Arterial Hypertension: Individual Therapeutic Approaches—From DNA Sequencing to Gender Differentiation and New Therapeutic Targets
Abstract
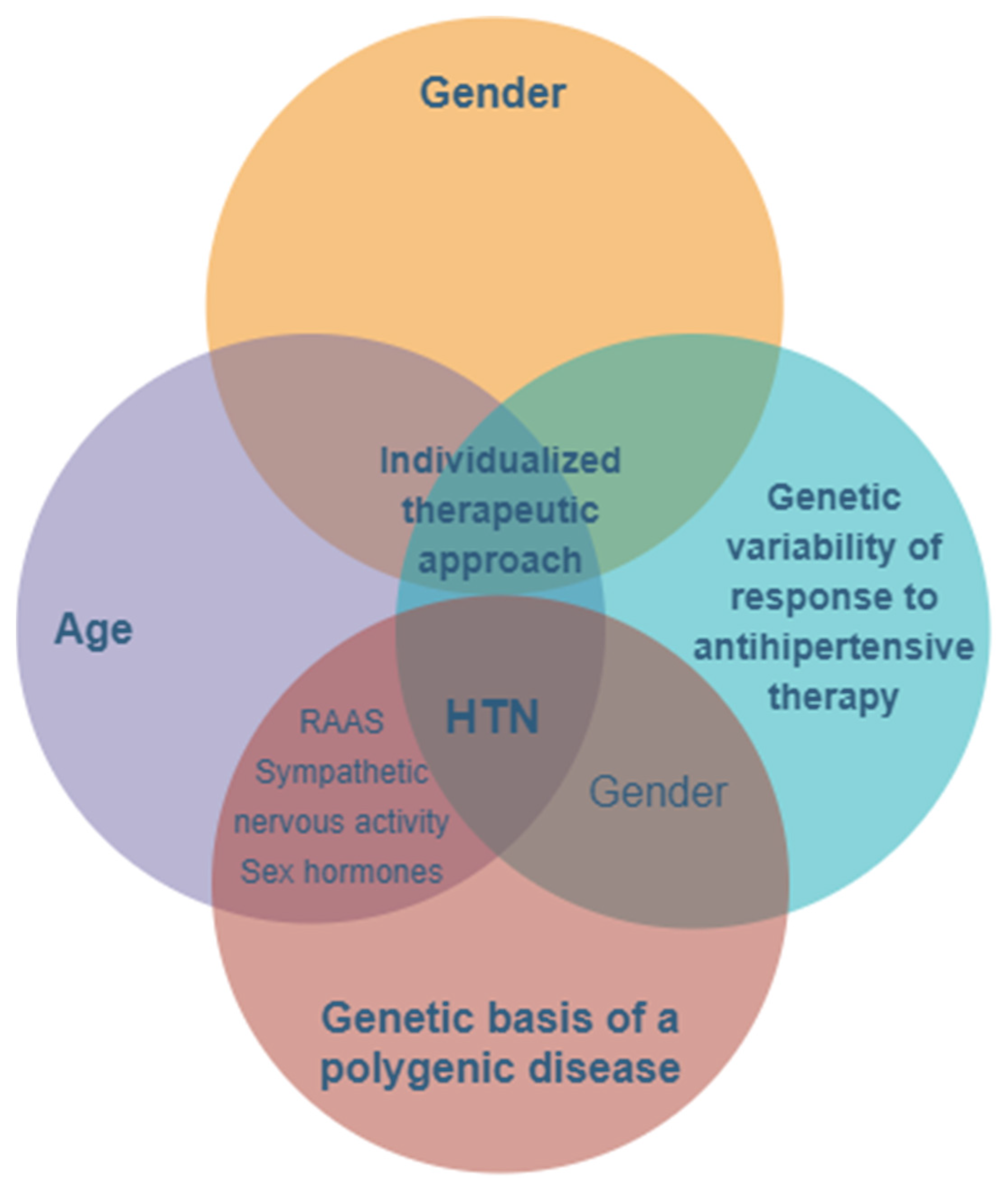
:1. Introduction
2. The Importance of Genetics in Arterial Hypertension
3. Gender Differentiation of Antihypertensive Treatment
4. The Past, the Present, and the Future Analysis
- Confirmation of the crucial role of the kidneys in the regulation of blood pressure (BP) and the pathogenesis of hypertension by the intervention of AT1A receptors for angiotensinogen in the proximal convoluted tubules in the regulation of “pressure natriuresis”, as well as by stimulating intrarenal sympathetic nerve activity associated with increased sodium reabsorption and hypertension;
- Deciphering the molecular mechanisms involved in peripheral vascular resistance, especially the signaling pathways activated by the receptors of hormonal mediators coupled with G proteins involved in the regulation of vascular tone and BP;
- Discovering the role of interstitial tissue in the skin as a “dynamic reservoir” of sodium, which buffers the impact of sodium accumulation on intravascular volume and BP;
- Identifying the important role of inflammation and the immune system in the development of hypertension, which also becomes an autoimmune disease (endothelial immunogens cause the infiltration and activation of T lymphocytes in the vascular adventitia, followed by the release of cytokines that increase BP).
