Surfactant-Assisted Distal Pulmonary Distribution of Budesonide Revealed by Mass Spectrometry Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Animal Experiments
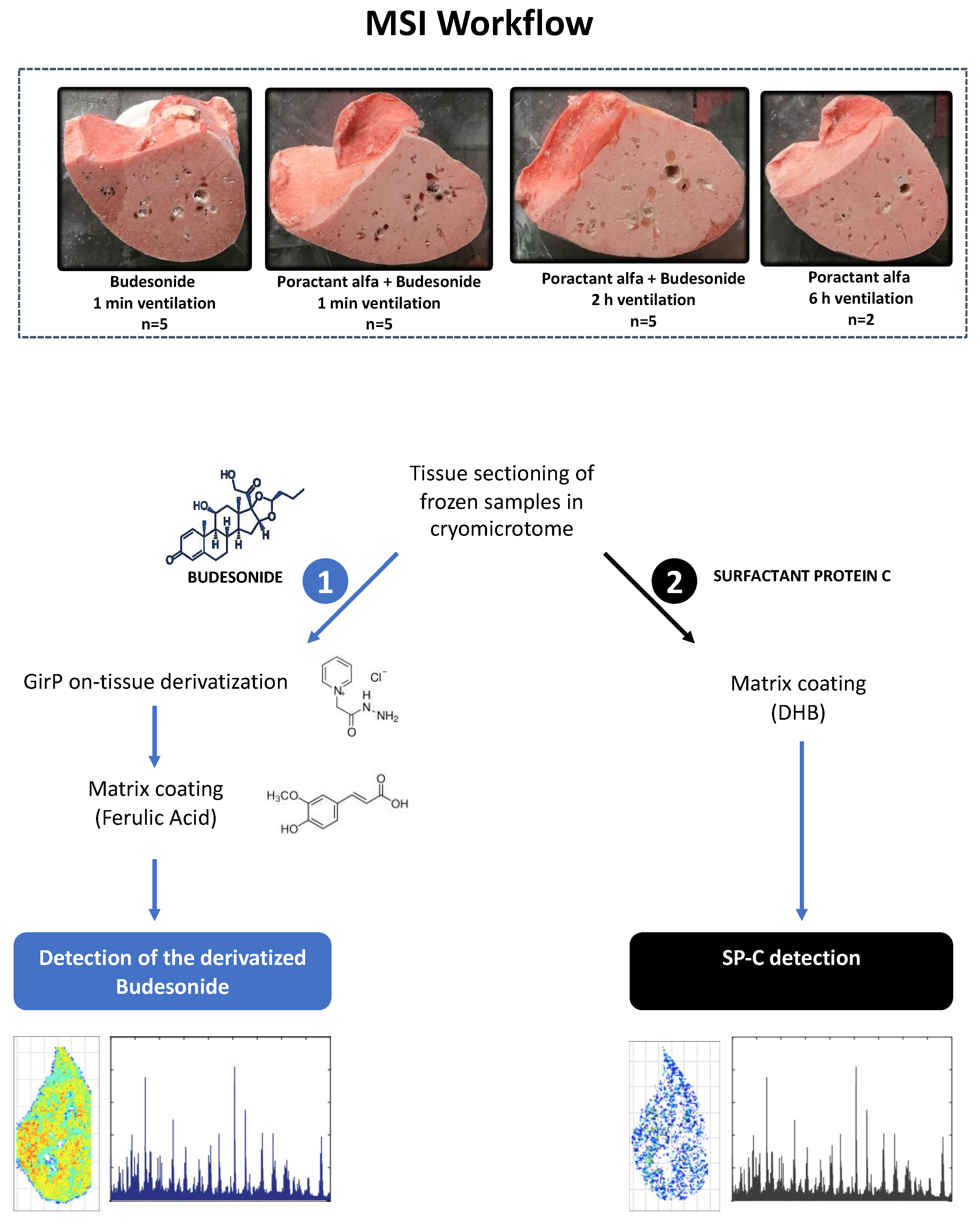
2.3. Tissue Sectioning
2.4. Sample Preparation and MALDI-MSI Analysis for Budesonide Detection
2.5. Sample Preparation and MALDI-MSI Analysis for SP-C Detection
2.6. Spatial Statistics
3. Results
3.1. Animal Characteristics
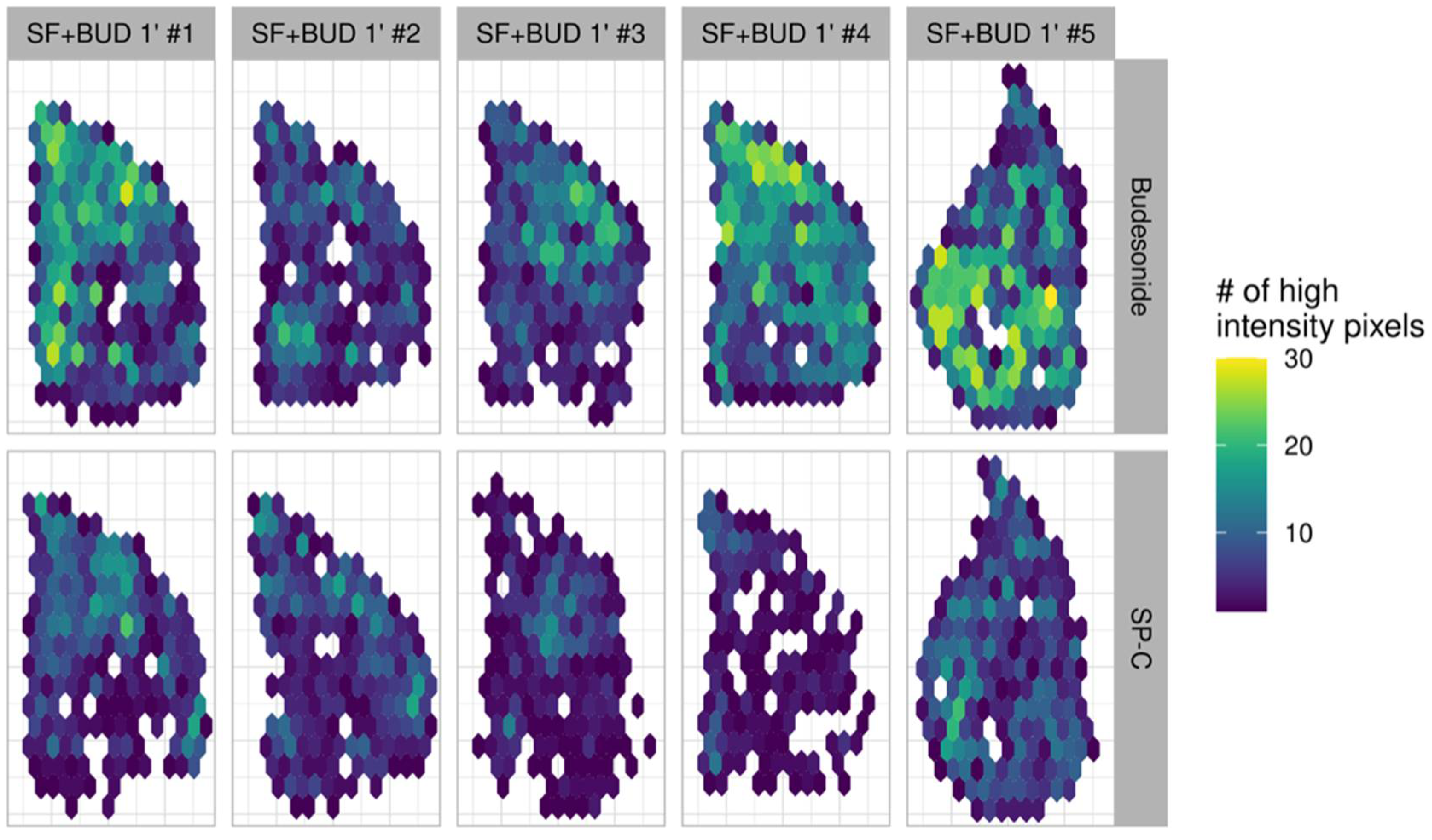
3.2. Budesonide Detection in Transverse Tissue Sections of the Right Lower Lobe
3.3. Comparison of the Lung Localization of Budesonide and SP-C
3.4. Statistical Analysis of Budesonide Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Prim. 2019, 5, 78. [Google Scholar] [CrossRef]
- Speer, C.P. Pulmonary inflammation and bronchopulmonary dysplasia. J. Perinatol. 2006, 26, S57–S62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bancalari, E. Bronchopulmonary Dysplasia. In Encyclopedia of Respiratory Medicine; Elsevier: Amsterdam, The Netherlands, 2006; pp. 300–305. ISBN 9780123708793. [Google Scholar]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef] [Green Version]
- Bassler, D.; Plavka, R.; Shinwell, E.S.; Hallman, M.; Jarreau, P.H.; Carnielli, V.; Van den Anker, J.N.; Meisner, C.; Engel, C.; Schwab, M.; et al. Early Inhaled Budesonide for the Prevention of Bronchopulmonary Dysplasia. N. Engl. J. Med. 2015, 373, 1497–1506. [Google Scholar] [CrossRef] [Green Version]
