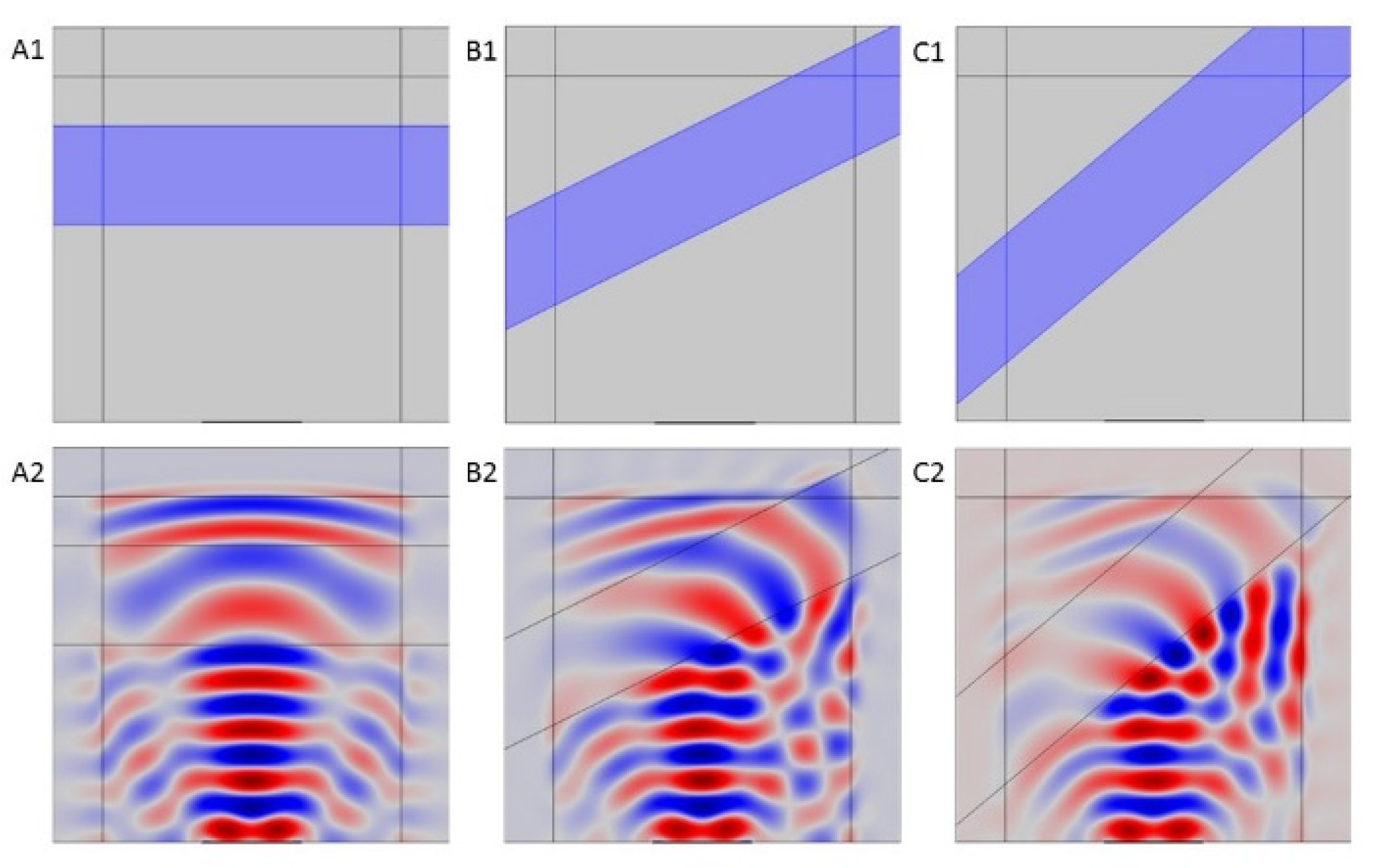
3.2.2. Constraints of Simultaneous Exposure to Light and US: Modelling US Nonnormal Application
3.2.3. Other Constraints and Device Fabrication
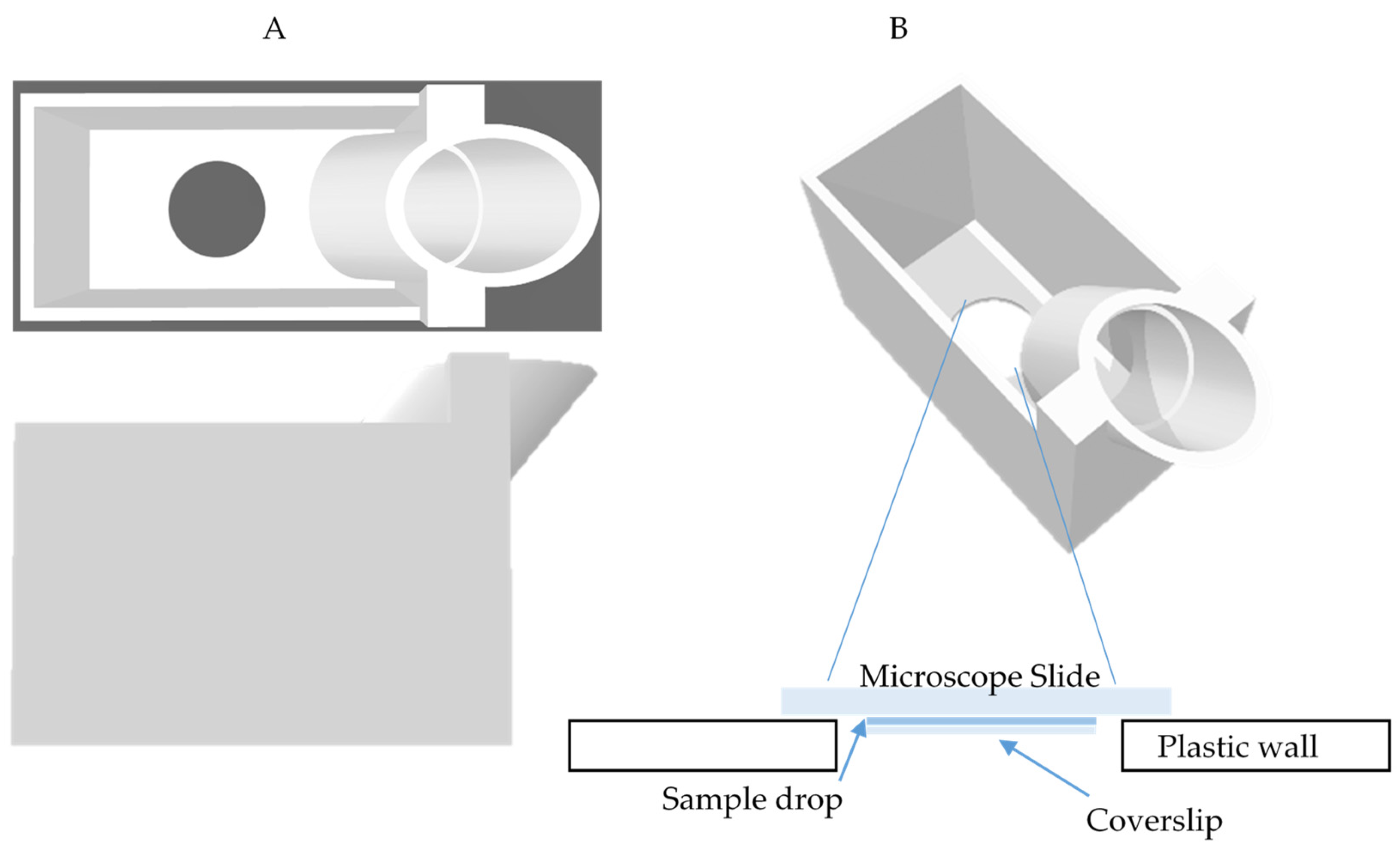
3.2.4. Sample Positioning
3.3. Device Experimental Calibrations
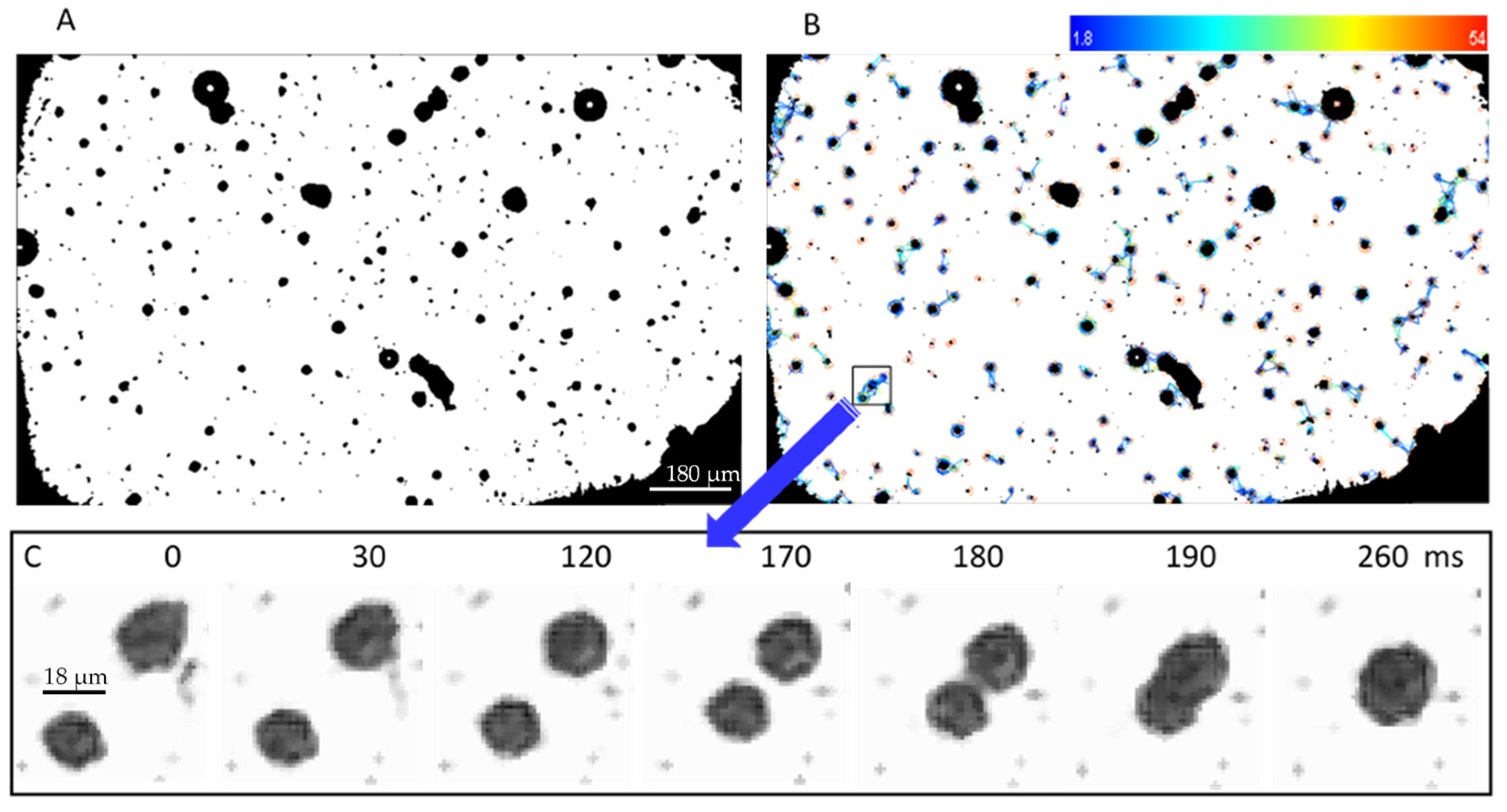
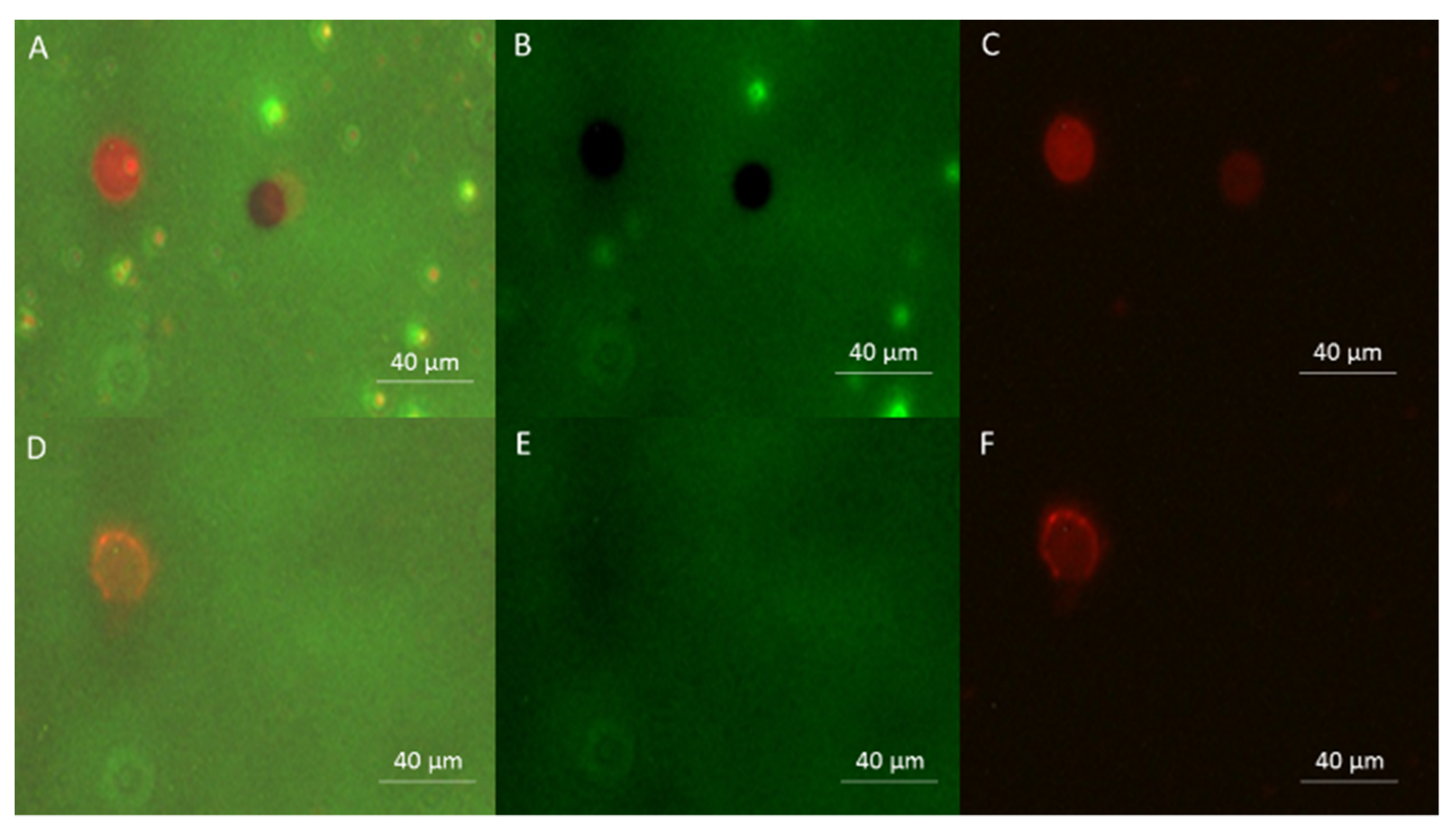
3.4. Experimental Results Using the New Setup
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Analytical Description
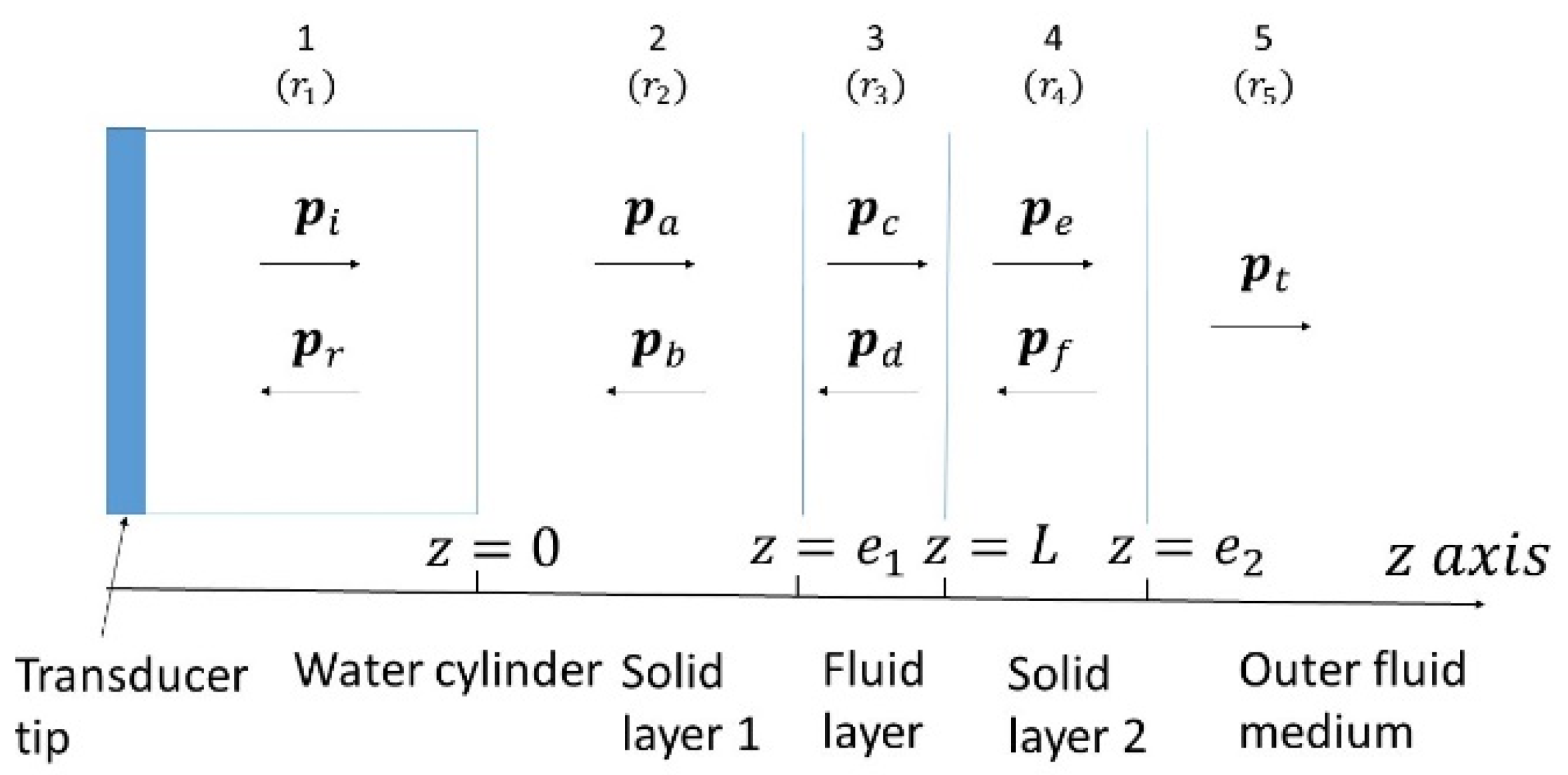
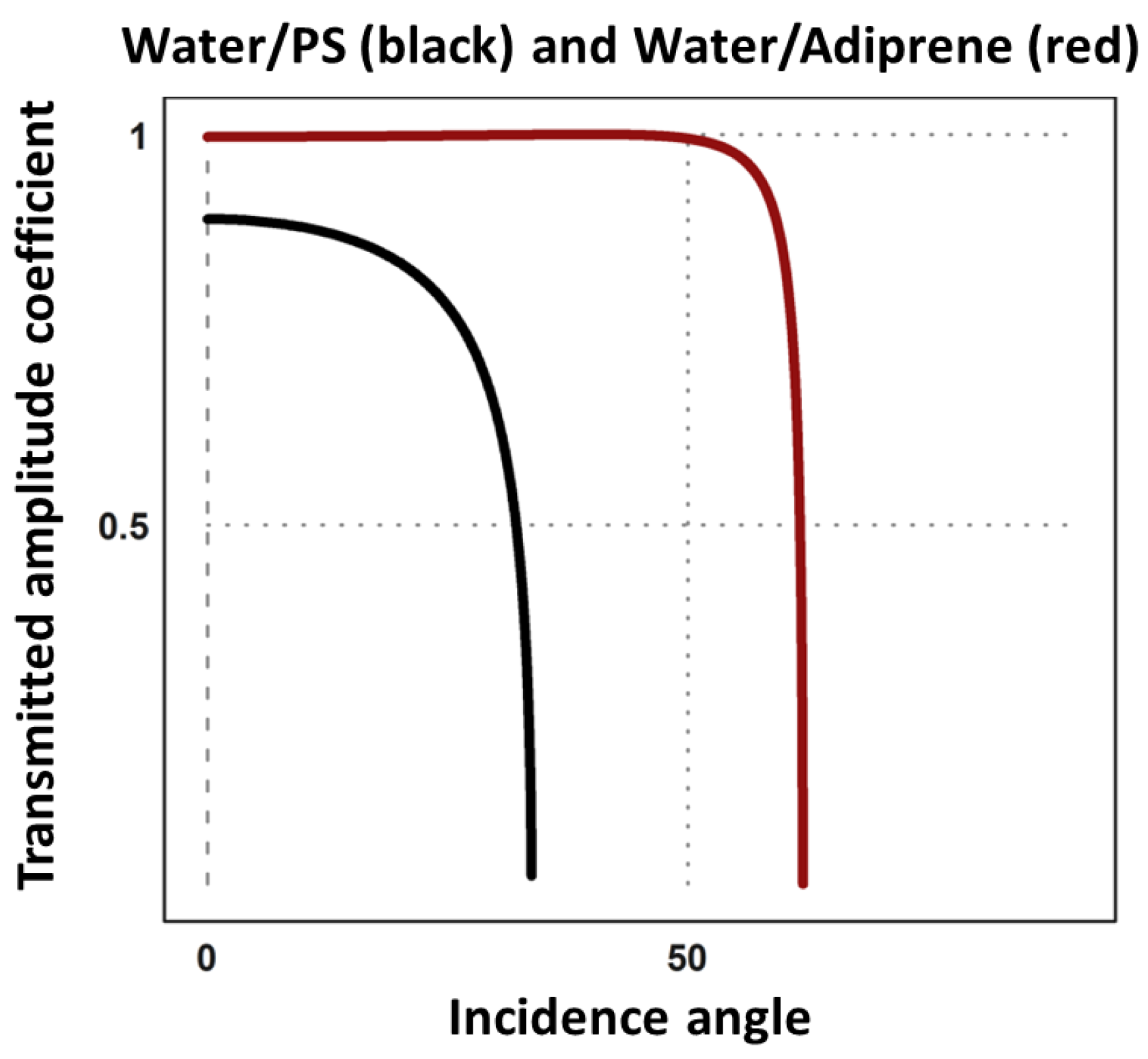
Appendix A.2. Influence of the Slides Materials


Appendix A.3. Intromission Angle
References
- Brayman, A.A.; Coppage, M.L.; Vaidya, S.; Miller, M.W. Transient poration and cell surface receptor removal from human lymphocytes in vitro by 1 MHz ultrasound. Ultrasound Med. Biol. 1999, 25, 999–1008. [Google Scholar] [CrossRef]
- Cheng, M.; Li, F.; Han, T.; Yu, A.C.H.; Qin, P. Effects of ultrasound pulse parameters on cavitation properties of flowing microbubbles under physiologically relevant conditions. Ultrason Sonochem. 2019, 52, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Shi, J.; Yao, Y.; Han, T.; Yu, A.C.H.; Qin, P. Plasma Membrane Blebbing Dynamics Involved in the Reversibly Perforated Cell by Ultrasound-Driven Microbubbles. Ultrasound Med. Biol. 2021, 47, 733–750. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Cai, C.; Tu, J.; Guo, X.; Zhang, D. Sonoporation-induced cell membrane permeabilization and cytoskeleton disassembly at varied acoustic and microbubble-cell parameters. Sci. Rep. 2018, 8, 3885. [Google Scholar] [CrossRef] [Green Version]
- Zou, P.; Li, M.; Wang, Z.; Zhang, G.; Jin, L.; Pang, Y.; Du, L.; Duan, Y.; Liu, Z.; Shi, Q. Micro-Particle Image Velocimetry Investigation of Flow Fields of SonoVue Microbubbles Mediated by Ultrasound and Their Relationship with Delivery. Front. Pharmacol. 2020, 10, 1651. [Google Scholar] [CrossRef]
- Fan, C.H.; Lin, Y.T.; Ho, Y.J.; Yeh, C.K. Spatial-Temporal Cellular Bioeffects from Acoustic Droplet Vaporization. Theranostics 2018, 8, 5731–5743. [Google Scholar] [CrossRef] [PubMed]
- Maciulevičius, M.; Tamošiūnas, M.; Venslauskas, M.S.; Šatkauskas, S. The relation of Bleomycin Delivery Efficiency to Microbubble Sonodestruction and Cavitation Spectral Characteristics. Sci. Rep. 2020, 10, 7743. [Google Scholar] [CrossRef]
- Morgan, K.E. Experimental and theoretical evaluation of microbubble behavior: Effect of transmitted phase and bubble size. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2000, 47, 1494–1509. [Google Scholar] [CrossRef] [PubMed]