- Platt (1947) measured BP in normotensive and hypertensive people as well as in their relatives, finding a bimodal distribution of BP (patients being a distinct subpopulation compared to normotensive), which led him to state that hypertension is a Mendelian monogenic disease caused by a single mutation
- Pickerring (1959) showed that BP has a quantitative, complex character with a continuous distribution (Gaussian) and polygenic determinism; hypertension (defined by systolic BP values ≥ 140 mm Hg) is only the upper portion (+2.5 standard deviations) of the continuous BP distribution curve. Over time, the Pickering hypothesis has been confirmed by extensive epidemiological studies, and it is now considered that the vast majority of hypertension cases have a multifactorial origin, being produced by the intervention of several susceptibility genes whose effects are modulated by interactions between different genes (epistaxis) as well as between genes and the environment. However, the Platt hypothesis cannot be completely ruled out, as there are rare forms of hypertension and hypotension that are caused by rare monogenic mutations with high penetrance and significant effects. BP certainly shows a phenotypic and genotypic heterogeneity.
5. Identification of Susceptibility Genes Involved in Multifactorial Hypertension
6. Susceptible Genes and their Function
7. Discussion
8. Further Directions—Genetic Testing, Advantages, and Limitations in an HTN Setting
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banegas, J.R.; Lopez-Garcia, E.; Dallongeville, J.; Guallar, E.; Halcox, J.P.; Borghi, C.; Masso-Gonzalez, E.L.; Jimenez, F.J.; Perk, J.; Steg, P.G.; et al. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: The EURIKA study. Eur. Heart J. 2011, 32, 2143–2152. [Google Scholar] [CrossRef]
- Chow, C.K.; Teo, K.K.; Rangarajan, S.; Islam, S.; Gupta, R.; Avezum, A.; Bahonar, A.; Chifamba, J.; Dagenais, G.; Diaz, R.; et al. Prevalence, Awareness, Treatment, and Control of Hypertension in Rural and Urban Communities in High-, Middle-, and Low-Income Countries. JAMA 2013, 310, 959–968. [Google Scholar] [CrossRef] [Green Version]
- Falaschetti, E.; Mindell, J.; Knott, C.; Poulter, N. Hypertension management in England: A serial cross-sectional study from 1994 to 2011. Lancet 2014, 383, 1912–1919. [Google Scholar] [CrossRef]
- Tocci, G.; Rosei, E.A.; Ambrosioni, E.; Borghi, C.; Ferri, C.; Ferrucci, A.; Mancia, G.; Morganti, A.; Pontremoli, R.; Trimarco, B.; et al. Blood pressure control in Italy: Analysis of clinical data from 2005–2011 surveys on hypertension. J. Hypertens. 2012, 30, 1065–1074. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Caulfield, M.J.; Dominiczak, A.F. Genetic and Molecular Aspects of Hypertension. Circ. Res. 2015, 116, 937–959. [Google Scholar] [CrossRef] [Green Version]
- Ezzati, M.; Obermeyer, Z.; Tzoulaki, I.; Mayosi, B.M.; Elliott, P.; Leon, D.A. Contributions of risk factors and medical care to cardiovascular mortality trends. Nat. Rev. Cardiol. 2015, 12, 508–530. [Google Scholar] [CrossRef] [Green Version]
- Tzoulaki, I.; Elliott, P.; Kontis, V.; Ezzati, M. Worldwide Exposures to Cardiovascular Risk Factors and Associated Health Effects. Circulation 2016, 133, 2314–2333. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.; Di Gaetano, C.; Cugliari, G.; Matullo, G. Advances in the Genetics of Hypertension: The Effect of Rare Variants. Int. J. Mol. Sci. 2018, 19, 688. [Google Scholar] [CrossRef] [Green Version]
