Cell Replacement Therapy for Retinal and Optic Nerve Diseases: Cell Sources, Clinical Trials and Challenges
Abstract
:1. Introduction
2. Search Strategy and Selection Criteria
3. Cell Sources
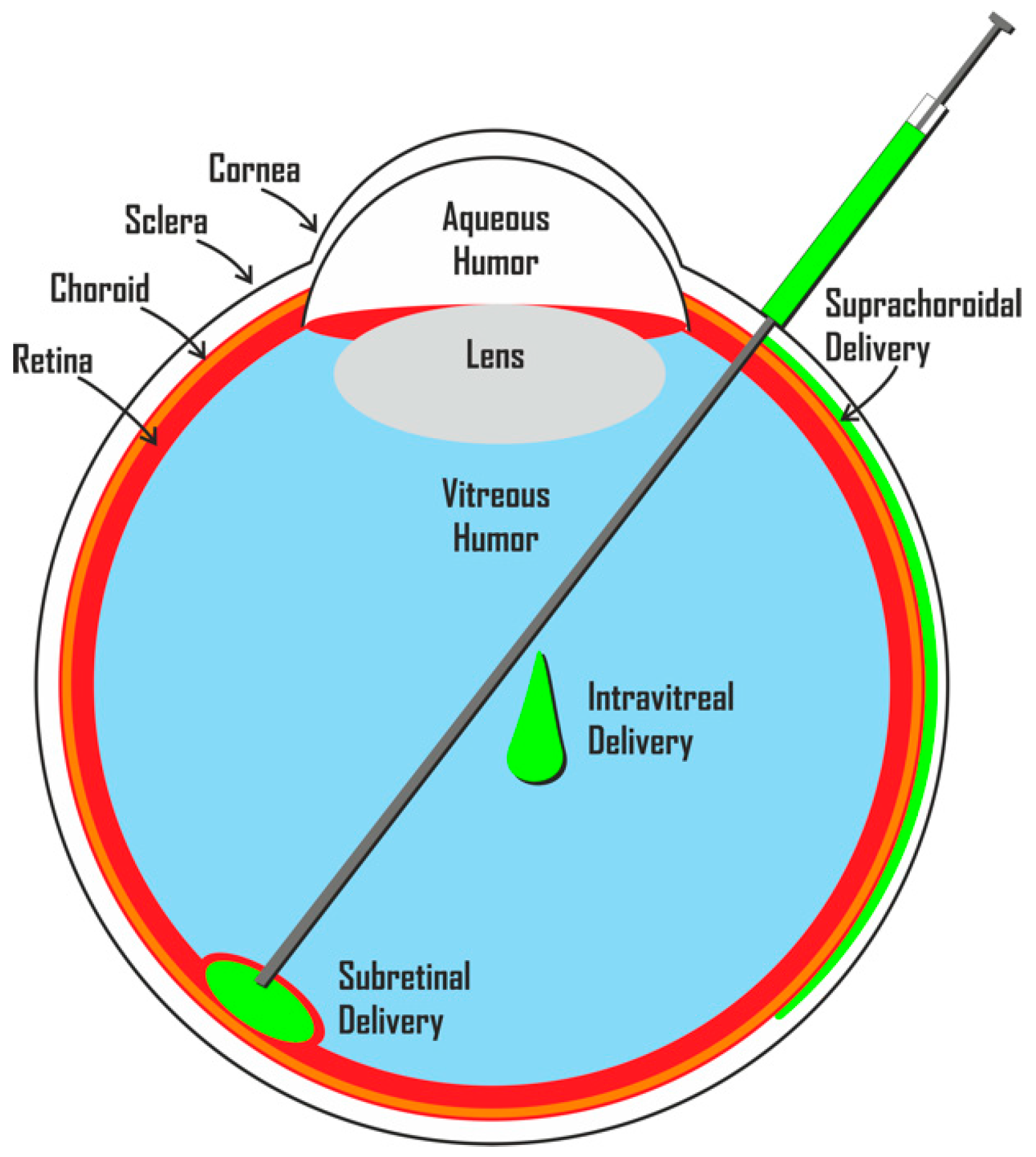
4. Direct Cell Replacement Therapy for Retinal and Optic Nerve Diseases
4.1. RPE Replacement
4.2. Photoreceptor Replacement
4.3. Ganglion Cell Replacement and Cell Therapy for Optic Nerve Diseases (ONDs)
4.4. Cell Therapy for Retinal Vascular Diseases
5. Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Jong, P.T. Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Di Iorio, E.; Barbaro, V.; Ponzin, D.; Sorrentino, F.S.; Parmeggiani, F. Retinitis pigmentosa: Genes and disease mechanisms. Curr. Genom. 2011, 12, 238–249. [Google Scholar] [CrossRef]
- Osborne, N.N.; Casson, R.J.; Wood, J.P.; Chidlow, G.; Graham, M.; Melena, J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef] [PubMed]
- Barber, A. A new view of diabetic retinopathy: A neurodegenerative disease of the eye. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 283–290. [Google Scholar] [CrossRef]
- Zarbin, M. Cell-Based Therapy for Retinal Disease: The New Frontier. Methods Mol. Biol. 2019, 1834, 367–381. [Google Scholar] [CrossRef]
- Labrador-Velandia, S.; Alonso-Alonso, M.L.; Alvarez-Sanchez, S.; González-Zamora, J.; Carretero-Barrio, I.; Pastor, J.C.; Fernandez-Bueno, I.; Srivastava, G.K. Mesenchymal stem cell therapy in retinal and optic nerve diseases: An update of clinical trials. World J. Stem Cells 2016, 8, 376–383. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Kwok, S.S.; Chan, Y.K.; Lai, J.S.; Pan, W.; Nie, L.; Shih, K.C. Therapeutic Strategies for Attenuation of Retinal Ganglion Cell Injury in Optic Neuropathies: Concepts in Translational Research and Therapeutic Implications. BioMed Res. Int. 2019, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- DeBusk, A.; Moster, M.L. Gene therapy in optic nerve disease. Curr. Opin. Ophthalmol. 2018, 29, 234–238. [Google Scholar] [CrossRef]
- Moore, D.L.; Goldberg, J.L. Four steps to optic nerve regeneration. J. Neuroophthalmol. 2010, 30, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Laha, B.; Stafford, B.K.; Huberman, A.D. Regenerating optic pathways from the eye to the brain. Science 2017, 356, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Chun, B.Y.; Cestari, D.M. Advances in experimental optic nerve regeneration. Curr. Opin. Ophthalmol. 2017, 28, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Cenni, M.C.; Bonfanti, L.; Martinou, J.C.; Ratto, G.M.; Strettoi, E.; Maffei, L. Long-term survival of retinal ganglion cells following optic nerve section in adult bcl-2 transgenic mice. Eur. J. Neurosci. 1996, 8, 1735–1745. [Google Scholar] [CrossRef]
- Bonfanti, L.; Strettoi, E.; Chierzi, S.; Cenni, M.C.; Liu, X.H.; Martinou, J.-C.; Maffei, L.; Rabacchi, S.A. Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. J. Neurosci. 1996, 16, 4186–4194. [Google Scholar] [CrossRef]
- Maes, M.E.; Schlamp, C.L.; Nickells, R.W. BAX to basics: How the BCL2 gene family controls the death of retinal ganglion cells. Prog. Retin Eye Res. 2017, 57, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Puertas-Neyra, K.; Usategui-Martín, R.; Coco, R.M.; Fernandez-Bueno, I. Intravitreal stem cell paracrine properties as a potential neuroprotective therapy for retinal photoreceptor neurodegenerative diseases. Neural Regen. Res. 2020, 15, 1631–1638. [Google Scholar] [CrossRef]
- Shen, Y. Stem cell therapies for retinal diseases: From bench to bedside. J. Mol. Med. 2020, 98, 1347–1368. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S. Future vision 2020 and beyond. 5 critical trends in eye research. Asia Pac. J. Ophthalmol. 2020, 9, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Kannabiran, C.; Mariappan, I. Therapeutic avenues for hereditary forms of retinal blindness. J. Genet. 2018, 97, 341–352. [Google Scholar] [CrossRef]
- Salero, E.; Blenkinsop, T.A.; Corneo, B.; Harris, A.; Rabin, D.; Stern, J.H.; Temple, S. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell 2012, 10, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tang, Z.; Gu, P. Stem/progenitor cell-based transplantation for retinal degeneration: A review of clinical trials. Cell Death Dis. 2020, 11, 793. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, Y.; Wang, Y.; Zhang, D.; Shen, B.; Luo, M.; Gu, P. Progress of stem/progenitor cell-based therapy for retinal degeneration. J. Transl. Med. 2017, 15, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, A.; Deniis, J. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Chamberlian, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megaw, R.; Dhillon, B. Stem cell therapies in the management of diabetic retinopathy. Curr. Diab. Rep. 2014, 14, 498. [Google Scholar] [CrossRef]
- Alvarez-Palomo, A.B.; McLenachan, S.; Chen, F.K.; Da Cruz, L.; Dilley, R.J.; Requena, J.; Lucas, M.; Lucas, A.; Drukker, M.; Edel, M.J. Prospects for clinical use of IPCS. Fibrogenesis Tissue Repair 2015, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Mandai, M.; Kurimoto, Y.; Takahashi, M. Comment: Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 2017, 377, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Ando, S.; Takata, N.; Kawada, M.; Muguruma, K.; Sekiguchi, K.; Saito, K.; Yonemura, S.; Eiraku, M.; Sasai, K. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 2012, 10, 771–785. [Google Scholar] [CrossRef] [Green Version]