- Delalande, A.; Kotopoulis, S.; Postema, M.; Midoux, P.; Pichon, C. Sonoporation: Mechanistic insights and ongoing challenges for gene transfer. Gene 2013, 525, 191–199. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, T.; Skachkov, I.; Beekers, I.; Lattwein, K.R.; Voorneveld, J.D.; Kokhuis, T.J.A.; Bera, D.; Luan, Y.; van der Steen, A.F.W.; de Jong, N.; et al. Viability of endothelial cells after ultrasound-mediated sonoporation: Influence of targeting, oscillation, and displacement of microbubbles. J. Control Release 2016, 238, 197–211. [Google Scholar] [CrossRef]
- Qin, P.; Xu, L.; Han, T.; Du, L.; Yu, A.C.H. Effect of non-acoustic parameters on heterogeneous sonoporation mediated by single-pulse ultrasound and microbubbles. Ultrason. Sonochem. 2016, 31, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Helfield, B.; Chen, X.; Watkins, S.C.; Villanueva, F.S. Biophysical insight into mechanisms of sonoporation. Proc. Natl. Acad. Sci. USA 2016, 113, 9983–9988. [Google Scholar] [CrossRef] [Green Version]
- Jelenc, J. Low-Frequency Sonoporation in vitro: Experimental System Evaluation. J. Mech. Eng. 2012, 58, 319–326. [Google Scholar] [CrossRef]
- Bouakaz, A.; Lamanauskas, N.; Novell, A.; Venslauskas, M. Bleomycin delivery into cancer cells in vitro with ultrasound and SonoVue® or BR14® microbubbles. J. Drug Target. 2013, 2330, 407–414. [Google Scholar]