- Levy, D.; Ehret, G.B.; Rice, K.; Verwoert, G.C.; Launer, L.J.; Dehghan, A.; Glazer, N.L.; Morrison, A.C.; Johnson, A.D.; Aspelund, T.; et al. Genome-wide association study of blood pressure and hypertension. Nat. Genet. 2009, 41, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Ehret, G.B.; Munroe, P.B.; Rice, K.M.; Bochud, M.; Johnson, A.D.; Chasman, D.I.; Smith, A.V.; Tobin, M.D.; Verwoert, G.C.; Hwang, S.J.; et al. Genetic variants in novel pathways influence blood pressure andcardiovascular disease risk. Nature 2011, 478, 103–109. [Google Scholar]
- Ehret, G.B.; Ferreira, T.; Chasman, D.I.; Jackson, A.U.; Schmidt, E.M.; Johnson, T.; Thorleifsson, G.; Luan, J.; Donnelly, L.A.; Kanoni, S.; et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat. Genet. 2016, 48, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.J.; Ehret, G.B.; Nandakumar, P.; Ranatunga, D.; Schaefer, C.; Kwok, P.Y.; Iribarren, C.; Chakravarti, A.; Risch, N. Genome-wide association analyses using electronic health records identify new loci influencing blood pressure variation. Nat. Genet. 2017, 49, 54–64. [Google Scholar] [CrossRef] [Green Version]
- The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3000 shared controls. Nature 2007, 447, 661. [Google Scholar] [CrossRef] [Green Version]
- Seidel, E.; Scholl, U.I. Genetic mechanisms of human hypertension and their implications for blood pressure physiology. Physiol. Genom. 2017, 49, 630–652. [Google Scholar] [CrossRef] [Green Version]
- Newton-Cheh, C.; Johnson, T.; Gateva, V.; Tobin, M.D.; Bochud, M.; Coin, L.; Najjar, S.S.; Zhao, J.H.; Heath, S.C.; Eyheramendy, S.; et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat. Genet. 2009, 41, 666–676. [Google Scholar] [CrossRef]
- Wain, L.V.; Verwoert, G.C.; O’Reilly, P.F.; Shi, G.; Johnson, T.; Johnson, A.D.; Bochud, M.; Rice, K.M.; Henneman, P.; Smith, A.V.; et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat. Genet. 2011, 43, 1005–1011. [Google Scholar] [CrossRef]
- Civelek, M.; Lusis, A.J. Systems genetics approaches to understand complex traits. Nat. Rev. Genet. 2014, 15, 34–48. [Google Scholar] [CrossRef] [Green Version]
- Sung, Y.J.; Basson, J.; Cheng, N.; Nguyen, K.-D.H.; Nandakumar, P.; Hunt, S.C.; Arnett, N.K.; Dávila-Román, V.G.; Rao, D.C.; Chakravarti, A. The role of rare variants in systolic blood pressure: Analysis of ExomeChip data in HyperGEN African Americans. Hum. Hered. 2015, 79, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Tran, N.T.; Aslibekyan, S.; Tiwari, H.K.; Zhi, D.; Sung, Y.J.; Hunt, S.C.; Rao, D.C.; Broeckel, U.; Judd, S.E.; Muntner, P.; et al. PCSK9 variation and association with blood pressure in African Americans: Preliminary findings from the HyperGEN and REGARDS studies. Front. Genet. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Yasukochi, Y.; Sakuma, J.; Takeuchi, I.; Kato, K.; Oguri, M.; Fujimaki, T.; Horibe, H.; Yamada, Y. Longitudinal exo-me-wide association study to identify genetic susceptibility loci for hypertension in a Japanese population. Exp. Mol. Med. 2017, 49, e409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlsson, T.; Lindgren, A.; Engstrom, G.; Jern, C.; Melander, O. A stop-codon of the phosphodiesterase 11A gene is asso-ciated with elevated blood pressure and measures of obesity. J. Hypertens. 2016, 34, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Surendran, P.; Drenos, F.; Young, R.; Warren, H.; Cook, J.P.; Manning, A.K.; Grarup, N.; Sim, X.; Barnes, D.R.; Witkow-ska, K.; et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hy-pertension. Nat. Genet. 2016, 48, 1151–1161. [Google Scholar] [CrossRef]