- Reichman, S.; Slembrouck, A.; Gagliardi, G.; Chaffiol, A.; Terray, A.; Nanteau, C.; Potey, A.; Belle, M.; Rabesandratana, O.; Duebel, J.; et al. Generation of Storable Retinal Organoids and Retinal Pigmented Epithelium from Adherent Human iPS Cells in Xeno-Free and Feeder-Free Conditions. Stem Cells 2017, 35, 1176–1188. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Yokoi, T.; Tamalu, F.; Watanabe, S.-I.; Nishina, S.; Azuma, N. Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Sci. Rep. 2015, 5, 8344. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, J.J.; Yoon, Y.S. Emerging therapy for diabetic neuropathy: Cell therapy targeting vessels and nerves. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 168–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.S. Cell Therapy Applications for Retinal Vascular Diseases: Diabetic Retinopathy and Retinal Vein Occlusion. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFj1–ORSFj10. [Google Scholar] [CrossRef] [Green Version]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef]
- Mackie, A.R.; Losordo, D.W. CD34 positive stem cells in the treatment of heart and vascular disease in human beings. Tex. Heart Inst. J. 2011, 38, 474–485. [Google Scholar] [PubMed]
- Caballero, S.; Sengupta, N.; Afzal, A.; Chang, K.H.; Li Calzi, S.; Guberski, D.L.; Kern, T.S.; Grant, M.B. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes 2007, 56, 960–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenberg-Cohen, N.; Avraham-Lubin, B.C.; Sadikov, T.; Askenasy, N. Effect of co-administration of neuronal growth factors on neuroglial differentiation of bone marrow-derived stem cells in the ischemic retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 502–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, R.J.; O’Neill, C.L.; Humphreys, M.W.; Gardiner, T.A.; Stitt, A.W. Outgrowth endothelial cells: Characterization and their potential for reversing ischemic retinopathy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5906–5913. [Google Scholar] [CrossRef] [Green Version]
- Mendel, T.A.; Clabough, E.B.; Kao, D.S.; Demidova-Rice, T.N.; Durham, J.T.; Zotter, B.C.; Seaman, S.A.; Cronk, S.M.; Rakoczy, E.P.; Katz, A.J.; et al. Pericytes derived from adipose-derived stem cells protect against retinal vasculopathy. PLoS ONE 2013, 8, e65691. [Google Scholar] [CrossRef]
- Prasain, N.; Lii, M.R.; Vemula, S.; Meador, J.L.; Yoshimoto, M.; Ferkowicz, M.J.; Fett, A.; Gupta, M.; Rapp, B.M.; Saadatzadeh, M.R.; et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat. Biotechnol. 2014, 32, 1151–1157. [Google Scholar] [CrossRef]
- Park, T.S.; Bhutto, I.; Zimmerlin, L.; Huo, J.S.; Nagaria, P.; Miller, D.; Rufaihah, A.J.; Talbot, C.; Aguilar, J.; Grebe, R.; et al. Vascular progenitors from cord blood-derived induced pluripotent stem cells possess augmented capacity for regenerating ischemic retinal vasculature. Circulation 2014, 129, 359–372. [Google Scholar] [CrossRef] [Green Version]
- Moisseiev, E.; Anderson, J.D.; Oltjen, S.; Goswami, M.; Zawadzki, R.J.; Nolta, J.A.; Park, S.S. Protective Effect of Intravitreal Administration of Exosomes Derived from Mesenchymal Stem Cells on Retinal Ischemia. Curr. Eye Res. 2017, 42, 1358–1367. [Google Scholar] [CrossRef]
- Safwat, A.; Sabry, D.; Ragiae, A.; Amer, E.; Mahmoud, R.H.; Shamardan, R.M. Adipose mesenchymal stem cells-derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J. Circ. Biomark. 2018, 7, 1849454418807827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, P.; Thomson, H.A.; Luff, A.J.; Lotery, A.J. Retinal pigment epithelium transplantation: Concepts, challenges, and future prospects. Eye 2015, 29, 992–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, S.; Stolba, U.; Krebs, I.; Kellner, L.; Jahn, C.; Feichtinger, H.; Povelka, M.; Frohner, U.; Kruger, A.; Hilgers, R.D.; et al. Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age-related macular degeneration: A pilot study. Am. J. Ophthalmol. 2002, 133, 215–225. [Google Scholar] [CrossRef]
- Li, L.X.; Turner, J.E. Inherited retinal dystrophy in the RCS rat: Prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp. Eye Res. 1988, 47, 911–917. [Google Scholar] [CrossRef]
- Uyama, H.; Mandai, M.; Takahashi, M. Stem Cell-Based Therapies for Retinal Degenerative Diseases: Current Challenges in the Establishment of New Treatment Strategies. Dev. Growth Differ. 2020, 63, 59–71. [Google Scholar] [CrossRef]
- Satarian, L.; Nourinia, R.; Safi, S.; Kanavi, M.R.; Jarughi, N.; Daftarian, N.; Arab, L.; Aghdami, N.; Ahmadieh, H.; Baharvand, H. Intravitreal injection of bone marrow mesenchymal stem cells in patients with advanced retinitis pigmentosa; a safety study. J. Ophthalmic Vis. Res. 2017, 12, 58–64. [Google Scholar] [CrossRef]
- Egypt Al-Azhar University. Safety Study of Use of Autologuous Bone Marrow Derived Stem Cell in Treatment of Age Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/study/NCT02016508 (accessed on 2 May 2021).