- Dini, L.; Abbro, L. Bioeffects of moderate-intensity static magnetic fields on cell cultures. Micron 2005, 36, 195–217. [Google Scholar] [CrossRef]
- Delalande, A.D.; Leduc, C.; Midoux, P.; Postema, M.; Pichon, C. Efficient Gene Delivery by Sonoporation Is Associated with Microbubble Entry into Cells and the Clathrin-Dependent Endocytosis Pathway. Ultrasound Med. Biol. 2015, 41, 1913–1926. [Google Scholar] [CrossRef]
- Deng, Q.; Chen, J.; Zhou, Q.; Hu, B.O.; Chen, Q.; Huang, J.I.A.; Guo, R. Ultrasound microbubbles combined with the NF κ B binding motif increase transfection efficiency by enhancing the cytoplasmic and nuclear import of plasmid DNA. Mol. Med. Rep. 2013, 8, 1439–1445. [Google Scholar] [CrossRef] [Green Version]
- Qin, D.; Zhang, L.; Chang, N.; Ni, P.; Zong, Y.; Bouakaz, A.; Wan, M.; Feng, Y. In situ observation of single cell response to acoustic droplet vaporization: Membrane deformation, permeabilization and blebbing. Ultrason. Sonochem. 2018, 47, 141–150. [Google Scholar] [CrossRef]
- Keller, S.; Bruce, M.; Averkiou, M.A. Ultrasound Imaging of Microbubble Activity during Sonoporation Pulse Sequences. Ultrasound Med. Biol. 2019, 45, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Beekers, I.; van Rooij, T.; van der Steen, A.F.W.; de Jong, N.; Verweij, M.D.; Kooiman, K. Acoustic Characterization of the CLINIcell for Ultrasound Contrast Agent Studies. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 244–246. [Google Scholar] [CrossRef] [Green Version]
- Angelova, M.I.; Dimitrov, D.S. Liposome electroformation. Faraday Discuss. Chem. Soc. 1986, 81, 303–311. [Google Scholar] [CrossRef]
- Breton, M.; Amirkavei, M.; Mir, L.M. Optimization of the Electroformation of Giant Unilamellar Vesicles (GUVs) with Unsaturated Phospholipids. J. Membr. Biol. 2015, 248, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Tinevez, J.Y.; Perry, N.; Schindelin, J.; Hoopes, G.M.; Reynolds, G.D.; Laplantine, E.; Bednarek, S.Y.; Shorte, S.L.; Eliceiri, K.W. TrackMate: An open and extensible platform for single-particle tracking. Methods 2017, 115, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Sassaroli, E.; O’Neill, B.E.; Li, K.C. Biphasic models of soft tissues for ultrasound applications. In Proceedings of the Meetings on Acousticst, Miami, FL, USA, 10–14 November 2008; Acoustical Society of America through the American Institute of Physics: Melville, NY, USA, 2009; Volume 5. [Google Scholar] [CrossRef] [Green Version]
- Kotopoulis, S.; Delalande, A.; Pichon, C. Real-time sonoporation through HeLa cells. In Nonlinear Acoustics State-of-the-Art and Perspectives: 19th International Symposium on Nonlinear Acoustics, Proceedings of the AIP Conference Proceedings, Tokyo, Japan, 21–24 May 2012; American Institute of Physics: Melville, NY, USA, 2012; Volume 1474, pp. 271–274. [Google Scholar]
- Escoffre, J.; Novell, A.; Serrière, S.; Lecomte, T.; Bouakaz, A. Irinotecan delivery by microbubble-assisted ultrasound: In vitro validation and a pilot preclinical study. Mol. Pharm. 2013, 1, 2667–2675. [Google Scholar] [CrossRef]
- Doinikov, A.A. Bjerknes forces and translational bubble dynamics. In Bubble and Particle Dynamics in Acoustic Fields: Modern Trends and Applications; Research Signpost: Thiruvananthapuram, India, 2005; pp. 2–49. [Google Scholar]
- Maciulevičius, M.; Tamošiūnas, M.; Jakštys, B.; Jurkonis, R.; Venslauskas, M.; Šatkauskas, S. Investigation of microbubble cavitation-induced calcein release from cells in vitro. Ultrasound Med. Biol. 2016, 42, 2990–3000. [Google Scholar] [CrossRef]
- Dalmay, C.; De Menorval, M.A.; Français, O.; Mir, L.M.; Le Pioufle, B. A microfluidic device with removable packaging for the real time visualisation of intracellular effects of nanosecond electrical pulses on adherent cells. Lab Chip 2012, 12, 