Recent Advances in Polymer-Based Vaginal Drug Delivery Systems
Abstract
:1. Introduction
2. Anatomy and Physiology of the Vagina
3. Polymers Used in Vaginal Drug Delivery Systems
3.1. Polymers of Natural Origin
3.1.1. Polymers from Plant Sources
Cellulose and Its Derivatives
- Pectin
Alginates
- Starch
- Carrageenans
3.1.2. Polymers Derived from Animal Sources
Chitosan
Hyaluronic Acid
Gelatin
3.1.3. Microbial Polymers
Gellan Gum
Xanthan Gum
3.2. Synthetic Polymers
3.2.1. Poloxamers
3.2.2. Polyacrylates
3.2.3. Polyvinylpyrrolidone
3.2.4. Polyethylene Glycol
4. The Examples of Polymer-Based Vaginal Formulations
4.1. Semisolid Formulations
4.1.1. Gels
4.1.2. Mucoadhesive Drug Delivery Systems
4.1.3. Thermosensitive Dosage Forms
4.2. Suppositories, Tablets, and Pessaries
4.3. Vaginal Rings
4.4. Microspheres
4.5. Pellets
4.6. Nanoparticles
4.6.1. Poly(lactic-co-glycolic) Acid
4.6.2. Polyethylene Glycol
4.6.3. (Meth)acrylate Polymers
4.6.4. Polyesters (Polycaprolactone)
4.6.5. Polymers of a Natural Origin
4.7. Vaginal Films
5. Conclusions and Future Directions
| API(s) | Formulation | Polymer(s) | References |
|---|---|---|---|
| --- | bioadhesive tablets | Carbopol®934, pectin, PVP, ethyl anhydrated maleic resins | Baloğlu et al. (2003) [230] |
| --- | dendrimers | SPL7013 BHA.lys15lys16(NHCOCH2O)1-(3,6-naphth(SO3Na)32 (BHA: benzhydrylamine) | Gong et al. (2005) [306] |
| --- | gel | Pluronics® F127 and F68, HPMC chitosan-thioglycolic acid conjugates; polycarbophil, Pluronic F 127 | Aka-Any-Grah et al. (2010) [213] Friedl et al. (2013) [307] Podaralla et al. (2014) [308] |
| --- | gel-microemulsions | carageenan, xanthan gum | D’Cruz et al. (2001) [309] |
| --- | microparticles | CMC | Kejdušová et al. (2015) [310] |
| --- | mucoadhesive sponges | HEC 250M | Furst et al. (2015) [77] |
| --- | nanoparticles | chitosan, poly(isobutylcyanoacrylate); | Pradines et al. (2015) [311] |
| --- | peptide-derivatized dendrimers | --- | Luganini (2011) [312] |
| --- | tablets | hyaluronic acid | Ekin et al. (2011) [229] |
| 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) | film | PVA; HPMC E5 | Zhang et al. (2013) [275] |
| abacavir | bioadhesive film | Alg-Na, HPMC | Ghosal et al. (2014) [289] |
| film | Alg-Na; HPMC E5; HPMC-PVP blend | Ghosal et al. (2014) [289] | |
| acyclovir | in situ gel | poloxamer, carageenan, Carbopol 934p-NF | Liu et al. (2009) [214] |
| insitu forming hydrogel | hyaluronic acid, poloxamer F127 F68 | Mayol et al. (2008) [166] | |
| acyclovir, ciprofloxacin | gel | chitosan citrate | Bonferoni et al. (2008) [208] |
| amoxicilin | hydrogel | PEG-dendrimercrosslinks | Navath et al. (2011) [313] |
| fast-dissolving matrix | PVP | Rossi et al. (2017) [261] | |
| amphotericin B | insitu gel | poloxamer 407, HPCD | Kim et al. (2010) [314] |
| amphotericin, fluconazole | liquid crystal precursor mucoadhesive system | chitosan, poloxamer | Salmazi et al. (2015) [315] |
| arctigenin | liposome-based gel | pH-sensitive liposomes | Chen et al. (2012) [316] |
| baicalein | insitu gel | poloxamer, HPCD | Zhou et al. (2013) [215] |
| benzydamine HCl | tablets | HPMC, Carbopol 940 | Perioli et al. (2011) [228] |
| camptothecin | nanoparticles | PLGA | Blum et al. (2011) [317] |
| chlorhexidine | inserts | chitosan, CMC | Bigucci et al. (2015) [318] |
| chlorhexidine digluconate | freeze-dried polimer complexes | Alg-Na, chitosan | Abruzzo et al. (2013) [319] |
| cisplatin | nanofibersgels, films | PLA, PEO, HPMC, Carbopol | Zong et al. (2015) [320] |
| clindamycin phosphate | bioadhesive system | HPC, xanthan gum | Dobaria and Mashru (2010) [321] |
| clomiphenecitrate | gel | polycarbophil-cysteinę and chitosan-thioglycolic acid conjugates | Cevher et al. (2008) [211] |
| clotrimazole | gel | Pluronic®F127, polycarbophil, Carbopol. HPC, PVP poloxamers 407 and 188 | Bilensoy et al. (2006) [212] Chang et al. (2002) [322] |
| film | HPC, Alg-Na | Mishra et al. (2016) [323] | |
| nanocapsules | Eudragit RS100 | Santos et al. (2014) [251] | |
| tablets | chitosan, (silicified MCC, potato starch, | Szymańska et al. (2014) [220] | |
| tablets with microspheres | Eudragit RS-100 and RL-100 | Gupta et al. (2013) [219] | |
| clotrimazole, metronidazole | acid-buferring tablet | polycarbophil, HMPC | Alam et al. (2007) [222] |
| coumarin-6 | nanoparticles | PLGA | Cu et al. (2011) [324] |
| CSIC | film | PVA-HPMC K4M blend; PEG 4000 | Gong et al. (2017) [276] |
| dapivirine | film | PVA, HPMC 4000, PEG 8000 PEO, HPC | Akil et al. (2011) [278] Regev et al. (2019) [283] |
| nanoparticles | poly(ε-caprolactone) PLGA | Neves et al. (2014) [325] Neves and Sarmento (2015) [245] | |
| dapivirine and tenofovir | film | PVA | Akil et al. (2014) [266] |
| disulfiram | tablets | MCC, maize starch | Baffoe et al. (2014) [232] |
| doxorubicin | nanoparticles | carboxyl modified polystyrene | Ensign et al. (2013) [326] |
| econazole | film | gelatin, PVP, Soluplus®, and Gelucire® evaluated for solid dispersions | Dolci et al. (2020) [292] |
| microparticle-loaded gel | chitosan lactate, poloxamer 407, Eudragit RS | Parodi et al. (2013) [327] | |
| econazole and miconazole nitrate | gel | chitosan | Şenyigit et al. (2014) [209] |
| econazole nitrate, miconazole nitrate | tablets | thiolated poly(acrylic acid)-cysteine (PAA-Cys) conjugate | Baloglu et al. (2011) [221] |
| econazole nitrate | microparticles | chitosan, Na-CMC, poloxamers | Albertini et al. (2009) [240] |
| Efda and 5-chloro-3-phenylsulfonylindole-2-carboxamide (CSIC) | film | PVA, HPMC E5, PEG 4000 | Zhang et al. (2015) [277] |
| fluconazole | film | HPMC chitosan:pectin (75:25) | Kumar et al. (2013) [273] Mishra et al. (2017) [291] |
| fluorescent labeled NPs | film | PVA, carrageenan, PEG | Traore et al. (2018) [267] |
| FSAD S-nitrosoglutathione (GSNO) | film | Carbopol 934P, HPMC, PEG | Yoo et al. (2009) [279] |
| griffithsin/carrageenan | fast-dissolving insert | carrageenan, HEC, xanthan gum | Lal et al. (2018) [113] |
| griffithsin/carrageenan | fast-dissolving insert | carrageenan | Derby et al. (2018) [328] |
| hexylaminolevulinate hydrochloridum | pellets | MCC, Carbopol | Hiorth et al. (2012) [243] |
| bioadhesive mini-tablets | MC, HEC, HPC, MCC | Hiorth et al. (2014) [231] | |
| HIV; IQP-0528 | film | PLGA:Eudragit S 100 nanoparticle encapsulated drug in polymeric films | Srinivasan et al. (2016) [329] |
| HIV and VC; Ebselen | rapidly soluble film | β-cyclodextrin, PVA, Soluplus® | Vartak et al. (2020) [269] |
| HIV-1 reverse transcriptase inhibitors UC781, tenofovir | gel | HEC, Carbopol®974P | Mahalingam et al. (2010) [330] |
| IQP-0528 (non-nucleoside reverse transcriptase inhibitor) | osmotic pump tablets | HPC, CAP, Carbopol 974P | Rastogi et al. (2013) [227] |
| itraconazole | bioadhesive tablets | cyclodextrins | Cevher et al. (2014) [218] |
| film | HPC, PEG 400 | Dobaria et al. (2009) [331] | |
| insitu gel | HPMC E50, poloxamers 188 and 407 | Karavana et al. (2012) [332] | |
| itraconazole, tea tree oil | thermosensitive gel | Lutrol®F127 | Mirza et al. (2013) [333] |
| lactic acid | gel | chitosan poloxamer 408, chitosan | Bonferoni et al. (2006) [207] Rossi et al. (2014) [201] |
| tablets | MC, chitosan | Małolepsza-Jarmołowska (2007) [334] | |