- Yeh, T.F.; Chen, C.M.; Wu, S.Y.; Husan, Z.; Li, T.C.; Hsieh, W.S.; Tsai, C.H.; Lin, H.C. Intratracheal administration of budesonide/surfactant to prevent bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2016, 193, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kothe, T.B.; Sadiq, F.H.; Burleyson, N.; Williams, H.L.; Anderson, C.; Hillman, N.H. Surfactant and budesonide for respiratory distress syndrome: An observational study. Pediatr. Res. 2020, 87, 940–945. [Google Scholar] [CrossRef]
- Donnelly, R.; Seale, J.P. Clinical pharmacokinetics of inhaled budesonide. Clin. Pharmacokinet. 2001, 40, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, C.; Feng, X.; Du, Y.; Zhang, Z.; Zhang, Y. Effects of intratracheal budesonide during early postnatal life on lung maturity of premature fetal rabbits. Pediatr. Pulmonol. 2018, 53, 28–35. [Google Scholar] [CrossRef]
- Brett Kothe, T.; Kemp, M.W.; Schmidt, A.; Royse, E.; Salomone, F.; Clarke, M.W.; Musk, G.C.; Jobe, A.H.; Hillman, N.H. Surfactant plus budesonide decreases lung and systemic inflammation in mechanically ventilated preterm sheep. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L888–L893. [Google Scholar] [CrossRef]
- Bianco, F.; Salomone, F.; Milesi, I.; Murgia, X.; Bonelli, S.; Pasini, E.; Dellacà, R.; Ventura, M.L.; Pillow, J. Aerosol drug delivery to spontaneously-breathing preterm neonates: Lessons learned. Respir. Res. 2021, 22, 71. [Google Scholar] [CrossRef]