4709–4715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postema, M.; van Wamel, A.; Cate, F.J.; De Jong, N. High-speed photography during ultrasound illustrates potential therapeutic applications of microbubbles High-speed photography during ultrasound illustrates potential therapeutic applications of microbubbles. Med. Phys. 2015, 32, 3707–3711. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.V. Sensitivity of photoacoustic microscopy. Photoacoustics 2014, 2, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Ranasinghesagara, J.C.; Jian, Y.; Chen, X.; Mathewson, K.; Zemp, R.J. Photoacoustic technique for assessing optical scattering properties of turbid media. J. Biomed. Opt. 2009, 14, 040504. [Google Scholar] [CrossRef] [Green Version]
- Shutilov, V.A. Fundamental Physics of Ultrasound, 1st ed.; Gordon and Breach Science Publishers: Amsterdam, The Netherlands, 1988; pp. 172–259. [Google Scholar]
- Kinsler, L.E.; Frey, A.R.; Coppens, A.B.; Sanders, J.V. Fundamentals of Acoustics, 4th ed.; Wiley: Hoboken, NJ, USA, 2000; pp. 149–167. [Google Scholar]


| Medium # in a Setup | (1) | (2) | (3) | (4) | (5) |
|---|---|---|---|---|---|
| Water-bath setup | Cell media | Water bath support | Air | ||
| Clinicell™ setup | water | Clinicell™ material (flexible) | Cell media uncontrolled thickness | Clinicell™ material (flexible) | Fluid immersion |