| M48U1 anti-HIV microbicide | gel | Pluronic®F127, F68, HPMC | Bouchemal et al. (2013) [335] |
| maraviroc and emtricitabine | non-aqueous gels | silicone elastomer | Forbes et al. (2014) [336] |
| metronidazole | film | HPMC E5 S-protected gellan gum | Gahlot and Maheshwari (2018) [274] Jalil et al. (2019) [337] |
| gel | chitosan, HEC, 5-methylpyrrolidinone-chitosan (MPCS); PF-127 | Perioli et al. (2008) [338] Ibrahim et al. (2012) [339] | |
| tablets | chitosan, Alg-Na, MCC, CMC; chitosan (FG90C), polyvinylpyrrolidone (PVPK90) and polycarbophil (PCPAA1) | El-Kamel et al. (2002) [340] Perioli et al. (2009) [341] | |
| tablets with preliposomes | MCC, starch, pectin, chitosan | Vanić et al. (2014) [342] | |
| microbicidal-STD pathogens (HIV, HSC); bacteria associated with BV Cellulose acetate phthalate (CAP) | film | HPC | Neurath et al. (2003) [272] |
| microbicidal for HIV and HSV; mAB VRC01-N; mAB HSV8-N | film | PVA, maltitol, polysorbate 20 | Politch et al. (2021) [270] |
| icrobicides PHI-113, PHI-346, PHI-443 | self-emulsyfying gel | PEG 400, MCC, xanthan gum | D’Cruz et al. (2005) [343] |
| MIV-150/zinc acetate/carrageenan | gel | carrageenan | Friedland et al. (2016) [344] |
| MIV-150/zinc acetate/carrageenan | gel | carrageenan | Kenney et al. (2012) [345] |
| maraviroc | electrospun fibers | PVP, PEO | Ball andWoodrow (2014) [346] |
| Na fluorescein, nile red | nanoparticles | Eudragit S-100, PVP | Yoo et al. (2011) [167] |
| natamycin | tablets | HPMC, xanthan gum, Carbopol 934 P, cyclodextrins | Cevher et al. (2008) [347] |
| nile red | polymeric nanocapsules in hydrogel | chitosan, Eudragit | Frank et al. (2014) [172] |
| nystatin | gel | poly(acrylic acid)-cysteine conjugate and the new poly(acrylic acid)-cysteamineconjugate | Hombach et al. (2009) [348] |
| microparticles | Alg-Na, poloxamer 407, chitosan | Martín-Villena et al. (2013) [349] | |
| ovoalbumin | microparticles | PLGA | Kuo-Haller et al. (2010) [350] |
| gel | chitosan, HPMC K100M, Pluronic F 127 | Tuğcu-Demiroz et al. (2013) [210] | |
| polyherbal microbicides | cream | Alg-Na, xanthan gum | Talwar et al. (2008|) [351] |
| polystyrene sulfonate (PSS) | film | HPMC, HEC, PVA | Garg et al. (2005) [194] |
| probiotic microorganisms | microparticles | pectinate, hyaluronic acid | Pliszczak et al. (2011) [237] |
| progesterone | hydrogel | glycolchitin | Almomen et al. (2015) [352] |
| mucoadhesive emulsion | cyclomethicone pentamer | Campaña-Seoane (2014) [353] | |
| propranolol HCl | gel | guar gum, Alg-Na, xanthan gum, HPMC 4000, Na-CMC, carbomer 934, 940 | Tasdighi et al. (2012) [354] |
| proteins, insulin | flux controlled pump, pellets | HEC, HPC, CG, | Teller et al. (2014) [355] |
| pyrimidinedione IQP-0528 | film | PVA | Ham et al. (2012) [271] |
| raltegravir + efavirenz | nanoparticles loaded gel | Pluronic®F127 and F68 | Date et al. (2012) [356] |
| saquinavir | nanoparticles loaded gel | HEC, PLGA, PVA | Yang et al. (2013) [357] |
| sertaconazole | microemulsion-based gel | Carbopol 940 | PatelandPatel (2012) [358] |
| sertaconazole | tablets | Cbp 934P, CH, CMC-Na, Alg-Na, MC, HPMC, HPC | Patel et al. (2011) [359] |
| siRNA-loaded nanoparticles with anti-HLA-DR antibody (siRNA-NP-Ab) | film | PLGA-PEG/PEI/siRNA-NP PVA-λ-carageenan film | Gu et al. (2015) [246] |
| SPL7013 sulphonated dendrimer | gel | Carbopol® | Mumper et al. (2009) [360] |
| STDs sodium dodecyl sulfate (SDS) | film | Carbopol 934P, HPMC, PEG | Yoo et al. (2006) [263] |
| Streptococcus vaccine | microparticles | Resomer, RG 503 PLG | Hunter et al. (2001) [361] |
| tebuconazole | nanoparticles | tetraethylorthosilicate | Mas et al. (2014) [362] |
| tenofovir | film | drug-loaded PLGA/SA composite NPs incorporated into a PVA-HPMC film; EC: xanthan gum (2:1) | Machado et al. (2016) [253] Cazorla-Luna et al. (2020) [363] |
| microparticles | Eudragit S-100 sodium salt | Zhang et al. (2013) [239] | |
| nanoparticles | chitosan; hyaluronic acid | Meng et al. (2011); Meng et al. (2014) [364,365] Agrahari et al. (2014) [366] | |
| tablets | HPMC, Kollidon SR | McConville et al. (2013) [224] | |
| SLN | PAA | Alukda et al. (2011) [367] | |
| films | HPMC-Zein (1:5) blend, PEG Eudragit RL, RS, L and S | Notario-Perez et al. (2019) [280] Notario-Perez et al. (2021) [301] | |
| tenofovir + efavirenz | film | drug-loaded PLGA NPs in HPMC-PVA films | Cunha-Reis et al. (2016) [249] |
| tenofovir disoproxil fumarate and emricitabine | film | Eudragit®L100 NPs in PVA films | Cautela et a. (2019) [268] |
| tenofovir, emtricitabine | tablets | microcrystalline cellulose, crospovidone, hydroxyethyl cellulose | Clark et al. (2014) [368] |
| tenofovir, maraviroc | dendrimers | carbosilane | Sepúlveda-Crespo et al. (2014) [369] |
| tenofovir, maraviroc, dapivirine | film | sodium CMC, HPMC, HEC; PVA, PVP-K90, PVP-K30 | Akil et al. (2015) [370] |
| tenofovir, tenofovirdisoproxil fumarate | nanoparticles | PLGA, Eudragit | Zhang et al. (2011) [371] |
| tioconazole | film | chitosan-HPMC, PEG 400 | Calvo et al. (2019) [372] |
| UAMC01398 | solid dispersion film | HPMC, PEG 400 | Grammen et al. (2014) [373] |
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMB | Amphotericin B; |
| BV | Bacterial vaginosis; |
| CD | Cyclodextrins; |
| CLSM | Confocal scanning laser microscopy |
| CMC | Carboxymethyl cellulose; |
| CP | Conventional particles; |
| EC | Ethyl cellulose; |
| EC | Ethylcellulose; |
| EVA | Ethylenacetate of vinyl; |
| FSAD | Female sexual arousal disorder; |
| HA | Hyaluronic acid; |
| HEC | Hydroxyethyl cellulose; |
| HIV | Human immunodeficiency virus; |
| HLB | Hydrophilic-lipophilic balance; |
| HME | Hot-melt extrusion; |
| HPC | Hydroxypropyl cellulose; |
| HPMC | Hydroxypropyl methylcellulose; |
| HSV | Herpes simplex virus; |
| LIPs | Liposomes; |
| mAB | Monoclonal antibody; |
| MC | Methylcellulose; |
| MCC | Microcrystalline cellulose; |
| MPP | Mucus penetrating particles; |
| Na-CMC | Sodium carboxymethyl cellulose; |
| NC | Nanocapsules; |
| NP | Nanoparticles; |
| NPs | Polymeric nanoparticles; |
| NS | Nanospheres; |
| OPTs | Osmotic pump tablets; |
| PCL | Polycaprolactone; |
| PEG | Poly(ethylene glycol); |
| PEI | Polyethylenimine; |
| PEO | Poly(ethylene oxide); |
| PEO | Polyethylene oxide; |
| PLGA | Poly(lactic-co-glycolic acid); |
| PLGA | Poly(lactic-co-glycolic) acid; |
| PPO | Poly(propylene oxide); |
| PrEP | Pre-exposure prophylaxis of sexual transmission; |
| PVA | Polyvinyl alcohol; |
| PVP | Polyvinylpyrrolidone; |
| QbD | Quality-by-design; |
| RVVC | Recurrent vulvovaginal candidiasis; |
| SC | Solvent casted; |
| siRNA | Small interfering RNA; |
| STD | Sexually transmitted disease; |
| STIs | Sexuallytransmitted infections; |
| VC | Vaginal candidiasis; |
| VFS | Vaginal fluid stimulant |
References
- Stevens, J.M. Gynaecology from Ancient Egypt: The papyrus Kahun: A translation of the oldest treatise on gynaecology that has survived from the ancient world. Med. J. Aust. 1975, 2, 949–952. [Google Scholar] [CrossRef]
- Contraception—An Ancient Interest—Contraceptive, Women, Methods, and Practice. Available online: https://science.jrank.org/pages/1761/Contraception-An-ancient-interest.html (accessed on 12 February 2020).