- Yeh, T.F.; Lin, H.C.; Chang, C.H.; Wu, T.S.; Su, B.H.; Li, T.C.; Pyati, S.; Tsai, C.H. Early intratracheal instillation of budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants: A pilot study. Pediatrics 2008, 121, e1310–e1318. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.T.; Yeh, T.F.; Kuo, Y.L.; Chen, P.C.; Chen, C.M. Effect of surfactant and budesonide on the pulmonary distribution of fluorescent dye in mice. Pediatr. Neonatol. 2015, 56, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-M.; Chang, C.-H.; Chao, C.-H.; Wang, M.-H.; Yeh, T.-F. Biophysical and chemical stability of surfactant/budesonide and the pulmonary distribution following intra-tracheal administration. Drug Deliv. 2019, 26, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Fajardo, C.; Levin, D.; Garcia, M.; Abrams, D.; Adamson, I. Surfactant versus Saline as a Vehicle for Corticosteroid Delivery to the Lungs of Ventilated Rabbits. Pediatr. Res. 1998, 43, 542–547. [Google Scholar] [CrossRef] [Green Version]
- Zecchi, R.; Franceschi, P.; Tigli, L.; Amidani, D.; Catozzi, C.; Ricci, F.; Salomone, F.; Pieraccini, G.; Pioselli, B.; Mileo, V. Sample preparation strategy for the detection of steroids-like compounds using MALDI mass spectrometry imaging: Pulmonary distribution of budesonide as a case study. Anal. Bioanal. Chem. 2021. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Auguie, B. egg: Extensions for “ggplot2”: Custom Geom, Custom Themes, Plot Alignment, Labelled Panels, Symmetric Scales, and Fixed Panel Size. Available online: https://rdrr.io/cran/egg/ (accessed on 11 June 2021).