- Liu, C.; Kraja, A.T.; Smith, J.A.; Brody, J.A.; Franceschini, N.; Bis, J.C.; Rice, K.; Morrison, A.C.; Lu, Y.; Weiss, S.; et al. Me-ta-analysis identifies common and rare variants influencing blood pressure and overlapping withmetabolic trait loci. Nat. Genet. 2016, 48, 1162–1170. [Google Scholar] [CrossRef]
- Nandakumar, P.; Lee, D.; Richard, M.A.; Tekola-Ayele, F.; Tayo, B.O.; Ware, E.; Sung, Y.J.; Salako, B.; Ogunniyi, A.; Gu, C.C.; et al. Rare coding variants associated with blood pressure variation in 15 914 individuals of African ancestry. J. Hypertens. 2017, 35, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- He, K.Y.; Wang, H.; Cade, B.E.; Nandakumar, P.; Giri, A.; Ware, E.B.; Haessler, J.; Liang, J.; Smith, J.A.; Franceschini, N.; et al. Rare variants in fox-1 homolog A (RBFOX1) are associated with lower blood pressure. PLoS Genet. 2017, 13, e1006678. [Google Scholar] [CrossRef]
- Kraja, A.T.; Cook, J.P.; Warren, H.R.; Surendran, P.; Liu, C.; Evangelou, E.; Manning, A.K.; Grarup, N.; Drenos, F.; Sim, X.; et al. New Blood Pressure–Associated Loci Identified in Meta-Analyses of 475,000 Individuals. Circ. Cardiovasc. Genet. 2017, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Warren, H.R.; Evangelou, E.; Cabrera, C.P.; Gao, H.; Ren, M.; Mifsud, B.; Ntalla, I.; Surendran, P.; Liu, C.; Cook, J.P.; et al. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardio-vascular risk. Nat. Genet. 2017, 49, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Hwang, M.Y.; Kim, Y.J.; Moon, S.; Han, S.; Kim, B.-J. Evaluation of pleiotropic effects among common genetic loci identified for cardio-metabolic traits in a Korean population. Cardiovasc. Diabetol. 2016, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vynckier, P.; Ferrannini, G.; Rydén, L.; Jankowski, P.; De Backer, T.; Gevaert, S.; De Bacquer, D.; De Smedt, D. Gender gap in risk factor control of coronary patients far from closing: Results from the European Society of Cardiology EUROASPIRE V registry. Eur. J. Prev. Cardiol. 2020, in press. [Google Scholar] [CrossRef]
- De Smedt, D.; De Bacquer, D.; De Sutter, J.; Dallongeville, J.; Gevaert, S.; De Backer, G.; Bruthans, J.; Kotseva, K.; Reiner, Ž.; Tokgözoğlu, L.; et al. The gender gap in risk factor control: Effects of age and education on the control of cardiovascular risk factors in male and female coronary patients. The EUROASPIRE IV study by the European Society of Cardiology. Int. J. Cardiol. 2016, 209, 284–290. [Google Scholar] [CrossRef]
- Hambraeus, K.; Tydén, P.; Lindahl, B. Time trends and gender differences in prevention guideline adherence and outcome after myocardial infarction: Data from the SWEDEHEART registry. Eur. J. Prev. Cardiol. 2016, 23, 340–348. [Google Scholar] [CrossRef]
- Zhao, M.; Vaartjes, I.; Graham, I.; Grobbee, D.; Spiering, W.; Klipstein-Grobusch, K.; Woodward, M.; Peters, S.A. Sex differences in risk factor management of coronary heart disease across three regions. Hear. 2017, 103, 1587–1594. [Google Scholar] [CrossRef] [Green Version]