- Smith, L. The Kahun Gynaecological Papyrus: Ancient Egyptian medicine. J. Fam. Plan. Reprod. Health Care 2011, 37, 54–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, I.; Zulkifle, M.; Ansari, A.H.; Sherwani, A.M.K.; Shakir, M. History of Ancient Egyptian Obstetrics & Gynecology: A Review. J. Microb. Biotechnol.Res. 2011, 1, 35–39. [Google Scholar]
- Britton, L.E.; Alspaugh, A.; Greene, M.Z.; McLemore, M.R. CE: An evidence-based update on contraception. Am. J. Nurs. 2020, 120, 22–33. [Google Scholar] [CrossRef] [PubMed]
- New Non-Hormonal Contraceptive Gel Found to Be Effective. Available online: https://www.pharmacytimes.com/view/new-non-hormonal-contraceptive-found-to-be-effective (accessed on 24 May 2021).
- Whorton, J.C. The Arsenic Century: How Victorian Britain was Poisoned at Home, Work, and Play; Oxford University Press: Oxford, UK, 2011; p. 412. [Google Scholar]
- Donders, G. Diagnosis and management of bacterial vaginosis and other types of abnormal vaginal bacterial flora: A review. Obstet. Gynecol. Surv. 2010, 65, 462–473. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20723268 (accessed on 12 February 2020). [CrossRef]
- Macklaim, J.M.; Clemente, J.C.; Knight, R.; Gloor, G.B.; Reid, G. Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb. Ecol. Health Dis. 2015, 26, 27799. Available online: http://www.microbecolhealthdis.net/index.php/mehd/article/view/27799 (accessed on 12 February 2020). [CrossRef] [PubMed]
- Tanphaichitr, N.; Srakaew, N.; Alonzi, R.; Kiattiburut, W.; Kongmanas, K.; Zhi, R.; Li, W.; Baker, M.; Wang, G.; Hickling, D. Potential use of antimicrobial peptides as vaginal spermicides/microbicides. Pharmaceuticals 2016, 9, 13. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26978373 (accessed on 12 February 2020). [CrossRef] [PubMed] [Green Version]
- Johal, H.S.; Garg, T.; Rath, G.; Goyal, A.K. Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. 2016, 23, 550–563. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef]
- Baptista, M.; Tavares, R.; Ramalho-Santos, J. Spermicidal and microbicidal compounds: In search of an efficient multipurpose strategy. Curr. Med. Chem. 2014, 21, 3693–3700. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25174922 (accessed on 12 February 2020). [CrossRef]
- Subramanian, B.; Agarwal, T.; Ghorai, S.K.; Mandal, P.; Chattopadhyay, S.; Basak, P.; Maiti, T.K.; Guha, S.K. Biocompatible polyvinyl alcohol and RISUG® blend polymeric films with spermicidal potential. Biomed. Mater. 2019, 14, 035017. [Google Scholar] [CrossRef]
- Daniel, S.; Rotem, R.; Koren, G.; Lunenfeld, E.; Levy, A. Vaginal antimycotics and the risk for spontaneous abortions. Am. J. Obstet. Gynecol. 2018, 218, 601.e1–601.e7. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29510088 (accessed on 13 February 2020). [CrossRef] [PubMed]
- El-Hammadi, M.; Arias, J. Nano-sized platforms for vaginal drug delivery. Curr. Pharm. Des. 2015, 21, 1633–1644. [Google Scholar] [CrossRef]
- Gupta, S.; Gabrani, R.; Ali, J.; Dang, S. Exploring novel approaches to vaginal drug delivery. Recent Pat. Drug Deliv. Formul. 2011, 5, 82–94. [Google Scholar] [CrossRef]
- Bassi, P.; Kaur, G. Innovations in bioadhesive vaginal drug delivery system. Expert Opin. Ther. Pat. 2012, 22, 1019–1032. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22860765 (accessed on 13 February 2020). [CrossRef]
- Naumova, I.; Castelo-Branco, C. Current treatment options for postmenopausal vaginal atrophy. Int.J. Womens Health 2018, 10, 387–395. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30104904 (accessed on 13 February 2020). [CrossRef] [Green Version]
- Veres, S.; Miller, L.; Burington, B. A comparison between the vaginal ring and oral contraceptives. Obstet. Gynecol. 2004, 104, 555–563. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15339769 (accessed on 13 February 2020). [CrossRef] [PubMed]
- Gao, Y.; Yuan, A.; Chuchuen, O.; Ham, A.; Yang, K.H.; Katz, D.F. Vaginal deployment and tenofovir delivery by microbicide gels. Drug Deliv. Transl. Res. 2015, 5, 279–294. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25874971 (accessed on 13 February 2020). [CrossRef] [Green Version]
- Ferguson, L.M.; Rohan, L.C. The importance of the vaginal delivery route for antiretrovirals in HIV prevention. Ther. Deliv. 2011, 2, 1535–1550. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22468220 (accessed on 13 February 2020). [CrossRef] [Green Version]
- Madanchi, H.; Shoushtari, M.; Kashani, H.; Sardari, S. Antimicrobial peptides of the vaginal innate immunity and their role in the fight against sexually transmitted diseases. N. Microbes N. Infect. 2020, 34, 100627. [Google Scholar] [CrossRef] [PubMed]
- Novetsky, A.P.; Keller, M.J.; Gradissimo, A.; Chen, Z.; Morgan, S.L.; Xue, X.; Strickler, H.D.; Fernández-Romero, J.A.; Burk, R.; Einstein, M.H. In vitro inhibition of human papillomavirus following use of a carrageenan-containing vaginal gel. Gynecol. Oncol. 2016, 143, 313–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, A.; Kleinbeck, K.; Mizenina, O.; Kizima, L.; Levendosky, K.; Jean-Pierre, N.; Villegas, G.; Ford, B.E.; Cooney, M.L.; Teleshova, N.; et al. In vitro and in vivo evaluation of two carrageenan-based formulations to prevent HPV acquisition. Antivir. Res. 2014, 108, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baptista, M.; Ramalho-Santos, J. Spermicides, microbicides and antiviral agents: Recent advances in the development of novel multi-functional compounds. Mini Rev. Med. Chem. 2009, 9, 1556–1567. Available online: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1389-5575&volume=9&issue=13&spage=1556 (accessed on 13 February 2020). [CrossRef] [PubMed]
- First Multipurpose Gel Designed to Prevent HIV, HSV, and HPV Simultaneously in Women and Men Advances in Clinical Trials. Population Council. Available online: https://www.popcouncil.org/news/first-multipurpose-gel-designed-to-prevent-hiv-hsv-and-hpv-simultaneously-i (accessed on 24 May 2021).