- Schwartz, S.D.; Hubschman, J.-P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R.; et al. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012, 379, 713–720. [Google Scholar] [CrossRef]
- Schwartz, S.; Regillo, C.; Lam, B.; Eliott, D.; Rosenfeld, P.; Gregori, N.; Hubschman, J.-P.; Davis, J.; Heilwell, G.; Spirn, M. Human embryonic stem cell-derived retinal pigment epithelium in patients with age related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Mehat, M.S.; Sundaram, V.; Ripamonti, C. Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in Macular Degeneration. Ophthalmology 2018, 125, 1765–1775. [Google Scholar] [CrossRef] [Green Version]
- Song, W.K.; Park, K.M.; Kim, H.J.; Lee, J.H.; Choi, J.; Chong, S.Y.; Shim, S.H.; Del Priore, L.V.; Lanza, R. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: Preliminary results in Asian patients. Stem Cell Rep. 2015, 4, 860–872. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.W.; Wang, L.; Li, S.Y.; Zhao, C.J.; Hao, J.; Li, Q.Y.; Zhao, T.T.; Wu, W.; Wang, Y.; et al. Human embryonic stem cell-derived retinal pigment epithelium transplants as a potential treatment for wet age-related macular degeneration. Cell Discov. 2018, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous induced stem-cell–derived retinal cells for macular degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Production of iPSC Derived RPE Cells for Transplantation in AMD. ClinicalTrials.gov. Identifier: NCT02464956. Last Updated: 8 June 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02464956 (accessed on 2 May 2021).
- Xu, H.; Wang, B.; Ono, M.; Kagita, A.; Fujii, K.; Sasakawa, N.; Ueda, T.; Gee, P.; Nishikawa, M.; Nomura, M.; et al. Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility. Cell Stem Cell 2019, 24, 566–578. [Google Scholar] [CrossRef] [Green Version]
- Maeda, T.; Lee, M.J.; Palczewska, G.; Marsili, S.; Tesar, P.J.; Palczewski, K.; Takahashi, M.; Maeda, A. Retinal pigmented epithelial cells obtained from human induced pluripotent stem cells possess functional visual cycle enzymes in vitro and in vivo. J. Biol. Chem. 2013, 288, 34484–34493. [Google Scholar] [CrossRef] [Green Version]
- Kamao, H.; Mandai, M.; Okamoto, S.; Sakai, N.; Suga, A.; Sugita, S.; Kiryu, J.; Takahashi, M. Characterization of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium Cell Sheets Aiming for Clinical Application. Stem Cell Rep. 2014, 2, 205–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben M’Barek, K.; Habeler, W.; Monville, C. Stem Cell-Based RPE Therapy for Retinal Diseases: Engineering 3D Tissues Amenable for Regenerative Medicine. Adv. Exp. Med. Biol. 2018, 1074, 625–632. [Google Scholar] [CrossRef]
- Kashani, A.H.; Uang, J.; Mert, M.; Rahhal, F.; Chan, C.; Avery, R.L.; Dugel, P.; Chen, S.; Lebkowski, J.; Clegg, D.O.; et al. Surgical Method for Implantation of a Biosynthetic Retinal Pigment Epithelium Monolayer for Geographic Atrophy: Experience from a Phase 1/2a Study. Ophthalmol. Retin. 2020, 4, 264–273. [Google Scholar] [CrossRef]
- da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Wang, Q.; Temple, S. Stem cell therapies for retinal diseases: Recapitulating development to replace degenerated cells. Development 2017, 144, 1368–1381. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Deng, X.; Spee, C.; Sonoda, S.; Hsieh, C.L.; Barron, E.; Pera, M.; Hinton, D.R. Polarized secretion of PEDF from human embryonic stem cell-derived RPE promotes retinal progenitor cell survival. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1573–1585. [Google Scholar] [CrossRef]
- Radtke, N.D.; Aramant, R.B.; Seiler, M.J.; Petry, H.M.; Pidwell, D. Vision change after sheet transplant of fetal retina with retinal pigment epithelium to a patient with retinitis pigmentosa. Arch. Ophthalmol. 2004, 122, 1159–1165. [Google Scholar] [CrossRef] [Green Version]
- Aramant, R.B.; Seiler, M.J. Progress in retinal sheet transplantation. Prog. Retin Eye Res. 2004, 23, 475–494. [Google Scholar] [CrossRef]
- Cordero, A.; West, E.L.; Pearson, R.A.; Duran, Y.; Carvalho, L.S.; Chu, C.J.; Naeem, A.; Blackford, S.J.I.; Georgiadis, A.; Lakowski, J.; et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat. Biotechnol. 2013, 31, 741–747. [Google Scholar] [CrossRef] [Green Version]
- Klassen, H.J.; Ng, T.F.; Kurimoto, Y.; Kirov, I.; Shatos, M.; Coffey, P.; Young, M.J. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4167–4173. [Google Scholar] [CrossRef] [PubMed]
- Lamba, D.A.; Gust, J.; Reh, T.A. Transplantation of Human Embryonic Stem Cell-Derived Photoreceptors Restores Some Visual Function in Crx-Deficient Mice. Cell Stem Cell 2009, 4, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Tucker, B.A.; Park, I.H.; Qi, S.D.; Klassen, H.J.; Jiang, C.; Yao, J.; Redenti, S.; Daley, G.Q.; Young, M.J. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS ONE 2011, 6, e18992. [Google Scholar] [CrossRef] [Green Version]
- Homma, K.; Okamoto, S.; Mandai, M.; Gotoh, N.; Rajasimha, H.K.; Chang, Y.S.; Chen, S.; Li, W.; Cogliati, T.; Swaroop, A.; et al. Developing rods transplanted into the degenerating retina of Crx-knockout mice exhibit neural activity similar to native photoreceptors. Stem Cells 2013, 31, 1149–1159. [Google Scholar] [CrossRef] [Green Version]
- Santos-Ferreira, T.; Völkner, M.; Borsch, O.; Haas, J.; Cimalla, P.; Vasudevan, P.; Carmeliet, P.; Corbeil, D.; Michalakis, S.; Koch, E.; et al. Stem Cell-Derived Photoreceptor Transplants Differentially Integrate Into Mouse Models of Cone-Rod Dystrophy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3509–3520. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Aslam, S.; Duncan, I.; Cramer, A.; Barnard, A.; MacLaren, R. Cell fusion following photoreceptor transplantation into the non-degenerate retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3989. [Google Scholar]