| Proposed setup | water | Glass/PS (rigid) | Cell media Controlled thickness | Glass/PS (rigid) | Air |
| Focal Spot | Isosurfaces (mm²) | |||||
|---|---|---|---|---|---|---|
| Transducer | Focal Distance (mm) | Length (mm) | Width (mm) | 5% | 15% | 25% |
| Flat | 60 | 60 | 9 | 2.1 | 8.6 | 63.6 |
| Focused | 60 | 30 | 1.7 | 0.4 | 1.3 | 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gailliègue, F.N.; Tamošiūnas, M.; André, F.M.; Mir, L.M. A Setup for Microscopic Studies of Ultrasounds Effects on Microliters Scale Samples: Analytical, Numerical and Experimental Characterization. Pharmaceutics 2021, 13, 847. https://doi.org/10.3390/pharmaceutics13060847
Gailliègue FN, Tamošiūnas M, André FM, Mir LM. A Setup for Microscopic Studies of Ultrasounds Effects on Microliters Scale Samples: Analytical, Numerical and Experimental Characterization. Pharmaceutics. 2021; 13(6):847. https://doi.org/10.3390/pharmaceutics13060847
Chicago/Turabian StyleGailliègue, Florian N., Mindaugas Tamošiūnas, Franck M. André, and Lluis M. Mir. 2021. "A Setup for Microscopic Studies of Ultrasounds Effects on Microliters Scale Samples: Analytical, Numerical and Experimental Characterization" Pharmaceutics 13, no. 6: 847. https://doi.org/10.3390/pharmaceutics13060847