- Price, C.F.; Tyssen, D.; Sonza, S.; Davie, A.; Evans, S.; Lewis, G.R.; Xia, S.; Spelman, T.; Hodsman, P.; Moench, T.R.; et al. SPL7013 gel (vivagel®) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. PLoS ONE 2011, 6, 24095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, A.; Ahsan, F. The vagina as a route for systemic drug delivery. J. Control Release 2005, 103, 301–313. [Google Scholar] [CrossRef]
- Barton, D.L.; Shuster, L.T.; Dockter, T.; Atherton, P.J.; Thielen, J.; Birrell, S.N.; Sood, R.; Griffin, P.; Terstriep, S.A.; Mattar, B.; et al. Systemic and local effects of vaginal dehydroepiandrosterone (DHEA): NCCTG N10C1 (Alliance). Support. Care Cancer 2017, 26, 1335–1343. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29164377 (accessed on 13 February 2020). [CrossRef] [PubMed]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Vermani, K.; Garg, S. The scope and potential of vaginal drug delivery. Pharm. Sci. Technol. Today 2000, 3, 359–364. [Google Scholar] [CrossRef]
- Machado, R.M.; Palmeira-De-Oliveira, A.; Gaspar, C.; de Oliveira, J.M.; Palmeira-De-Oliveira, R. Studies and methodologies on vaginal drug permeation. Adv. Drug Deliv. Rev. 2015, 92, 14–26. [Google Scholar] [CrossRef]
- Machado, A.; das Neves, J. Tissue-based in vitro and ex vivo models for vaginal permeability studies. In Concepts and Models for Drug Permeability Studies: Cell and Tissue based In Vitro Culture Models; Elsevier: Amsterdam, The Netherlands, 2016; pp. 273–308. [Google Scholar]
- Morrow, R.J.; Woolfson, A.D.; Donnelly, L.; Curran, R.; Andrews, G.; Katinger, D.; Malcolm, R.K. Sustained release of proteins from a modified vaginal ring device. Eur. J. Pharm. Biopharm. 2011, 77, 3–10. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21055465 (accessed on 13 February 2020). [CrossRef] [Green Version]
- Srikrishna, S.; Cardozo, L. The vagina as a route for drug delivery: A review. Int. Urogynecology J. 2012, 24, 537–543. Available online: https://link.springer.com/article/10.1007/s00192-012-2009-3 (accessed on 20 May 2020). [CrossRef]
- Sherrard, J.; Wilson, J.; Donders, G.; Mendling, W.; Jensen, J.S. 2018 European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. Int. J. STD AIDS 2018, 29, 1258–1272. Available online: https://pubmed.ncbi.nlm.nih.gov/30049258/ (accessed on 20 May 2020). [CrossRef] [PubMed]
- CDC—Bacterial Vaginosis Statistics. Available online: https://www.cdc.gov/std/bv/stats.htm (accessed on 20 May 2021).
- Vaginal and Vulvar Cancers Statistics. Available online: https://www.cdc.gov/cancer/vagvulv/statistics/index.htm (accessed on 20 May 2021).
- Koumans, E.H.; Sternberg, M.; Bruce, C.; McQuillan, G.; Kendrick, J.; Sutton, M.; Markowitz, L.E. The prevalence of bacterial vaginosis in the United States, 2001-2004; associations with symptoms, sexual behaviors, and reproductive health. Sex. Transm. Dis. 2007, 34, 864–869. Available online: https://pubmed.ncbi.nlm.nih.gov/17621244/ (accessed on 13 February 2020). [CrossRef] [PubMed]
- Katz, D.F.; Yuan, A.; Gao, Y. Vaginal drug distribution modeling. Adv. Drug Deliv. Rev. 2015, 92, 2–13. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25933938 (accessed on 13 February 2020). [CrossRef] [PubMed] [Green Version]
- Melis, G.B.; Ibba, M.T.; Steri, B.; Kotsonis, P.; Matta, V.; Paoletti, A.M. Role of pH as a regulator of vaginal physiological environment. Minerva Ginecol. 2000, 52, 111–121. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10900941 (accessed on 13 February 2020). [PubMed]
- Mercer, B.M.; Miodovnik, M.; Thurnau, G.R.; Goldenberg, R.L.; Das, A.F.; Ramsey, R.D.; A Rabello, Y.; Meis, P.J.; Moawad, A.H.; Iams, J.D.; et al. The preterm prediction study: Significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am.J. Obs. Gynecol. 1995, 173, 1231–1235. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7485327 (accessed on 13 February 2020).
- Stojanović, N.; Plećaš, D.; Plešinac, S. Normal vaginal flora, disorders and application of probiotics in pregnancy. Arch. Gynecol. Obstet. 2012, 286, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, K.T.; Izquierdo, A.; Pretorius, E.S.; Shera, D.M.; Shabbout, M.; Shaunik, A. Baseline dimensions of the human vagina. Hum. Reprod. 2006, 21, 1618–1622. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16478763 (accessed on 13 February 2020). [CrossRef] [Green Version]
- Graziottin, A.; Gambini, D. Anatomy and physiology of genital organs—women. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 39–60. [Google Scholar]
- Hickey, M.; Pillai, G.; Higham, J.; Sullivan, M.; Horncastle, D.; Doherty, D.; Stamp, G. Changes in endometrial blood vessels in the endometrium of women with hormone replacement therapy-related irregular bleeding. Hum. Reprod. 2003, 18, 1100–1106. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12721191 (accessed on 13 February 2020). [CrossRef] [Green Version]
- Cunha, G.R. The dual origin of vaginal epithelium. Am. J. Anat. 1975, 143, 387–392. [Google Scholar] [CrossRef]
- Nilsson, K.; Risberg, B.; Heimer, G. The vaginal epithelium in the postmenopause—cytology, histology and pH as methods of assessment. Maturitas 1995, 21, 51–56. Available online: http://www.ncbi.nlm.nih.gov/pubmed/7731384 (accessed on 13 February 2020). [CrossRef]
- Boutin, E.L.; Cunha, G.R. Estrogen-induced epithelial proliferation and cornification are uncoupled in sinus vaginal epithelium associated with uterine stroma. Differentiation 1998, 62, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.; Maibach, H. Lifetime changes in the vulva and vagina. Arch. Gynecol. Obstet. 2006, 273, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Mårdh, P.A. The vaginal ecosystem. Am. J. Obstet. Gynecol. 1991, 165, 1163–1168. [Google Scholar] [CrossRef]
- Valore, E.V.; Park, C.H.; Igreti, S.L.; Ganz, T. Antimicrobial components of vaginal fluid. Am. J. Obstet. Gynecol. 2002, 187, 561–568. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12237628 (accessed on 13 February 2020). [CrossRef] [PubMed]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Chen, K.C.; Forsyth, P.S.; Buchanan, T.M.; Holmes, K.K. Amine content of vaginal fluid from untreated and treated patients with nonspecific vaginitis. J. Clin. Investig. 1979, 63, 828–835. Available online: http://www.ncbi.nlm.nih.gov/pubmed/447831 (accessed on 13 February 2020). [CrossRef] [PubMed]
- Rajan, N.; Cao, Q.; Anderson, B.E.; Pruden, D.L.; Sensibar, J.; Duncan, J.L.; Schaeffer, A.J. Roles of glycoproteins and oligosaccharides found in human vaginal fluid in bacterial adherence. Infect. Immun. 1999, 67, 5027–5032. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10496874 (accessed on 20 February 2020). [CrossRef] [Green Version]
- Sobel, J.D.; Faro, S.; Force, R.W.; Foxman, B.; Ledger, W.; Nyirjesy, P.R.; Reed, B.D.; Summers, P.R. Vulvovaginal candidiasis: Epidemiologic, diagnostic, and therapeutic considerations. Am. J. Obstet. Gynecol. 1998, 178, 203–211. [Google Scholar] [CrossRef]
- Heine, P.; McGregor, J.A. Trichomonas vaginalis: A reemerging pathogen. Clin. Obstet. Gynecol. 1993, 36, 137–144. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8435938 (accessed on 20 February 2020). [CrossRef]
- Pascual, L.M.; Daniele, M.B.; Pájaro, C.; Barberis, L. Lactobacillus species isolated from the vagina: Identification, hydrogen peroxide production and nonoxynol-9 resistance. Contraception 2006, 73, 78–81. [Google Scholar] [CrossRef]
- Linhares, I.M.; Summers, P.R.; Larsen, B.; Giraldo, P.C.; Witkin, S.S. Contemporary perspectives on vaginal pH and lactobacilli. Am. J. Obstet. Gynecol. 2011, 204, 120.e1–120.e5. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20832044 (accessed on 13 February 2020). [CrossRef] [PubMed]
- Caillouette, J.C.; Sharp, J.; Zimmerman, G.J.; Roy, S. Vaginal pH as a marker for bacterial pathogens and menopausal status. Am. J. Obstet. Gynecol. 1997, 176, 1270–1277. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18984051 (accessed on 13 February 2020). [CrossRef] [PubMed]
- Kale, V. Vaginal mucosa—A promising site for drug therapy. Br. J. Pharm. Res. 2013, 3, 983–1000. [Google Scholar] [CrossRef]
- Sigurdsson, H.H.; Kirch, J.; Lehr, C.-M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. 2013, 453, 56–64. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0378517313004572 (accessed on 13 February 2020). [CrossRef] [PubMed]