- Ortin-Martinez, A.; Tsai, E.L.; Nickerson, P.E.; Bergeret, M.; Lu, Y.; Smiley, S.; Comanita, L.; Wallace, V.A. A Reinterpretation of Cell Transplantation: GFP Transfer From Donor to Host Photoreceptors. Stem Cells 2017, 35, 932–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, R.C.; Messias, A.; Messias, K.; Arcieri, R.S.; Ruiz, M.A.; Souza, N.F.; Martins, L.C.; Jorge, R. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell -clinical trial). Stem Cell Res. Ther. 2015, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrell, D.; Comander, J. Current Stem-Cell Approaches for the treatment of inherited retinal degenerations. Semin. Ophthalmol. 2019, 34, 287–292. [Google Scholar] [CrossRef]
- Zheng, A.; Li, Y.; Tsang, S.H. Personalized therapeutic strategies for patients with retinitis pigmentosa. Expert Opin. Biol. Ther. 2015, 15, 391–402. [Google Scholar] [CrossRef] [Green Version]
- Cai, B.; Sun, S.; Li, Z.; Zhang, X.; Ke, Y.; Yang, J.; Li, X. Application of CRISPR/Cas9 technologies combined with iPSCs in the study and treatment of retinal degenerative diseases. Hum. Genet. 2018, 137, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Burnight, E.R.; Wiley, L.A.; Mullins, R.F.; Stone, E.M.; Tucker, B.A. Gene therapy using stem cells. Cold Spring Harb. Perspect. Med. 2014, 5, a017434. [Google Scholar] [CrossRef] [Green Version]
- Chuang, K.; Fields, M.A.; Del Priore, L.V. Potential of Gene Editing and Induced Pluripotent Stem Cells (iPSCs) in Treatment of Retinal Diseases. Yale J. Biol. Med. 2017, 90, 635–642. [Google Scholar]
- Liao, H.K.; Hatanaka, F.; Araoka, T.; Reddy, P.; Wu, M.Z.; Sui, Y.; Yamauchi, T.; Sakurai, M.; O’Keefe, D.D.; Núñez-Delicado, E.; et al. In Vivo Target Gene Activation via CRISPR/Cas9-Mediated Trans-epigenetic Modulation. Cell 2017, 171, 1495–1507. [Google Scholar] [CrossRef] [Green Version]
- Santiago, C.P.; Keuthan, C.J.; Boye, S.L.; Boye, S.E.; Imam, A.A.; Ash, J.D. A Drug-Tunable Gene Therapy for Broad-Spectrum Protection against Retinal Degeneration. Mol. Ther. 2018, 26, 2407–2417. [Google Scholar] [CrossRef] [Green Version]
- Cereso, N.; Pequignot, M.O.; Robert, L.; Becker, F.; De Luca, V.; Nabholz, N.; Rigau, V.; De Vos, J.; Hamel, C.P.; Kalatzis, V. Proof of concept for AAV2/5-mediated gene therapy in iPSC-derived retinal pigment epithelium of a choroideremia patient. Mol. Ther. Methods Clin. Dev. 2014, 1, 14011. [Google Scholar] [CrossRef]
- Burnight, E.R.; Wiley, L.A.; Drack, A.V.; Braun, T.A.; Anfinson, K.R.; Kaalberg, E.E.; Halder, J.A.; Affatigato, L.M.; Mullins, R.F.; Stone, E.M.; et al. CEP290 gene transfer rescues Leber congenital amaurosis cellular phenotype. Gene Ther. 2014, 21, 662–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassuk, A.G.; Zheng, A.; Li, Y.; Tsang, S.H.; Mahajan, V.B. Precision Medicine: Genetic Repair of Retinitis Pigmentosa in Patient-Derived Stem Cells. Sci. Rep. 2016, 6, 19969. [Google Scholar] [CrossRef] [Green Version]
- Garita-Hernandez, M.; Lampič, M.; Chaffiol, A.; Guibbal, L.; Routet, F.; Santos-Ferreira, T.; Gasparini, S.; Borsch, O.; Gagliardi, G.; Reichman, S.; et al. Restoration of visual function by transplantation of optogenetically engineered photoreceptors. Nat. Commun. 2019, 10, 4524. [Google Scholar] [CrossRef] [Green Version]
- Drori, T.; Chapman, J. Diagnosis and classification of neuromyelitis optica (Devic’s syndrome). Autoimmun Rev. 2014, 13, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Mallick, J.; Devi, L.; Malik, P.K.; Mallick, J. Update on Normal Tension Glaucoma. J. Ophthalmic Vis. Res. 2016, 11, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Kauper, K.; McGovern, C.; Sherman, S.; Heatherton, P.; Rapoza, R.; Stabila, P.; Dean, B.; Lee, A.; Borges, S.; Bouchard, B.; et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7484–7491. [Google Scholar] [CrossRef]
- Birch, D.G.; Weleber, R.G.; Duncan, J.L.; Jaffe, G.J.; Tao, W. Ciliary Neurotrophic Factor Retinitis Pigmentosa Study Groups. Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. Am. J. Ophthalmol. 2013, 156, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Levison, S.W.; Ducceschi, M.H.; Young, G.M.; Wood, T.L. Acute exposure to CNTF in vivo induces multiple components of reactive gliosis. Exp. Neurol. 1996, 141, 256–268. [Google Scholar] [CrossRef]
- Amore, G.; Romagnoli, M.; Carbonelli, M.; Barboni, P.; Carelli, V.; La Morgia, C. Therapeutic Options in Hereditary Optic Neuropathies. Drugs 2021, 81, 57–86. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, Y.; Tang, L.; Li, Y.; Fan, F.; Jiang, B. Protective effects of human umbilical cord blood stem cell intravitreal transplantation against optic nerve injury in rats. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1021–1028. [Google Scholar] [CrossRef]
- Lopez Sanchez, M.I.; Crowston, J.G.; Mackey, D.A.; Trounce, I.A. Emerging Mitochondrial Therapeutic Targets in Optic Neuropathies. Pharmacol. Ther. 2016, 165, 132–152. [Google Scholar] [CrossRef]
- Usategui-Martín, R.; Puertas-Neyra, K.; García-Gutiérrez, M.T.; Fuentes, M.; Pastor, J.C.; Fernandez-Bueno, I. Human Mesenchymal Stem Cell Secretome Exhibits a Neuroprotective Effect over In Vitro Retinal Photoreceptor Degeneration. Mol. Ther. Methods Clin. Dev. 2020, 17, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Labrador-Velandia, S.; Alonso-Alonso, M.L.; Di Lauro, S.; García-Gutierrez, M.T.; Srivastava, G.K.; Pastor, J.C.; Fernandez-Bueno, I. Mesenchymal stem cells provide paracrine neuroprotective resources that delay degeneration of co-cultured organotypic neuroretinal cultures. Exp. Eye Res. 2019, 185, 107671. [Google Scholar] [CrossRef]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone marrow derived stem cells in the treatment of Dominant Optic Atrophy. Stem Cell Investig. 2019, 6, 41. [Google Scholar] [CrossRef]