- Leifheit-Limson, E.C.; D’Onofrio, G.; Daneshvar, M.; Geda, M.; Bueno, H.; Spertus, J.A.; Krumholz, H.M.; Lichtman, J.H. Sex Differences in Cardiac Risk Factors, Perceived Risk, and Health Care Provider Discussion of Risk and Risk Modification Among Young Patients With Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2015, 66, 1949–1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redfors, B.; Angerås, O.; Råmunddal, T.; Petursson, P.; Haraldsson, I.; Dworeck, C.; Odenstedt, J.; Ioaness, D.; Ravn-Fischer, A.; Wellin, P.; et al. Trends in Gender Differences in Cardiac Care and Outcome After Acute Myocardial Infarction in Western Sweden: A Report From the Swedish Web System for Enhancement of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). J. Am. Heart. Assoc. 2015, 4, e001995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrell, J.; Zeymer, U.; Baumgartner, I.; Limbourg, T.; Röther, J.; Bhatt, D.L.; Steg, P.G. Differences in management and outcomes between male and female patients with atherothrombotic disease: Results from the REACH Registry in Europe. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 270–277. [Google Scholar] [CrossRef]
- Ames, M.K.; Atkins, C.E.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. J. Veter. Intern. Med. 2019, 33, 363–382. [Google Scholar] [CrossRef] [Green Version]
- Riet, L.T.; Van Esch, J.H.M.; Roks, A.J.M.; Meiracker, A.H.V.D.; Danser, A.J. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Reckelhoff, J.F. Gender differences in hypertension. Curr. Opin. Nephrol. Hypertens. 2018, 27, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Kim, H.C.; Kang, D.R. Sex differences in hypertension prevalence and control: Analysis of the 2010-2014 Korea National Health and Nutrition Examination Survey. PLoS ONE 2017, 12, e0178334. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Lu, Y.; Wang, X.; Li, X.; Linderman, G.C.; Wu, C.; Cheng, X.; Mu, L.; Zhang, H.; Liu, J.; et al. Prevalence, awareness, treatment, and control of hypertension in China: Data from 1·7 million adults in a population-based screening study (China PEACE Million Persons Project). Lancet 2017, 390, 2549–2558. [Google Scholar] [CrossRef]
- Zhang, Y.; Moran, A.E. Trends in the Prevalence, Awareness, Treatment, and Control of Hypertension Among Young Adults in the United States, 1999 to 2014. Hypertens. 2017, 70, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Niiranen, T.J.; Rader, F.; Henglin, M.; Kim, A.; Ebinger, J.E.; Claggett, B.; Merz, C.N.B.; Cheng, S. Sex Differences in Blood Pressure Associations with Cardiovascular Outcomes. Circulation 2021, 143, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Coffman, T.M. Under pressure: The search for the essential mechanisms of hypertension. Nat. Med. 2011, 17, 1402–1409. [Google Scholar] [CrossRef]
- Hirschhorn, J.N.; Gajdos, Z.K.Z. Genome-Wide Association Studies: Results from the First Few Years and Potential Implications for Clinical Medicine. Annu. Rev. Med. 2011, 62, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Padmanabhan, S.; Newton-Cheh, C.; Dominiczak, A.F. Genetic basis of blood pressure and hypertension. Trends Genet. 2012, 28, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Dodoo, S.N.; Benjamin, I.J. Genomic Approaches to Hypertension. Cardiol. Clin. 2017, 35, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, A.K.; Pandey, P.; Chandra, S.; Singh, K.A.; Gambhir, I.S. Molecular genetics of essential hypertension. Clin. Exp. Hypertens. 2016, 38, 268–277. [Google Scholar] [CrossRef]