- Smart, J.D.; Kellaway, I.W.; Worthington, H.E.C. An in-vitro investigation of mucosa-adhesive materials for use in controlled drug delivery. Pharm. Pharmacol. 2011, 36, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Pal, K.; Anis, A.; Pramanik, K.; Prabhakar, B. Polymers in mucoadhesive drug-delivery systems: A brief note. Monomers Polym. 2009, 12, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Sriamornsak, P.; Wattanakorn, N.; Takeuchi, H. Study on the mucoadhesion mechanism of pectin by atomic force microscopy and mucin-particle method. Carbohydr. Polym. 2010, 79, 54–59. [Google Scholar] [CrossRef]
- Hartman, C.G. The permeability of the vaginal mucosa. Ann. N. Y. Acad. Sci. 1959, 83, 318–327. Available online: http://www.ncbi.nlm.nih.gov/pubmed/14400131 (accessed on 13 February 2020). [CrossRef]
- Das Neves, J.; Bahia, M.F. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006, 318, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Van der Bijl, P.; van Eyk, A.D. Comparative in vitro permeability of human vaginal, small intestinal and colonic mucosa. Int. J. Pharm. 2003, 261, 147–152. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12878403 (accessed on 13 February 2020). [CrossRef]
- Der Bijl, P.; van Eyk, A.D.; Thompson, I.O.C.; Stander, I.A. Diffusion rates of vasopressin through human vaginal and buccal mucosa. Eur. J. Oral. Sci. 1998, 106, 958–962. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9786326 (accessed on 13 February 2020). [CrossRef] [PubMed]
- Van Eyk, A.D.; Van der Bijl, P. Comparative permeability of various chemical markers through human vaginal and buccal mucosa as well as porcine buccal and mouth floor mucosa. Arch. Oral Biol. 2004, 49, 387–392. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15041486 (accessed on 13 February 2020). [CrossRef]
- Shokri, J.; Adibki, K. Application of cellulose and cellulose derivatives in pharmaceutical industries. In Cellulose—Medical, Pharmaceutical and Electronic Applications; InTech: London, UK, 2013. [Google Scholar]
- Sahin, H.T.; Arslan, M.B. A study on physical and chemical properties of cellulose paper immersed in various solvent mixtures. Int. J. Mol. Sci. 2008, 9, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Burchard, W. Solubility and solution structure of cellulose derivatives. Cellulose 2003, 10, 213–225. [Google Scholar] [CrossRef]
- Kristl, J.; Peppas, N.A.; Baumgartner, S. Network structure of cellulose ethers used in pharmaceutical applications during swelling and at equilibrium. Pharm Res. 2002, 19, 1084–1090. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12240932 (accessed on 13 February 2020).
- Furst, T.; Piette, M.; Lechanteur, A.; Evrard, B.; Piel, G. Mucoadhesive cellulosic derivative sponges as drug delivery system for vaginal application. Eur. J. Pharm. Biopharm. 2015, 95, 128–135. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25660908 (accessed on 13 February 2020). [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.K.; Gupta, A.B.; Kumar, R.; Yadav, J.S.; Kumar, B. Mucoadhesive polymers: Means of improving the mucoadhesive properties of drug delivery system. J. Chem. Pharm. Res. 2010, 2, 418–432. [Google Scholar]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.M.; El-Gendy, A.A. Pharmaceutical significance of cellulose: A review. Express Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Jain, S.; Sandhu, P.S.; Malvi, R.; Gupta, B. Cellulose derivatives as thermoresponsive polymer: An overview. J. Appl. Pharm. Sci. 2013, 3, 139–144. [Google Scholar]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Round, A.N.; Rigby, N.M.; MacDougall, A.; Morris, V.J. A new view of pectin structure revealed by acid hydrolysis and atomic force microscopy. Carbohydr. Res. 2010, 345, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.J.; Gromer, A.; Kirby, A.R.; Bongaerts, R.J.; Gunning, A.P. Using AFM and force spectroscopy to determine pectin structure and (bio) functionality. Food Hydrocoll. 2011, 25, 230–237. [Google Scholar] [CrossRef]
- Ralet-Renard, M.-C.; Lerouge, P.; Quéméner, B. Mass spectrometry for pectin structure analysis. Carbohydr. Res. 2009, 344, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Klemetsrud, T.; Jonassen, H.; Hiorth, M.; Kjøniksen, A.-L.; Smistad, G. Studies on pectin-coated liposomes and their interaction with mucin. Colloids Surf. B Biointerfaces 2013, 103, 158–165. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23201733 (accessed on 13 February 2020). [CrossRef]
- Gacesa, P. Alginates. Carbohydr. Polym. 1988, 8, 161–182. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural characterization of sodium alginate and calcium alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Johnson, F.A.; Craig, D.Q.M.; Mercer, A.D. Characterization of the block structure and molecular weight of sodium alginates. J. Pharm. Pharmacol. 2011, 49, 639–643. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9255704 (accessed on 13 February 2020). [CrossRef]
- Sutherland, I.W. Alginates. In Biomaterials; Palgrave Macmillan: London, UK, 1991; pp. 307–331. [Google Scholar] [CrossRef]
- Poncelet, D.; Babak, V.; Dulieu, C.; Picot, A. A physico-chemical approach to production of alginate beads by emulsification-internal ionotropic gelation. Colloids Surf. A Physicochem. Eng. Asp. 1999, 155, 171–176. [Google Scholar] [CrossRef]
- Leong, J.-Y.; Lam, W.-H.; Ho, K.-W.; Voo, W.-P.; Lee, M.F.-X.; Lim, H.P.; Lim, S.-L.; Tey, B.-T.; Poncelet, D.; Chan, E.-S. Advances in fabricating spherical alginate hydrogels with controlled particle designs by ionotropic gelation as encapsulation systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12149954 (accessed on 13 February 2020). [CrossRef] [PubMed]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25976619 (accessed on 13 February 2020). [CrossRef] [PubMed]
- Boyd, J.; Turvey, J.R. Structural studies of alginic acid, using a bacterial poly-α-L-guluronate lyase. Carbohydr. Res. 1978, 66, 187–194. [Google Scholar] [CrossRef]
- Ingar Draget, K.; Østgaard, K.; Smidsrød, O. Homogeneous alginate gels: A technical approach. Carbohydr. Polym. 1990, 14, 159–178. [Google Scholar] [CrossRef]
- Zhu, F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Wu, A.C.; Li, E.; Gilbert, R.G. Exploring extraction/dissolution procedures for analysis of starch chain-length distributions. Carbohydr. Polym. 2014, 114, 36–42. [Google Scholar] [CrossRef]
- Cai, J.; Man, J.; Huang, J.; Liu, Q.; Wei, W.; Wei, C. Relationship between structure and functional properties of normal rice starches with different amylose contents. Carbohydr. Polym. 2015, 125, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Bemiller, J.N. Pasting, paste, and gel properties of starch-hydrocolloid combinations. Carbohydr. Polym. 2011, 86, 386–423. [Google Scholar] [CrossRef]
- Güler, M.A.; Gök, M.K.; Figen, A.K.; Özgümüş, S. Swelling, mechanical and mucoadhesion properties of Mt/starch-g-PMAA nanocomposite hydrogels. Appl. Clay. Sci. 2015, 112–113, 44–52. [Google Scholar] [CrossRef]
- Mehta, S.; Verstraelen, H.; Peremans, K.; Villeirs, G.; Vermeire, S.; De Vos, F.; Mehuys, E.; Remon, J.P.; Vervaet, C. Vaginal distribution and retention of a multiparticulate drug delivery system, assessed by gamma scintigraphy and magnetic resonance imaging. Int. J. Pharm. 2012, 426, 44–53. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22265911 (accessed on 14 February 2020). [CrossRef] [Green Version]
- Mehta, S.; Verstraelen, H.; Vandaele, L.; Mehuys, E.; Remon, J.P.; Vervaet, C. Vaginal distribution and retention of tablets comprising starch-based multiparticulates: Evaluation by colposcopy. Drug Dev. Ind. Pharm. 2012, 39, 1944–1950. [Google Scholar] [CrossRef]
- Poelvoorde, N.; Verstraelen, H.; Verhelst, R.; Saerens, B.; De Backer, E.; Santiago, G.L.D.S.; Vervaet, C.; Vaneechoutte, M.; De Boeck, F.; Van Bortel, L.; et al. In vivo evaluation of the vaginal distribution and retention of a multi-particulate pellet formulation. Eur. J. Pharm. Biopharm. 2009, 73, 280–284. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19524668 (accessed on 14 February 2020). [CrossRef]
- Carrageenan—An Overview. ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/neuroscience/carrageenan (accessed on 26 May 2021).