- Nascimento-Dos-Santos, G.; Teixeira-Pinheiro, L.C.; da Silva-Júnior, A.J.; Carvalho, L.R.P.; Mesentier-Louro, L.A.; Hauswirth, W.W.; Mendez-Otero, R.; Santiago, M.F.; Petrs-Silva, H. Effects of a combinatorial treatment with gene and cell therapy on retinal ganglion cell survival and axonal outgrowth after optic nerve injury. Gene Ther. 2020, 27, 27–39. [Google Scholar] [CrossRef]
- Huang, H.; Kolibabka, M.; Eshwaran, R.; Chatterjee, A.; Schlotterer, A.; Willer, H.; Bieback, K.; Hammes, H.P.; Feng, Y. Intravitreal injection of mesenchymal stem cells evokes retinal vascular damage in rats. FASEB J. 2019, 33, 14668–14679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labrador Velandia, S.; Di Lauro, S.; Alonso-Alonso, M.L.; Tabera Bartolome, S.; Srivastava, G.K.; Pastor, J.C.; Fernandez-Bueno, I. Biocompatibility of intravitreal injection of human mesenchymal stem cells in immunocompetent rabbits. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Bauer, G.; Abedi, M.; Pontow, S.; Panorgias, A.; Jonnal, R.; Zawadzki, R.J.; Werner, J.S.; Nolta, J. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: Preliminary phase 1 clinical trial findings. Investig. Ophthalmol Vis. Sci. 2014, 56, 81–89. [Google Scholar] [CrossRef]
- Gu, X.; Yu, X.; Zhao, C.; Duan, P.; Zhao, T.; Liu, Y.; Li, S.; Yang, Z.; Li, Y.; Qian, C.; et al. Efficacy and Safety of Autologous Bone Marrow Mesenchymal Stem Cell Transplantation in Patients with Diabetic Retinopathy. Cell Physiol. Biochem. 2018, 49, 40–52. [Google Scholar] [CrossRef]
- China. Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine. Role of the Serum Exosomal miRNA in Diabetic Retinopathy (DR). Available online: https://clinicaltrials.gov/ct2/show/NCT03264976 (accessed on 2 May 2021).
- Castanheira, P.; Torquetti, L.; Nehemy, M.B.; Goes, A.M. Retinal incorporation and differentiation of mesenchymal stem cells intravitreally injected in the injured retina of rats. Arq. Bras. Oftalmol. 2008, 71, 644–650. [Google Scholar] [CrossRef] [Green Version]
- van Zeeburg, E.J.; Maaijwee, K.J.; Missotten, T.O.; Heimann, H.; van Meurs, J.C. A free retinal pigment epithelium-choroid graft in patients with exudative age-related macular degeneration: Results up to 7 years. Am. J. Ophthalmol. 2012, 153, 120–127. [Google Scholar] [CrossRef]
- Ma, Z.; Han, L.; Wang, C.; Dou, H.; Hu, Y.; Feng, X.; Xu, Y.; Wang, Z.; Yin, Z.; Liu, Y. Autologous transplantation of retinal pigment epithelium-Bruch’s membrane complex for hemorrhagic age-related macular degeneration. Investig. Ophthalmol Vis. Sci. 2009, 50, 2975–2981. [Google Scholar] [CrossRef]
- Jung, Y.H.; Phillips, M.J.; Lee, J.; Xie, R.; Ludwig, A.L.; Chen, G.; Zheng, Q.; Kim, T.J.; Zhang, H.; Barney, P.; et al. 3D Microstructured Scaffolds to Support Photoreceptor Polarization and Maturation. Adv. Mater. 2018, 30, e1803550. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.S.; Higashi, Y.; Wood, K.J. Transplanting stem cells: Potential targets for immune attack. Modulating the immune response against embryonic stem cell transplantation. Adv. Drug Deliv. Rev. 2005, 57, 1944–1969. [Google Scholar] [CrossRef] [PubMed]
- Drukker, M.; Katz, G.; Urbach, A.; Schuldiner, M.; Markel, G.; Itskovitz-Eldor, J.; Reubinoff, B.; Mandelboim, O.; Benvenisty, N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 9864–9869. [Google Scholar] [CrossRef] [Green Version]
- Araki, R.; Uda, M.; Hoki, Y.; Sunayama, M.; Nakamura, M.; Ando, S.; Sugiura, M.; Ideno, H.; Shimada, A.; Nifuji, A.; et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 2013, 494, 100–104. [Google Scholar] [CrossRef]
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kamao, H.; Mandai, M.; Shiina, T.; Ogasawara, K.; Hirami, Y.; Kurimoto, Y.; Takahashi, M. Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models. Stem Cell Rep. 2016, 7, 635–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umekage, M.; Sato, Y.; Takasu, N. Overview: An iPS cell stock at CiRA. Inflamm. Regen. 2019, 39, 17. [Google Scholar] [CrossRef] [Green Version]
- Sugita, S.; Mandai, M.; Hirami, Y.; Takagi, S.; Maeda, T.; Fujihara, M.; Matsuzaki, M.; Yamamoto, M.; Iseki, K.; Hayashi, N.; et al. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med. 2020, 9, 2217. [Google Scholar] [CrossRef]
- Hazra, S.; Stepps, V.; Bhatwadekar, A.D.; Caballero, S.; Boulton, M.E.; Higgins, P.J.; Nikonova, E.V.; Pepine, C.J.; Thut, C.; Finney, E.M.; et al. Enhancing the function of CD34(+) cells by targeting plasminogen activator inhibitor-1. PLoS ONE 2013, 8, e79067. [Google Scholar] [CrossRef] [Green Version]
- Ueki, Y.; Wilken, M.S.; Cox, K.E.; Chipman, L.; Jorstad, N.; Sternhagen, K.; Simic, M.; Ullom, K.; Nakafuku, M.; Reh, T.A. Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice. Proc. Natl. Acad. Sci. USA 2015, 112, 13717–13722. [Google Scholar] [CrossRef] [Green Version]
- Mesentier-Louro, L.A.; Teixeira-Pinheiro, L.C.; Gubert, F.; Vasques, J.F.; Silva-Junior, A.J.; Chimeli-Ormonde, L.; Nascimento-Dos-Santo, G.; Mendez-Otero, R.; Santiago, M.F. Long-term neuronal survival, regeneration, and transient target reconnection after optic nerve crush and mesenchymal stem cell transplantation. Stem Cell Res. Ther. 2019, 10, 121. [Google Scholar] [CrossRef]
- Barber, A.; Farmer, K.; Martin, K.R.; Smith, P.D. Retinal regeneration mechanisms linked to multiple cancer molecules: A therapeutic conundrum. Prog. Retin. Eye Res. 2017, 56, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, O.W.; Brown, A.J.; Godwin, C.R.; Chimenti, M.S.; Boland, L.K.; Ankrum, J.A.; Kardon, R.H. Systemic Mesenchymal Stem Cell Treatment Mitigates Structural and Functional Retinal Ganglion Cell Degeneration in a Mouse Model of Multiple Sclerosis. Transl. Vis. Sci. Technol. 2020, 9, 16. [Google Scholar] [CrossRef] [PubMed]