- Wise, I.A.; Charchar, F.J. Epigenetic Modifications in Essential Hypertension. Int. J. Mol. Sci. 2016, 17, 451. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.M.; Musini, V.M.; Gill, R. First-line drugs for hypertension. Cochrane Database Syst. Rev. 2018, 4, CD001841. [Google Scholar] [CrossRef] [Green Version]
- Crişan, S.; Petriş, A.O.; Petrescu, L.; Luca, C.T. Current Perspectives in Facilitated Angioplasty. Am. J. Ther. 2019, 26, e208–e212. [Google Scholar] [CrossRef]
- Vacarescu, C.; Cozma, D.; Petrescu, L.; Dragan, S.; Mornos, C.; Crisan, S.; Feier, H.; Lazar, M.-A.; Cozlac, R.A.; Luca, C.T. Exercise test is essential in LV-only fusion CRT pacing without right ventricle lead. Clin. Interv. Aging 2019, 14, 969–975. [Google Scholar] [CrossRef] [Green Version]
- Mornoş, C.; Muntean, D.; Mornoş, A.; Crişan, S.; Petrescu, L.; Ionac, A.; Sosdean, R.; Cozma, D. Risk stratification in patients with heart failure: The value of considering both global longitudinal left ventricular strain and mechanical dispersion. Can. J. Physiol. Pharmacol. 2017, 95, 1360–1368. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.F.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-J.; Ma, Z.; Wang, J.; Chen, L.-X.; Zhong, J.-C. Gender Differences in Hypertension. J. Cardiovasc. Transl. Res. 2019, 13, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Williams, G.; Mamun, A. Gender differences in hypertension awareness, antihypertensive use and blood pressure control in Bangladeshi adults: Findings from a national cross-sectional survey. J. Heal. Popul. Nutr. 2017, 36, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kajiwara, A.; Saruwatari, J.; Kita, A.; Oniki, K.; Yamamura, M.; Murase, M.; Koda, H.; Hirota, S.; Ishizuka, T.; Nakagawa, K. Younger Females Are at Greater Risk of Vasodilation-Related Adverse Symptoms Caused by Dihydropyridine Calcium Channel Blockers: Results of a Study of 11,918 Japanese Patients. Clin. Drug Investig. 2014, 34, 431–435. [Google Scholar] [CrossRef]
- Shah, T.; Palaskas, N.; Ahmed, A. An Update on Gender Disparities in Coronary Heart Disease Care. Curr. Atheroscler. Rep. 2016, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.H.E.M.; van der Schouw, Y.T.; Regitz-Zagrosek, V.; Swahn, E.; Appelman, Y.E.; Pasterkamp, G.; ten Cate, H.; Nilsson, P.M.; Huisman, M.V.; Stam, H.C.G.; et al. Red alert for women’s heart: The urgent need for more research and knowledge on cardiovascular disease in women: Proceedings of the Workshop held in Brussels on Gender Differences in Cardiovascular disease, 29 September 2010. Eur. Heart J. 2011, 32, 1362–1368. [Google Scholar] [CrossRef] [Green Version]
- Melloni, C.; Berger, J.S.; Wang, T.Y.; Gunes, F.; Stebbins, A.; Pieper, K.S.; Dolor, R.J.; Douglas, P.S.; Mark, D.B.; Newby, L.K. Representation of Women in Randomized Clinical Trials of Cardiovascular Disease Prevention. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Stramba-Badiale, M. Women and research on cardiovascular diseases in Europe: A report from the European Heart Health Strategy (EuroHeart) project. Eur. Hear. J. 2010, 31, 1677–1681. [Google Scholar] [CrossRef]
- Wenger, N.K.; Ferdinand, K.C.; Merz, C.N.B.; Walsh, M.N.; Gulati, M.; Pepine, C.J. Women, Hypertension, and the Systolic Blood Pressure Intervention Trial. Am. J. Med. 2016, 129, 1030–1036. [Google Scholar] [CrossRef]