- Shen, Y.-R.; Kuo, M.-I. Effects of different carrageenan types on the rheological and water-holding properties of tofu. LWT 2017, 78, 122–128. [Google Scholar] [CrossRef]
- Therkelsen, G.H. Carrageenan. In Industrial Gums: Polysaccharides and Their Derivatives, 3rd ed; Elsevier: Amsterdam, The Netherlands, 2012; pp. 145–180. [Google Scholar]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Rubio, J.; Tamayo, A.; Veiga, M.-D. Carrageenan-based acyclovir mucoadhesive vaginal tablets for prevention of genital herpes. Mar. Drugs 2020, 18, 249. Available online: www.mdpi.com/journal/marinedrugs (accessed on 26 May 2021). [CrossRef]
- Elias, C.J.; Coggins, C.; Alvarez, F.; Brache, V.; Fraser, I.S.; Lacarra, M.; Lähteenmäkl, P.; Massai, R.; Mishell, D.R.; Phillips, D.M.; et al. Colposcopic evaluation of a vaginal gel formulation of iota-carrageenan. Contraception 1997, 56, 387–389. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.-P.; Martín-Illana, A.; Ruiz-Caro, R.; Bermejo, P.; Abad, M.-J.; Carro, R.; Bedoya, L.-M.; Tamayo, A.; Rubio, J.; Fernández-Ferreiro, A.; et al. Chitosan and kappa-carrageenan vaginal acyclovir formulations for prevention of genital herpes. In vitro and ex vivo evaluation. Mar. Drugs 2015, 13, 5976–5992. Available online: www.mdpi.com/journal/marinedrugsArticle (accessed on 26 May 2021). [CrossRef] [Green Version]
- Lal, M.; Lai, M.; Ugaonkar, S.; Wesenberg, A.; Kizima, L.; Rodriguez, A.; Levendosky, K.; Mizenina, O.; Fernández-Romero, J.; Zydowsky, T. Development of a vaginal fast-dissolving insert combining griffithsin and carrageenan for potential use against sexually transmitted infections. J. Pharm. Sci. 2018, 107, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Al Sagheer, F.; Al-Sughayer, M.; Muslim, S.; Elsabee, M. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and chitosan: Structure, properties and applications in biomedical engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- De Andrade, S.M.B.; Ladchumananandasivam, R.; da Rocha, B.G.; Belarmino, D.D.; Galvão, A.O. The use of exoskeletons of shrimp (Litopenaeus vanammei) and crab (Ucides cordatus) for the extraction of chitosan and production of nanomembrane. Mater. Sci. Appl. 2012, 3, 495–508. [Google Scholar]
- Puvvada, Y.S.; Vankayalapati, S.; Sukhavasi, S. Extraction of chitin from chitosan from exoskeleton of shrimp for application in the pharmaceutical industry. Int. Curr. Pharm. J. 2012, 1, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Pillai, C.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Ravindra, R.; Krovvidi, K.R.; Khan, A. Solubility parameter of chitin and chitosan. Carbohydr. Polym. 1998, 36, 121–127. [Google Scholar] [CrossRef]
- Riva, R.; Ragelle, H.; Rieux, A.D.; Duhem, N.; Jérôme, C.; Préat, V. Chitosan and chitosan derivatives in drug delivery and tissue engineering. Adv. Polym. Sci. 2011, 244, 19–44. [Google Scholar]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Applications of Chitosan and Chitosan Derivatives in Drug Delivery. Available online: https://www.researchgate.net/publication/228468813_Applications_of_Chitosan_and_Chitosan_Derivatives_in_Drug_Delivery (accessed on 21 February 2020).
- Illum, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9755881 (accessed on 21 February 2020). [CrossRef]
- Tang, H.; Zhang, P.; Kieft, T.L.; Ryan, S.J.; Baker, S.M.; Wiesmann, W.P.; Rogelj, S. Antibacterial action of a novel functionalized chitosan-arginine against Gram-negative bacteria. Acta Biomater. 2010, 6, 2562–2571. [Google Scholar] [CrossRef] [Green Version]
- Jegatheeswaran, S.; Bhanot, U.; Siriwardena, A. In vivo evaluation of the chitosan-based haemostatic agent omni-stat® in porcine liver resection and in liver injury. Eur. Surg. Res. 2012, 49, 73–79. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22906964 (accessed on 21 February 2020). [CrossRef] [PubMed]
- Zhang, H.-L.; Tao, Y.; Guo, J.; Hu, Y.-M.; Su, Z.-Q. Hypolipidemic effects of chitosan nanoparticles in hyperlipidemia rats induced by high fat diet. Int. Immunopharmacol. 2011, 11, 457–461. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21215349 (accessed on 21 February 2020). [CrossRef] [PubMed]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is chitosan mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18540644 (accessed on 21 February 2020). [CrossRef] [PubMed]
- Alsarra, I.A. Chitosan topical gel formulation in the management of burn wounds. Int. J. Biol. Macromol. 2009, 45, 16–21. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19447254 (accessed on 21 February 2020). [CrossRef] [PubMed]
- Lupo, N.; Fodor, B.; Muhammad, I.; Yaqoob, M.; Matuszczak, B.; Bernkop-Schnürch, A. Entirely S-protected chitosan: A promising mucoadhesive excipient for metronidazole vaginal tablets. Acta Biomater. 2017, 64, 106–115. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29030305 (accessed on 21 February 2020). [CrossRef] [PubMed]
- Dai, T.; Tanaka, M.; Huang, Y.-Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.; Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010, 28, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Ekici, S.; Ilgin, P.; Butun, S.; Sahiner, N. Hyaluronic acid hydrogel particles with tunable charges as potential drug delivery devices. Carbohydr. Polym. 2011, 84, 1306–1313. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm. Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2006, 29, 17–25. [Google Scholar] [CrossRef]
- Brown, M.; Jones, S.A. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 308–318. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15857456 (accessed on 21 February 2020). [CrossRef] [PubMed]
- Falcone, S.J.; Palmeri, D.M.; Berg, R.A. Rheological and cohesive properties of hyaluronic acid. J. Biomed. Mater. Res. Part A 2006, 76, 721–728. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16315193 (accessed on 21 February 2020). [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30960626 (accessed on 21 February 2020). [CrossRef] [PubMed] [Green Version]
- Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds Research. Available online: https://www.woundsresearch.com/article/hyaluronic-acid-inflammation-and-tissue-regeneration (accessed on 21 February 2020).
- Greenberg, D.; Stoker, A.; Kane, S.; Cockrell, M.; Cook, J. Biochemical effects of two different hyaluronic acid products in a co-culture model of osteoarthritis. Osteoarthr. Cartil. 2006, 14, 814–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lev-Sagie, A.; Nyirjesy, P.; Tarangelo, N.; Bongiovanni, A.M.; Bayer, C.; Linhares, I.M.; Giraldo, P.C.; Ledger, W.J.; Witkin, S.S. Hyaluronan in vaginal secretions: Association with recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2009, 201, 206.e1–206.e5. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19646572 (accessed on 24 February 2020). [CrossRef]
- Cermelli, C.; Cuoghi, A.; Scuri, M.; Bettua, C.; Neglia, R.G.; Ardizzoni, A.; Blasi, E.; Iannitti, T.; Palmieri, B. In vitro evaluation of antiviral and virucidal activity of a high molecular weight hyaluronic acid. Virol. J. 2011, 8, 141. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21439070 (accessed on 24 February 2020). [CrossRef] [Green Version]
- Nomura, K.; Murakami, K.; Shozu, M.; Nakama, T.; Yui, N.; Inoue, M. Local application of danazol-loaded hyaluronic acid hydrogel to endometriosis in a rat model. Fertil. Steril. 2006, 85, 1157–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef]
- Karim, A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- B. Braun Sharing Expertise. Available online: https://www.bbraun.co.uk/en.html (accessed on 6 November 2020).