| Stem Cell Source | Main Advantages and Disadvantages | Cell Type | Potential Applications |
|---|---|---|---|
| Retinal Progenitor Cells | |||
| Fetal stem cells |
| Retinal progenitor cells (RPCs) |
|
| Cortical progenitor cells (CPCs) |
| ||
| Pluripotent Stem Cells | |||
| Human embryonic stem cells |
| Human embryonic stem cell derived retinal pigment epitheliums (hESC-RPE) |
|
| Adult induced pluripotent stem cells |
| Adult induced pluripotent stem cells (iPSC) |
|
| Multipotent Stem Cells | |||
| Mesenchymal stem cells |
| Bone marrow-derived stem cells (BMSCs) |
|
| Adipose-derived stem cells (ADRCs) |
| ||
| Human umbilical multipotent stem cells retrieved from donor umbilical cords (hUTSCs) |
| |
| Other sources | Ciliary epithelium-derived stem cells (CESCs) |
| |
| Cells extracted from the adult human RPE, obtained from eye banks and activated in vitro into a stem cell state (RPESCs) |
| ||
| Reprogrammed endogenous Müller glia into RGCs (hMSCs) |
|
| Reference | Cell Type | Title | Disease | Administration Procedure | Status |
|---|---|---|---|---|---|
| NCT01226628 Phase I/IIa Study | Human umbilical multipotent stem cells retrieved from donor umbilical cords (hUTSCs) | A Safety Study of CNTO 2476 in Patients With Age-Related Macular Degeneration | Geographic atrophy due to age-related macular Degeneration | Subretinal with the iTrack Model 275 micro catheter | Completed |
| NCT01914913 Phase I/II Study | Autologous bone marrow derived mono nuclear stem cells (BMMNCs) | Clinical Study to Evaluate Safety and Efficacy of BMMNC in Retinitis Pigmentosa | Retinitis pigmentosa | Intravitreal | Unknown |
| NCT02280135 Phase I Study | Autologous bone marrow stem cells | Clinical Trial of Intravitreal Injection of Autologous Bone Marrow Stem Cells in Patients With Retinitis Pigmentosa | Retinitis pigmentosa | Intravitreal | Completed |
| NCT01560715 Phase II Study | Autologous bone marrow stem cells | Autologous Bone Marrow-Derived Stem Cells Transplantation For Retinitis Pigmentosa | Retinitis pigmentosa | Intravitreal | Completed |
| NCT01531348 Phase I Study | Human bone marrow-derived mesenchymal stem cells | Feasibility and Safety of Human Bone Marrow-derived Mesenchymal Stem Cells by Intravitreal Injection in Patients With Retinitis Pigmentosa | Retinitis pigmentosa | Subretinal | Unknown |
| NCT02016508 Phase I/II Study | Autologous bone marrow derived stem cells | Safety Study of Use of Autologous Bone Marrow Derived Stem Cell in Treatment of Age Related Macular Degeneration | Age-related macular degeneration | Intravitreal | Unknown |
| NCT03944239 Phase I Study | Retinal pigment epithelial (RPE) cells derived from human embryonic stem cells (hESC) | Safety and Efficacy of Subretinal Transplantation of Clinical Human Embryonic Stem Cell Derived Retinal Pigment Epitheliums in Treatment of Retinitis Pigmentosa | Retinitis pigmentosa | Subretinal | Recruiting |
| NCT02749734 Phase I/II Study | Human embryonic stem cell derived retinal pigment epitheliums (hESC-RPE) | Clinical Study of Subretinal Transplantation of Human Embryo Stem Cell Derived Retinal Pigment Epitheliums in Treatment of Macular Degeneration Diseases | Macular degeneration and Stargardt’s macular dystrophy | Subretinal | Unknown |
| NCT03046407 Phase I/II Study | Human embryonic stem cell derived retinal pigment epitheliums (hESC-RPE) | Treatment of Dry Age Related Macular Degeneration Disease With Retinal Pigment Epithelium Derived From Human Embryonic Stem Cells | Dry age-related macular degeneration | Subretinal | Unknown |
| NCT03167203 Phase I/II Study | Human embryonic stem cell derived retinal pigment epitheliums (hESC-RPE) | A Safety Surveillance Study in Subjects With Macular Degenerative Disease Treated With Human Embryonic Stem Cell-derived Retinal Pigment Epithelial Cell Therapy | Macular degenerative disease | Subretinal | Enrolling by invitation |
| NCT02941991 Follow up Study | Human embryonic stem cell derived retinal pigment epitheliums (hESC-RPE) | A Follow up Study to Determine the Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigmented Epithelial (hESC-RPE) Cells in Patients With Stargardt’s Macular Dystrophy (SMD) | Stargardt’s macular dystrophy | Biological: hESC-RPE | Completed |
| NCT02903576 Phase I/II Study |
| Stem Cell Therapy for Outer Retinal Degenerations |
| Subretinal | Completed |
| NCT01345006 Phase I/II Study | Human embryonic stem cell derived retinal pigment epithelium cells Biological: (MA09-hRPE) | Sub-retinal Transplantation of hESC Derived RPE(MA09-hRPE) Cells in Patients With Stargardt’s Macular Dystrophy | Stargardt’s macular dystrophy | Subretinal | Completed |
| NCT01469832 Phase I/II Study | Human embryonic stem cell derived retinal pigment epithelium cells Biological: (MA09-hRPE) | Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigmented Epithelial (hESC-RPE) Cells in Patients With Stargardt’s Macular Dystrophy (SMD) | Stargardt’s macular dystrophy | Subretinal | Completed |
| NCT01344993 Phase I/II Study | Human embryonic stem cell derived retinal pigment epithelium cells Biological: (MA09-hRPE) | Safety and Tolerability of Sub-retinal Transplantation of hESC Derived RPE (MA09-hRPE) Cells in Patients With Advanced Dry Age Related Macular Degeneration | Dry age-related macular degeneration | Subretinal | Completed |
| NCT01344993 Phase I/II Study | Human embryonic stem cell derived retinal pigment epithelium cells Biological: (MA09-hRPE) | Safety and Tolerability of Sub-retinal Transplantation of hESC Derived RPE (MA09-hRPE) Cells in Patients With Advanced Dry Age Related Macular Degeneration | Dry age-related macular degeneration | Subretinal | Completed |