- Ambrosius, W.T.; Sink, K.M.; Foy, C.G.; Berlowitz, D.R.; Cheung, A.K.; Cushman, W.C.; Fine, L.J.; Goff, D.C.; Johnson, K.C.; Killeen, A.A.; et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin. Trials 2014, 11, 532–546. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.T., Jr.; Cushman, W.; Oparil, S.; Cheung, A.K.; Rocco, M.; Reboussin, D.M.; Fine, L.; Kimmel, P.; Ryan, L.; Launer, L.; et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Con-trol. N. Engl. J. Med. 2015, 373, 2103–2116. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y. Tracking of Blood Pressure From Childhood to Adulthood: A systematic review and meta-regression analysis. Circulation 2008, 117, 3171–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelinka, T.; Vicha, A.; Musil, Z.; Widimsky, J. RESULTS OF GENETIC TESTING IN PATIENTS WITH PHEOCHROMOCYTOMA/PARAGANGLIOMA. J. Hypertens. 2019, 37, e40. [Google Scholar] [CrossRef]
- Rossi, G.P.; Ceolotto, G.; Caroccia, B.; Lenzini, L. Genetic screening in arterial hypertension. Nat. Rev. Endocrinol. 2017, 13, 289–298. [Google Scholar] [CrossRef]
- Phillips, K.A.; Deverka, P.A.; Hooker, G.W.; Douglas, M.P. Genetic Test Availability And Spending: Where Are We Now? Where Are We Going? Health Aff. 2018, 37, 710–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Factors Influencing Genetic Variability of Response to Antihypertensive Therapy | |
|---|---|
| 1 | Genetic variations of metabolizing enzymes |
| 2 | Genetic variability of sodium sensitivity |
| 3 | Genetic variability of proteins regulating renal tubules ion transport |
| BP Implication | Genes Involved | References |
|---|---|---|
| Systolic BP | ATP2B1, CYP17A1, PLEKHA7, SH2B3 | Levy et al., 2009 [9] |
| Diastolic BP | ATP2B1, CACNB2, CSK-ULK3, SH2B3, TBX3-TBX5, ULK4 | Levy et al., 2009 [9] |
| Systolic or diastolic BP | CYP17A1, CYP1A2, FGF5, SH2B3, MTHFR, c10orf107, PLCD3 | Newton-Cheh et al., 2009 [15] |
| Pulse pressure | CHIC2/PDGFRA, PIK3CG, NOV, ADAMTS8 | Wain et al., 2011 [16] |
| Mean arterial pressure | CHIC2/PDGFRA, PIK3CG, NOV, ADAMTS8 | Wain et al., 2011 [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luca, C.-T.; Crisan, S.; Cozma, D.; Negru, A.; Lazar, M.-A.; Vacarescu, C.; Trofenciuc, M.; Rachieru, C.; Craciun, L.M.; Gaita, D.; et al. Arterial Hypertension: Individual Therapeutic Approaches—From DNA Sequencing to Gender Differentiation and New Therapeutic Targets. Pharmaceutics 2021, 13, 856. https://doi.org/10.3390/pharmaceutics13060856
Luca C-T, Crisan S, Cozma D, Negru A, Lazar M-A, Vacarescu C, Trofenciuc M, Rachieru C, Craciun LM, Gaita D, et al. Arterial Hypertension: Individual Therapeutic Approaches—From DNA Sequencing to Gender Differentiation and New Therapeutic Targets. Pharmaceutics. 2021; 13(6):856. https://doi.org/10.3390/pharmaceutics13060856
Chicago/Turabian StyleLuca, Constantin-Tudor, Simina Crisan, Dragos Cozma, Alina Negru, Mihai-Andrei Lazar, Cristina Vacarescu, Mihai Trofenciuc, Ciprian Rachieru, Laura Maria Craciun, Dan Gaita, and et al. 2021. "Arterial Hypertension: Individual Therapeutic Approaches—From DNA Sequencing to Gender Differentiation and New Therapeutic Targets" Pharmaceutics 13, no. 6: 856. https://doi.org/10.3390/pharmaceutics13060856
APA StyleLuca, C.-T., Crisan, S., Cozma, D., Negru, A., Lazar, M.-A., Vacarescu, C., Trofenciuc, M., Rachieru, C., Craciun, L. M., Gaita, D., Petrescu, L., Mischie, A., & Iurciuc, S. (2021). Arterial Hypertension: Individual Therapeutic Approaches—From DNA Sequencing to Gender Differentiation and New Therapeutic Targets. Pharmaceutics, 13(6), 856. https://doi.org/10.3390/pharmaceutics13060856