- Yavuz, S.T.; Sahiner, U.M.; Sekerel, B.E.; Tuncer, A.; Kalayci, O.; Sackesen, C. Anaphylactic reactions to measles-mumps-rubella vaccine in three children with allergies to hen’s egg and cow’s milk. Acta Paediatr. 2011, 100, e94–e96. [Google Scholar] [CrossRef]
- Bacelar, A.H.D.; Correia, J.S.; Oliveira, J.M.; Reis, R.L. Recent progress in gellan gum hydrogels provided by functionalization strategies. J. Mater. Chem. B 2016, 4, 6164–6174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Ochoa, F.; Santos, V.E.; Casas, J.; Gomez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Rodríguez Couto, S.; Sanromán, M.Á. Application of solid-state fermentation to food industry—A review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Rosalam, S.; England, R. Review of xanthan gum production from unmodified starches by Xanthomonas campestris SP. Enzym. Microb. Technol. 2006, 39, 197–207. [Google Scholar] [CrossRef]
- Bejenariu, A.; Popa, M.; Le Cerf, D.; Picton, L. Stiffness xanthan hydrogels: Synthesis, swelling characteristics and controlled release properties. Polym. Bull. 2008, 61, 631–641. [Google Scholar] [CrossRef]
- Bodratti, A.; Alexandridis, P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djekic, L.; Čalija, B.; Medarević, Đ. Gelation behavior, drug solubilization capacity and release kinetics of poloxamer 407 aqueous solutions: The combined effect of copolymer, cosolvent and hydrophobic drug. J. Mol. Liq. 2020, 303, 112639. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer hydrogels for biomedical applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Schmolka, I.R. A review of block polymer surfactants. J. Am. Oil Chem. Soc. 1977, 54, 110–116. [Google Scholar] [CrossRef]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified poloxamer 407. Heliyon 2017, 3, e00390. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Liu, M.; Fu, H.; Zhang, W.; Peng, G.; Zhang, Y.; Cao, H.; Luo, L. Novel thermosensitive in situ gel based on poloxamer for uterus delivery. Eur. J. Pharm. Sci. 2015, 77, 24–28. [Google Scholar] [CrossRef]
- Yuan, Y.; Cui, Y.; Zhang, L.; Zhu, H.-P.; Guo, Y.-S.; Zhong, B.; Hu, X.; Zhang, L.; Wang, X.-H.; Chen, L. Thermosensitive and mucoadhesive in situ gel based on poloxamer as new carrier for rectal administration of nimesulide. Int. J. Pharm. 2012, 430, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Zaki, N.M.; A Awad, G.; Mortada, N.D.; El Hady, S.S.A. Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesive in situ gel with modulated rheological and mucociliary transport properties. Eur. J. Pharm. Sci. 2007, 32, 296–307. [Google Scholar] [CrossRef]
- Xuan, J.-J.; Balakrishnan, P.; Oh, D.H.; Yeo, W.H.; Park, S.M.; Yong, C.S.; Choi, H.-G. Rheological characterization and in vivo evaluation of thermosensitive poloxamer-based hydrogel for intramuscular injection of piroxicam. Int. J. Pharm. 2010, 395, 317–323. [Google Scholar] [CrossRef]
- Kojarunchitt, T.; Hook, S.; Rizwan, S.; Rades, T.; Baldursdottir, S. Development and characterisation of modified poloxamer 407 thermoresponsive depot systems containing cubosomes. Int. J. Pharm. 2011, 408, 20–26. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Thanasanchokpibull, S.; Fuongfuchat, A.; Veeranondha, S. Optimization and evaluation of thermoresponsive diclofenac sodium ophthalmic in situ gels. Int. J. Pharm. 2011, 411, 128–135. [Google Scholar] [CrossRef]
- Soliman, K.A.; Ullah, K.; Shah, A.; Jones, D.S.; Singh, T.R. Poloxamer-based in situ gelling thermoresponsive systems for ocular drug delivery applications. Drug Discov. Today 2019, 24, 1575–1586. [Google Scholar] [CrossRef]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; I La Rotonda, M. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef]
- Yoo, J.W.; Giri, N.; Lee, C.H. PH-sensitive Eudragit nanoparticles for mucosal drug delivery. Int. J. Pharm. 2011, 403, 262–267. [Google Scholar] [CrossRef]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Expert Opin. Drug Deliv. 2012, 10, 131–149. [Google Scholar] [CrossRef]
- Ivanova, N.A.; Trapani, A.; Di Franco, C.; Mandracchia, D.; Trapani, G.; Franchini, C.; Corbo, F.; Tripodo, G.; Kolev, I.N.; Stoyanov, G.S.; et al. In vitro and ex vivo studies on diltiazem hydrochloride-loaded microsponges in rectal gels for chronic anal fissures treatment. Int. J. Pharm. 2019, 557, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Ramineni, S.K.; Dziubla, T.D.; Cunningham, L.L.; Puleo, D.A. Local delivery of imiquimod in hamsters using mucoadhesive films and their residence time in human patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 665–673. [Google Scholar] [CrossRef]
- Chantasart, D.; Tocanitchart, P.; Wongrakpanich, A.; Teeranachaideekul, V.; Junyaprasert, V.B. Fabrication and evaluation of Eudragit® polymeric films for transdermal delivery of piroxicam. Pharm. Dev. Technol. 2017, 23, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Guterres, S.; Frank, L.A.; Sandri, G.; D’Autilia, F.; Contri, R.V.; Bonferoni, M.C.; Caramella, C.; Frank, A.G.; Pohlmann, A.R. Chitosan gel containing polymeric nanocapsules: A new formulation for vaginal drug delivery. Int. J. Nanomed. 2014, 9, 3151–3161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, F.; Zhang, X.; Ping, Q. New method for ophthalmic delivery of azithromycin by poloxamer/carbopol-based in situ gelling system. Drug Deliv. 2010, 17, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Rodríguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Rheological characterization of topical carbomer gels neutralized to different pH. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef] [Green Version]
- Shahin, M.; Hady, S.A.; Hammad, M.; Mortada, N. Optimized formulation for topical administration of clotrimazole using Pemulen polymeric emulsifier. Drug Dev. Ind. Pharm. 2010, 37, 559–568. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, X.; Wang, X.; Wang, H.; Mao, S. Tunable and sustained-release characteristics of venlafaxine hydrochloride from chitosan–carbomer matrix tablets based on in situ formed polyelectrolyte complex film coating. Asian J. Pharm. Sci. 2018, 13, 566–574. [Google Scholar] [CrossRef]
- Rashad, A.A.; El-Helaly, S.N.; El Rehim, R.T.A.; El Gazayerly, O. Core-in-cup/liquisol dual tackling effect on azelnidipine buccoadhesive tablet micromeritics, in vitro release, and mucoadhesive strength. Acta Pharm. 2019, 69, 381–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, J.T.; Thomas, G.A.O.; Rhodes, J.; Evans, B.K.; Russell, M.A.H.; Feyerabend, C.; Fuller, G.S.; Newcombe, R.G.; Sandborn, W.J. Pharmacokinetics of nicotinic carbomer enemas: A new treatment modality for ulcerative colitis. Clin. Pharmacol. Ther. 1997, 61, 340–3488. [Google Scholar] [CrossRef]
- Wang, L.; Tang, X. A novel ketoconazole bioadhesive effervescent tablet for vaginal delivery: Design, in vitro and “in vivo” evaluation. Int. J. Pharm. 2008, 350, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.K.; Chawla, M.; Singh, A. Potential applications of carbomer in oral mucoadhesive controlled drug delivery system: A review. Drug Dev. Ind. Pharm. 2000, 26, 913–924. [Google Scholar] [CrossRef]
- Michálek, J.; Vacík, J.; Kúdelková, J.; Ježová, N. Hydrogels for biomedical use based on 1-vinyl-2-pyrrolidone crosslinked with macromonomers. Angew. Makromol. Chem. 1996, 239, 151–160. [Google Scholar] [CrossRef]
- Goodwin, M.J.; Musa, O.M.; Berry, D.J.; Steed, J.W. Small-molecule povidone analogues in coamorphous pharmaceutical phases. Cryst. Growth Des. 2018, 18, 701–709. [Google Scholar] [CrossRef]
- Bühler, V. Polyvinylpyrrolidone Excipients for Pharmaceuticals: Povidone, Crospovidone and Copovidone. Springer: Berlin/Heidelberg, Germany, 2005; pp. 5–120. [Google Scholar]
- D’Souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Noda, Y.; Watanabe, K.; Sanagawa, A.; Sobajima, Y.; Fujii, S. Physicochemical properties of macrogol ointment and emulsion ointment blend developed for regulation of water absorption. Int. J. Pharm. 2011, 419, 131–136. [Google Scholar] [CrossRef]