| NCT02463344 Phase I/II Study | Human embryonic stem cell derived retinal pigment epithelium cells Biological: (MA09-hRPE) | Long Term Follow Up of Sub-retinal Transplantation of hESC Derived RPE Cells in Patients With AMD | Age-related macular degeneration | Subretinal | Completed |
| NCT01345006 Phase I/II Study | Human embryonic stem cell derived retinal pigment epithelium cells Biological: (MA09-hRPE) | Sub-retinal Transplantation of hESC Derived RPE(MA09-hRPE)Cells in Patients With Stargardt’s Macular Dystrophy | Stargardt’s macular dystrophy | Subretinal | Completed |
| NCT02445612 Long term follow up | Human embryonic stem cell derived retinal pigment epithelium cells Biological: (MA09-hRPE) | Long Term Follow Up of Sub-retinal Transplantation of hESC Derived RPE Cells in Stargardt Macular Dystrophy Patients | Stargardt’s macular dystrophy | Subretinal | Completed |
| NCT01469832 Phase I/II Study | Human embryonic stem cell derived retinal pigment epithelium cells Biological: (MA09-hRPE) | Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigmented Epithelial (hESC-RPE) Cells in Patients With Stargardt’s Macular Dystrophy (SMD) | Stargardt’s macular dystrophy | Subretinal | Completed |
| NCT01625559 Phase I/II Study | Human embryonic stem cell derived retinal pigment epithelium cells Biological: (MA09-hRPE) | Safety and Tolerability of MA09-hRPE Cells in Patients With Stargardt’s Macular Dystrophy(SMD) | Stargardt’s macular dystrophy | Subretinal | Unknown |
| NCT01674829 Phase I/II Study | Human embryonic stem cell derived retinal pigment epithelium cells Biological: (MA09-hRPE) | A Phase I/IIa, Open-Label, Single-Center, Prospective Study to Determine the Safety and Tolerability of Sub-retinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigmented Epithelial(MA09-hRPE) Cells in Patients With Advanced Dry Age-related Macular Degeneration(AMD) | Dry age-related macular degeneration | Subretinal | Active, not recruiting |
| NCT02286089 Phase I/II Study | Retinal pigment epithelial (RPE) cells derived from human embryonic stem cells (hESC) Biological: OpRegen: cell suspension either in ophthalmic Balanced Salt Solution Plus (BSS Plus) or in CryoStor® 5 (Thaw-and-Inject, TAI) | Safety and Efficacy Study of OpRegen for Treatment of Advanced Dry-Form Age-Related Macular Degeneration | Age-related macular degeneration | Subretinal | Active, not recruiting |
| NCT03963154 Phase I/II Study | Human embryonic stem cell derived retinal pigment epithelium (RPE) Investigational Medicinal Product: ISTEM-01 | Interventional Study of Implantation of hESC-derived RPE in Patients With RP Due to Monogenic Mutation | Retinitis pigmentosa | Subretinal | Recruiting |
| NCT02590692 Phase I/II Study | Human embryonic stem cell-derived RPE cells Biological: CPCB-RPE1 | Study of Subretinal Implantation of Human Embryonic Stem Cell-Derived RPE Cells in Advanced Dry AMD |
| Subretinal | Active, not recruiting |
| NCT03102138 Safety follow up Study | Human embryonic stem cell-derived RPE cells Biological: PF-05206388 | Retinal Pigment Epithelium Safety Study For Patients In B4711001 | Age-related macular degeneration | Intravitreal | Active, not recruiting |
| NCT01691261 Phase I Study | Human embryonic stem cell derived retinal pigment epithelium (RPE) living tissue equivalent Biological: PF-05206388: monolayer of RPE cells immobilized on a polyester membrane | A Study Of Implantation Of Retinal Pigment Epithelium In Subjects With Acute Wet Age Related Macular Degeneration | Age-related macular degeneration | Intraocular | Active, not recruiting |
| NCT02464956 Feasibility of production of these cells | Induced pluripotent stem cell (iPSC)-derived RPE cells from a patient’s own skin or blood | Production of iPSC Derived RPE Cells for Transplantation in AMD | Age-related macular degeneration | None | Unknown |
| Reference | Cell Type | Title | Disease | Administration Procedure | Status |
|---|---|---|---|---|---|
| NCT02464436 Phase I/IIa Study | Human retinal progenitor cells (hRPC) | Safety and Tolerability of hRPC in Retinitis Pigmentosa | Retinitis pigmentosa | Subretinal | Recruiting |
| NCT01068561 Phase I Study | Autologous bone marrow-derived stem cells | Autologous Bone Marrow-Derived Stem Cells Transplantation For Retinitis Pigmentosa | Retinitis pigmentosa | Intravitreal | Completed |
| NCT01560715 Phase II Study | Autologous bone marrow stem cells | Autologous Bone Marrow-Derived Stem Cells Transplantation For Retinitis Pigmentosa | Retinitis pigmentosa | Intravitreal | Completed |
| NCT01518127 Phase I/II Study | Autologous bone marrow stem cells | Intravitreal Bone Marrow-Derived Stem Cells in Patients With Macular Degeneration | Age-related macular degeneration and Stargartd | Intravitreal | Completed |
| NCT03437759 Phase I Study | Biological: exosomes derived from mesenchymal stem cells (MSC-Exo) | MSC-Exos Promote Healing of MHs | Macular holes | Intravitreal during a vitrectomy and the aid of endotamponades | Active, not recruiting |
| NCT03853252 Not applicable (proof of concept) | Autologous skin biopsy to get cells from choroideremia patients | iPS Cells of Patients for Models of Retinal Dystrophies | Retinal dystrophies: choroideremia | Other: create cell models of disease | Recruiting |
| Reference | Disease | Cell Type | Administration Route | Study Start Date | Status |
|---|---|---|---|---|---|
| NCT01364246 Phase I/II Study | Multiple sclerosis and neuromyelitis optica | Human umbilical multipotent stem cells retrieved from donor umbilical cords (hUTSCs) | Transplantation | January 2010 | Unknown |
| NCT01834079 Phase I/II Study | Optic nerve atrophy | Autologous bone marrow derived stem cells | Intrathecal injection | September 2014 | Unknown |
| NCT02249676 Phase II Study | Progressive and refractory neuromyelitis optica spectrum disorders | Autologous mesenchymal stem cells | Intravenous infusion of MSC a day-case 2.0 × 106 cells/kg | January 2013 | Unknown |