- Pebesma, E.J. Multivariable geostatistics in S: The gstat package. Comput. Geosci. 2004, 30, 683–691. [Google Scholar] [CrossRef]
- Csárdi, G.; Nepusz, T. The igraph software package for complex network research. Inter J. Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Bivand, R.; Rundel, C.; Pebesma, E.; Stuetz, R.; Hufthammer, K.O.; Giraudoux, P.; Davis, M.; Santilli, S. Rgeos: Interface to Geometry Engine—Open Source (’GEOS’). Available online: https://cran.r-project.org/package=rgeos (accessed on 11 June 2021).
- Pebesma, E.J.; Bivand, R. Classes and Methods for Spatial Data in R. Available online: https://cran.r-project.org/doc/Rnews/ (accessed on 11 June 2021).
- Pateiro-Lopez, B.; Rodriguez-Casal, A. Alphahull: Generalization of the Convex Hull of a Sample of Points in the Plane. Available online: https://cran.r-project.org/package=alphahull (accessed on 11 June 2021).
- Johansson, J.; Curstedt, T. Synthetic surfactants with SP-B and SP-C analogues to enable worldwide treatment of neonatal respiratory distress syndrome and other lung diseases. J. Intern. Med. 2019, 285, 165–186. [Google Scholar] [CrossRef] [Green Version]
- Heo, M.; Jeon, G.W. Intratracheal administration of budesonide with surfactant in very low birth weight infants to prevent bronchopulmonary dysplasia. Turk. J. Pediatr. 2020, 62, 551–559. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Ballard, P.L.; Ward, R.M.; Rower, J.E.; Wadhawan, R.; Hudak, M.L.; Weitkamp, J.H.; Harris, J.; Asselin, J.; Chapin, C.; et al. Dose-escalation trial of budesonide in surfactant for prevention of bronchopulmonary dysplasia in extremely low gestational age high-risk newborns (SASSIE). Pediatr. Res. 2020, 88, 629–636. [Google Scholar] [CrossRef]
- ACTRN12617000322336: Multicentre Randomised Controlled Trial of Surfactant Plus Budesonide to Improve Survival Free of Bronchopulmonary Dysplasia in Extremely Preterm Infants. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372110 (accessed on 11 June 2021).