| NCT03605238 Phase I Study | Relapsed and/or refractory AQP4-IgG seropositive neuromyelitis optica spectrum disorders | CD19/CD20 tanCAR T Cells | Intravenous infusion | August 2018 | Withdrawn |
| NCT02976441 Phase I Study | High grade gliomas | Autologous stem cell collection | Stem cell intravenous infusion prior chemoradiation and reinfused back after treatment | January 2017 | Withdrawn |
| NCT02144103 Phase I/II Study | Retinal degeneration and primary open-angle glaucoma | Autologous adipose-derived regenerative cells (ADRC) | Subtenon | May 2014 | Unknown |
| NTC 01339455 Phase I/II Study | Neuromyelitis optica | Autologous hematopoietic stem cells | Intravenous infusion | April 2011 | Terminated (recruitment failure) |
| Reference | Disease | Cell Type | Administration Route | Sponsor | Study Start Date | Status |
|---|---|---|---|---|---|---|
| NTC 02638714 Phase I/II Study | Optic nerve atrophy | Autologous bone marrow CD 34+, 133+, and 271+ stem cells | No site declared | Stem Cells Arabia | April 2013 | Recruiting |
| NTC 03173638 Phase II Study | Acute ischemic optic neuropathy nonarteritic | Allogenic mesenchymal stem (MSV) cells from bone marrow | Intravitreal injection | IOBA, Spain | March 2018 | Recruiting |
| NCT 022836771 Phase I Study | Neuromyelitis optica | Tolerogenic dendritic cells loaded with myelin peptides | Intravenous administration | Hospital Clinic of Barcelona, Spain | September 2015 | Completed |
| NTC 01920867 Phase (n/a) | Various ocular diseases including optic neuritis | Bone marrow derived stem cells (BMSC). Study I | Injections of BMSC retrobulbar, subtenon and intravenous | MD Stem Cells, USA | August 2012 | Enrolling by invitation |
| NTC 03011541 Phase (n/a) | Various ocular diseases including optic neuropathy Nonarteritic ischemic optic neuropathy Optic atrophy, optic nerve disease, glaucoma, Leber hereditary optic neuropathy | Bone marrow derived stem cells (BMSC). Study II | Injections of BMSC retrobulbar, subtenon and intravenous | MD Stem Cells, USA | January 2016 | Recruiting |
| NTC 00787722 Phase I/II Study | Devic neuromyelitis | High dose immunosuppressive therapy with hematopoietic stem cells transplantation | Intravenous infusion | Northwestern University, USA | October 2009 | Completed |
| NTC 00716066 Phase II Study | Neurologic autoimmune diseases, including neuromyelitis optica | High dose immunosuppressive therapy with autologous hematopoietic stem cell transplantation | Intravenous infusion | Fred Hutchinson Cancer Research Center National Cancer Institute, USA | June 2008 | Recruiting |
| NTC 04577300 Phase II Study | Glaucoma | Dual NT-501 CNTF encapsulated cell therapy | Intravitreal NT-501 implants | Stanford University, USA | October 2020 | Not yet recruiting |
| NTC 02862938 Phase II Study | Glaucoma | NT-501 CNTF encapsulated cell therapy | Intravitreal NT-501 implants | Stanford University, USA | August 2016 | Active, not recruiting |
| NTC 02330978 Phase I Study | Glaucoma | Autologous bone marrow-derived mesenchymal stem cell | Intravitreal | University of Sao Paulo, Brazil | July 2019 | Completed |
| Reference | Cell Type | Title | Disease | Administration Procedure | Status |
|---|---|---|---|---|---|
| NCT01518842 Not applicable | Bone marrow stem cells | Effect of Intravitreal Bone Marrow Stem Cells on Ischemic Retinopathy (RetinaCell) | Ischemic retinopathy, including diabetic retinopathy with severe loss of retinal capillaries | Intravitreal | Unknown |
| NCT01736059 Phase I Study | CD34+ autologous adult bone marrow stem cells intravitreal | Clinical Trial of Autologous Intravitreal Bone-marrow CD34+ Stem Cells for Retinopathy | Non-exudative age-related macular degeneration Diabetic retinopathy Retina vein occlusion Retinitis pigmentosa hereditary macular degeneration | Intravitreal | Enrolling by invitation |
| NCT03981549 Phase I/II Study | CD34+ autologous bone marrow stem cells versus sham therapy | Treatment of Central Retinal Vein Occlusion Using Stem Cells Study (TRUST) | Central retinal vein occlusion | Intravitreal | Recruiting |
| NCT03403699 Not applicable | Combination of CD34+CD45+ cells derived from human inducible pluripotent stem cells (iPSCs) with iPSCs derived from the mesoderm: vascular wall-derived progenitor cells or endothelial colony forming cells (ECFCs) subset (SSEA5-KNA+) | Human iPSC for Repair of Vasodegenerative Vessels in Diabetic Retinopathy | Diabetes complications Diabetic retinopathy | Others: to test if the hiPSC-derived-mesoderm subset (SSEA5-KNA+) can revascularize vasodegenerative capillaries and if their reparative action can be enhanced by coinjection of CD34+CD45+ cells intravitreally. | Recruiting |
| NCT03264976 Not applicable | None | Role of the Serum Exosomal miRNA in Diabetic Retinopathy (DR) | Diabetic retinopathy | Validation of a diagnostic test based on exosomal miRNAs in serum samples that will be sequenced | Not yet recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coco-Martin, R.M.; Pastor-Idoate, S.; Pastor, J.C. Cell Replacement Therapy for Retinal and Optic Nerve Diseases: Cell Sources, Clinical Trials and Challenges. Pharmaceutics 2021, 13, 865. https://doi.org/10.3390/pharmaceutics13060865
Coco-Martin RM, Pastor-Idoate S, Pastor JC. Cell Replacement Therapy for Retinal and Optic Nerve Diseases: Cell Sources, Clinical Trials and Challenges. Pharmaceutics. 2021; 13(6):865. https://doi.org/10.3390/pharmaceutics13060865
Chicago/Turabian StyleCoco-Martin, Rosa M., Salvador Pastor-Idoate, and Jose Carlos Pastor. 2021. "Cell Replacement Therapy for Retinal and Optic Nerve Diseases: Cell Sources, Clinical Trials and Challenges" Pharmaceutics 13, no. 6: 865. https://doi.org/10.3390/pharmaceutics13060865