- Zeng, L.; Tian, J.; Song, F.; Li, W.; Jiang, L.; Gui, G.; Zhang, Y.; Ge, L.; Shi, J.; Sun, X.; et al. Corticosteroids for the prevention of bronchopulmonary dysplasia in preterm infants: A network meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F506–F511. [Google Scholar] [CrossRef] [Green Version]
- Yeh, T.F.; Lin, Y.J.; Lin, H.C.; Huang, C.C.; Hsieh, W.S.; Lin, C.H.; Tsai, C.H. Outcomes at School Age after Postnatal Dexamethasone Therapy for Lung Disease of Prematurity. N. Engl. J. Med. 2004. [Google Scholar] [CrossRef] [PubMed]
- Bassler, D.; Shinwell, E.S.; Hallman, M.; Jarreau, P.-H.; Plavka, R.; Carnielli, V.; Meisner, C.; Engel, C.; Koch, A.; Kreutzer, K.; et al. Long-Term Effects of Inhaled Budesonide for Bronchopulmonary Dysplasia. N. Engl. J. Med. 2018, 378, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Fok, T.F.; Monkman, S.; Dolovich, M.; Gray, S.; Coates, G.; Paes, B.; Rashid, F.; Newhouse, M.; Kirpalani, H. Efficiency of aerosol medication delivery from a metered dose inhaler versus jet nebulizer in infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 1996, 21, 301–309. [Google Scholar] [CrossRef]
- Herting, E.; Gan, X.; Rauprich, P.; Jarstrand, C.; Robertson, B. Combined treatment with surfactant and specific immunoglobulin reduces bacterial proliferation in experimental neonatal group B streptococcal pneumonia. Am. J. Respir. Crit. Care Med. 1999, 159, 1862–1867. [Google Scholar] [CrossRef]
- van’t Veen, A.; Mouton, J.W.; Gommers, D.; Lachmann, B. Pulmonary surfactant as vehicle for intratracheally instilled tobramycin in mice infected with Klebsiella pneumoniae. Br. J. Pharmacol. 1996, 119, 1145–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basabe-Burgos, O.; Zebialowicz, J.; Stichtenoth, G.; Curstedt, T.; Bergman, P.; Johansson, J.; Rising, A. Natural derived surfactant preparation as a carrier of Polymyxin e for treatment of pseudomonas aeruginosa pneumonia in a near-term rabbit model. J. Aerosol Med. Pulm. Drug Deliv. 2019, 32, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Banaschewski, B.J.H.; Veldhuizen, E.J.A.; Keating, E.; Haagsman, H.P.; Zuo, Y.Y.; Yamashita, C.M.; Veldhuizen, R.A.W. Antimicrobial and biophysical properties of surfactant supplemented with an antimicrobial peptide for treatment of bacterial pneumonia. Antimicrob. Agents Chemother. 2015, 59, 3075–3083. [Google Scholar] [CrossRef] [Green Version]
- Katkin, J.P.; Husser, R.C.; Langston, C.; Welty, S.E. Exogenous surfactant enhances the delivery of recombinant adenoviral vectors to the lung. Hum. Gene Ther. 1997, 8, 171–176. [Google Scholar] [CrossRef]
- Hidalgo, A.; Garcia-Mouton, C.; Autilio, C.; Carravilla, P.; Orellana, G.; Islam, M.N.; Bhattacharya, J.; Bhattacharya, S.; Cruz, A.; Pérez-Gil, J. Pulmonary surfactant and drug delivery: Vehiculization, release and targeting of surfactant/tacrolimus formulations. J. Control. Release 2021, 329, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Kopincova, J.; Kolomaznik, M.; Mikolka, P.; Kosutova, P.; Topercerova, J.; Matasova, K.; Calkovska, A.; Mokra, D. Recombinant human superoxide dismutase and N-acetylcysteine addition to exogenous surfactant in the treatment of meconium aspiration syndrome. Molecules 2019, 24, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahl, H.B.; Hütten, M.C.; Monz, D.; Tutdibi, E.; Ophelders, D.; Nikiforou, M.; Tschernig, T.; Gortner, L.; Nohr, D.; Biesalski, H.K.; et al. Vitamin A supplementation by endotracheal application of a nano-encapsulated preparation is feasible in ventilated preterm lambs. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Curstedt, T.; Agarwal, B.; Prahaladan, V.M.; Ramirez, J.; Bhandari, S.; Syed, M.A.; Salomone, F.; Casiraghi, C.; Pelizzi, N.; et al. Small Molecule Inhibitor Adjuvant Surfactant Therapy Attenuates Ventilator- and Hyperoxia-Induced Lung Injury in Preterm Rabbits. Front. Physiol. 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Kopincova, J.; Mikolka, P.; Kolomaznik, M.; Kosutova, P.; Calkovska, A.; Mokra, D. Selective inhibition of NF-κB and surfactant therapy in experimental meconium-induced lung injury. Physiol. Res. 2017, 66, S227–S236. [Google Scholar] [CrossRef]
- Mikolka, P.; Kopincova, J.; Kosutova, P.; Kolomaznik, M.; Calkovska, A.; Mokra, D. Anti-IL-8 antibody potentiates the effect of exogenous surfactant in respiratory failure caused by meconium aspiration. Exp. Lung Res. 2018, 44, 40–50. [Google Scholar] [CrossRef]
- Nimmo, A.J.; Carstairs, J.R.; Patole, S.K.; Whitehall, J.; Davidson, K.; Vink, R. Intratracheal administration of glucocorticoids using surfactant as a vehicle. Clin. Exp. Pharmacol. Physiol. 2002, 29, 661–665. [Google Scholar] [CrossRef]
- Dani, C.; Corsini, I.; Burchielli, S.; Cangiamila, V.; Longini, M.; Paternostro, F.; Buonocore, G.; Rubatelli, F.F. Natural surfactant combined with beclomethasone decreases oxidative lung injury in the preterm lamb. Pediatr. Pulmonol. 2014, 44, 1159–1167. [Google Scholar] [CrossRef]
- Linner, R.; Perez-de-Sa, V.; Cunha-Goncalves, D. Lung Deposition of Nebulized Surfactant in Newborn Piglets. Neonatology 2015, 107, 277–282. [Google Scholar] [CrossRef]
- Hidalgo, A.; Cruz, A.; Pérez-Gil, J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur. J. Pharm. Biopharm. 2015, 95, 117–127. [Google Scholar] [CrossRef]
- Singh, N.; Hawley, K.L.; Viswanathan, K. Efficacy of Porcine Versus Bovine Surfactants for Preterm Newborns With Respiratory Distress Syndrome: Systematic Review and Meta-analysis. Pediatrics 2011, 128, e1588–e1595. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Catozzi, C.; Ravanetti, F.; Murgia, X.; D’Aló, F.; Macchidani, N.; Sgarbi, E.; Di Lallo, V.; Saccani, F.; Pertile, M. In vitro and in vivo characterization of poractant alfa supplemented with budesonide for safe and effective intratracheal administration. Pediatr. Res. 2017, 82, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Salomone, F.; Fresno, N.; Orellana, G.; Cruz, A.; Perez-Gil, J. Efficient Interfacially Driven Vehiculization of Corticosteroids by Pulmonary Surfactant. Langmuir 2017, 33, 7929–7939. [Google Scholar] [CrossRef]
- Gie, A.G.; Regin, Y.; Salaets, T.; Casiraghi, C.; Salomone, F.; Deprest, J.; Vanoirbeek, J.; Toelen, J. Intratracheal budesonide/surfactant attenuates hyperoxia-induced lung injury in preterm rabbits. Am. J. Physiol. Cell. Mol. Physiol. 2020, 319, L949–L956. [Google Scholar] [CrossRef]
- Hillman, N.H.; Abugisisa, L.; Royse, E.; Fee, E.; Kemp, M.W.; Kramer, B.W.; Schmidt, A.F.; Salomone, F.; Clarke, M.W.; Musk, G.C.; et al. Dose of budesonide with surfactant affects lung and systemic inflammation after normal and injurious ventilation in preterm lambs. Pediatr. Res. 2020, 88, 726–732. [Google Scholar] [CrossRef]
- Kothe, T.B.; Royse, E.; Kemp, M.W.; Schmidt, A.; Salomone, F.; Saito, M.; Usuda, H.; Watanabe, S.; Musk, G.C.; Jobe, A.H.; et al. Effects of budesonide and surfactant in preterm fetal sheep. Am. J. Physiol. Cell. Mol. Physiol. 2018, 315, L193–L201. [Google Scholar] [CrossRef]
- Hillman, N.H.; Kemp, M.W.; Fee, E.; Rittenschober-Böhm, J.; Royse, E.; Abugisisa, L.; Salomone, F.; Musk, G.C.; Jobe, A.H. Budesonide with surfactant decreases systemic responses in mechanically ventilated preterm lambs exposed to fetal intra-amniotic lipopolysaccharide. Pediatr. Res. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Ricci, F.; Bresesti, I.; LaVerde, P.A.M.; Salomone, F.; Casiraghi, C.; Mersanne, A.; Storti, M.; Catozzi, C.; Tigli, L.; Zecchi, R.; et al. Surfactant lung delivery with LISA and InSurE in adult rabbits with respiratory distress. Pediatr. Res. 2021, 1, 1–8. [Google Scholar] [CrossRef]
- Zecchi, R.; Franceschi, P.; Tigli, L.; Ricci, F.; Boscaro, F.; Pioselli, B.; Mileo, V.; Murgia, X.; Bianco, F.; Salomone, F.; et al. Mass spectrometry imaging as a tool for evaluating the pulmonary distribution of exogenous surfactant in premature lambs. Respir. Res. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller-Larsson, A.; Mattsson, H.; Hjertberg, E.; Dahlbäck, M.; Tunek, A.; Brattsand, R. Reversible fatty acid conjugation of budesonide: Novel mechanism for prolonged retention of topically applied steroid in airway tissue. Drug Metab. Dispos. 1998, 26, 623–630. [Google Scholar] [PubMed]



| BUD 1′ | SF+BUD 1′ | SF+BUD 120′ | |
|---|---|---|---|
| Birth weight (kg) | 3.41 ± 0.21 | 2.73 ± 0.23 | 3.20 ± 0.52 |
| Male:Female ratio | 1:4 | 1:4 | 1:4 |
| Gestational age (d) | 126 ± 1 | 126 ± 1 | 126 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zecchi, R.; Franceschi, P.; Tigli, L.; Pioselli, B.; Mileo, V.; Murgia, X.; Salomone, F.; Pieraccini, G.; Usada, H.; Schmidt, A.F.; et al. Surfactant-Assisted Distal Pulmonary Distribution of Budesonide Revealed by Mass Spectrometry Imaging. Pharmaceutics 2021, 13, 868. https://doi.org/10.3390/pharmaceutics13060868
Zecchi R, Franceschi P, Tigli L, Pioselli B, Mileo V, Murgia X, Salomone F, Pieraccini G, Usada H, Schmidt AF, et al. Surfactant-Assisted Distal Pulmonary Distribution of Budesonide Revealed by Mass Spectrometry Imaging. Pharmaceutics. 2021; 13(6):868. https://doi.org/10.3390/pharmaceutics13060868
Chicago/Turabian StyleZecchi, Riccardo, Pietro Franceschi, Laura Tigli, Barbara Pioselli, Valentina Mileo, Xabier Murgia, Fabrizio Salomone, Giuseppe Pieraccini, Haruo Usada, Augusto F. Schmidt, and et al. 2021. "Surfactant-Assisted Distal Pulmonary Distribution of Budesonide Revealed by Mass Spectrometry Imaging" Pharmaceutics 13, no. 6: 868. https://doi.org/10.3390/pharmaceutics13060868
APA StyleZecchi, R., Franceschi, P., Tigli, L., Pioselli, B., Mileo, V., Murgia, X., Salomone, F., Pieraccini, G., Usada, H., Schmidt, A. F., Hillman, N. H., Kemp, M. W., & Jobe, A. H. (2021). Surfactant-Assisted Distal Pulmonary Distribution of Budesonide Revealed by Mass Spectrometry Imaging. Pharmaceutics, 13(6), 868. https://doi.org/10.3390/pharmaceutics13060868