- Harris, J.M. Poly (Ethylene Glycol) Chemistry: Biotechnical and Biomedical Applicationse; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–385. [Google Scholar]
- Acarturk, F. Mucoadhesive Vaginal Drug Delivery Systems. Recent Pat. Drug Deliv. Formul. 2009, 3, 193–205. [Google Scholar] [CrossRef]
- Machado, R.M.; Palmeira-De-Oliveira, A.; Martinez-De-Oliveira, J.; Palmeira-De-Oliveira, R. Vaginal semisolid products: Technological performance considering physiologic parameters. Eur. J. Pharm. Sci. 2017, 109, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-De-Oliveira, R.; Duarte, P.; Palmeira-De-Oliveira, A.; Das Neves, J.; Amaral, M.H.; Breitenfeld, L.; Martinez-De-Oliveira, J. Women’s experiences, preferences and perceptions regarding vaginal products: Results from a cross-sectional web-based survey in Portugal. Eur. J. Contracept. Reprod. Health Care 2015, 20, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, E.; Bernkop-Schnürch, A.; Karavana, S.Y.; Senyigit, Z.A. Strategies to prolong the intravaginal residence time of drug delivery systems. J. Pharm. Pharm. Sci. 2009, 12, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-de-Oliveira, R.; Duarte, P.; Palmeira-de-Oliveira, A.; das Neves, J.; Amaral, M.; Breitenfeld, L.; Martinez-de-Oliveira, J. What do Portuguese women prefer regarding vaginal products? Results from a cross-sectional web-based survey. Pharmaceutics 2014, 6, 543–556. [Google Scholar] [CrossRef]
- Nappi, R.E.; Liekens, G.; Brandenburg, U. Attitudes, perceptions and knowledge about the vagina: The International Vagina Dialogue Survey. Contraception 2006, 73, 493–500. [Google Scholar] [CrossRef]
- Van Den Berg, J.J.; Rosen, R.K.; Bregman, D.E.; Thompson, L.A.; Jensen, K.M.; Kiser, P.F.; Katz, D.F.; Buckheit, K.; Buckheit, R.W., Jr.; Morrow, K.M. “set it and forget it”: Women’s perceptions and opinions of long-acting topical vaginal gels. AIDS Behav. 2014, 18, 862–870. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.; Vermani, K.; Garg, A.; Anderson, R.A.; Rencher, W.B.; Zaneveld, L.J.D. Development and characterization of bioadhesive vaginal films of sodium polystyrene sulfonate (PSS), a novel contraceptive antimicrobial agent. Pharm. Res. 2005, 22, 584–595. [Google Scholar] [CrossRef]
- Almdal, K.; Dyre, J.; Hvidt, S.; Kramer, O. Towards a phenomenological definition of the term “gel”. Polym. Gels Netw. 1993, 1, 5–17. [Google Scholar] [CrossRef]
- Rogovina, L.Z.; Vasil’Ev, V.G.; Braudo, E.E. Definition of the concept of polymer gel. Sci. Ser. C 2008, 50, 85–92. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G.; Zhang, D. Stimuli responsive gels based on low molecular weight gelators. J. Mater. Chem. 2012, 22, 38–50. [Google Scholar] [CrossRef]
- Salah, S.; Awad, G.E.; Makhlouf, A.I. Improved vaginal retention and enhanced antifungal activity of miconazole microsponges gel: Formulation development and in vivo therapeutic efficacy in rats. Eur. J. Pharm. Sci. 2018, 114, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Menard, J.P. Antibacterial treatment of bacterial vaginosis: Current and emerging therapies. Int. J. Women’s Health 2011, 3, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Friend, D.R. Advances in vaginal drug delivery. Drug Deliv. Transl. Res. 2011, 1, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G.; Faccendini, A.; Puccio, A.; Caramella, C. Comparison of poloxamer-and chitosan-based thermally sensitive gels for the treatment of vaginal mucositis. Drug Dev. Ind. Pharm. 2013, 40, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Vajdy, M. Mucosal HIV transmission and vaccination strategies through oral compared with vaginal and rectal routes. Expert Opin. Biol. Ther. 2010, 10, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Home—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 28 January 2021).
- De Araújo Pereira, R.R.; Bruschi, M.L. Vaginal mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 2012, 38, 643–652. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Giunchedi, P.; Scalia, S.; Rossi, S.; Sandri, G.; Caramella, C. Chitosan gels for the vaginal delivery of lactic acid: Relevance of formulation parameters to mucoadhesion and release mechanisms. AAPS PharmSciTech 2006, 7, E141–E147. [Google Scholar] [CrossRef] [Green Version]
- Bonferoni, M.C.; Sandri, G.; Rossi, S.; Ferrari, F.; Gibin, S.; Caramella, C. Chitosan citrate as multifunctional polymer for vaginal delivery. Evaluation of penetration enhancement and peptidase inhibition properties. Eur. J. Pharm. Sci. 2008, 33, 166–176. [Google Scholar] [CrossRef]
- Şenyiğit, Z.A.; Karavana, S.Y.; Eraç, B.; Gürsel, Ö.; Limoncu, M.H.; Baloğlu, E. Evaluation of chitosan based vaginal bioadhesive gel formulations for antifungal drugs. Acta Pharm. 2014, 64, 139–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demiröz, F.N.T.; Acartürk, F.; Erdoğan, D. Development of long-acting bioadhesive vaginal gels of oxybutynin: Formulation, in vitro and in vivo evaluations. Int. J. Pharm. 2013, 457, 25–39. [Google Scholar] [CrossRef]
- Cevher, E.; Sensoy, D.; Taha, M.A.M.; Araman, A. Effect of thiolated polymers to textural and mucoadhesive properties of vaginal gel Formulations prepared with polycarbophil and chitosan. AAPS PharmSciTech 2008, 9, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Bilensoy, E.; Rouf, M.A.; Vural, I.; Šen, M.; Hincal, A.A. Mucoadhesive, thermosensitive, prolonged-release vaginal gel for clotrimazole: β-cyclodextrin complex. AAPS PharmSciTech 2006, 7, E54–E60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aka-Any-Grah, A.; Bouchemal, K.; Koffi, A.; Agnely, F.; Zhang, M.; Djabourov, M.; Ponchel, G. Formulation of mucoadhesive vaginal hydrogels insensitive to dilution with vaginal fluids. Eur. J. Pharm. Biopharm. 2010, 76, 296–303. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Wei, G.; Lu, W. Effect of carrageenan on poloxamer-based in situ gel for vaginal use: Improved in vitro and in vivo sustained-release properties. Eur. J. Pharm. Sci. 2009, 37, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhong, L.; Wei, X.; Dou, W.; Chou, G.; Wang, Z. Baicalein and hydroxypropyl-γ-cyclodextrin complex in poloxamer thermal sensitive hydrogel for vaginal administration. Int. J. Pharm. 2013, 454, 125–134. [Google Scholar] [CrossRef]
- Deshkar, S.S.; Palve, V.K. Formulation and development of thermosensitive cyclodextrin-based in situ gel of voriconazole for vaginal delivery. J. Drug Deliv. Sci. Technol. 2019, 49, 277–285. [Google Scholar] [CrossRef]
- Rençber, S.; Karavana, S.Y.; Şenyiğit, Z.A.; Eraç, B.; Limoncu, M.H.; Baloğlu, E. Mucoadhesive in situ gel formulation for vaginal delivery of clotrimazole: Formulation, preparation, and in vitro/in vivo evaluation. Pharm. Dev. Technol. 2016, 22, 551–561. [Google Scholar] [CrossRef]
- Cevher, E.; Açma, A.; Sinani, G.; Aksu, B.; Zloh, M.; Mülazımoğlu, L. Bioadhesive tablets containing cyclodextrin complex of itraconazole for the treatment of vaginal candidiasis. Int. J. Biol. Macromol. 2014, 69, 124–136. [Google Scholar] [CrossRef]
- Gupta, N.V.; Natasha, S.; Getyala, A.; Bhat, R.S. Bioadhesive vaginal tablets containing spray dried microspheres loaded with clotrimazole for treatment of vaginal Candidiasis. Acta Pharm. 2013, 63, 359–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymańska, E.; Winnicka, K.; Amelian, A.; Cwalina, U. Vaginal chitosan tablets with clotrimazole-design and evaluation of mucoadhesive properties using porcine vaginal mucosa, mucin and gelatine. Chem. Pharm. Bull. 2014, 62, 160–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baloglu, E.; Senyıgıt, Z.A.; Karavana, S.Y.; Vetter, A.; Metın, D.Y.; Polat, S.H.; Guneri, T.; Bernkop-Schnurch, A. In vitro evaluation of mucoadhesive vaginal tablets of antifungal drugs prepared with thiolated polymer and development of a new dissolution technique for vaginal formulations. Chem. Pharm. Bull. 2011, 59, 952–958. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.A.; Ahmad, F.J.; Khan, Z.I.; Khar, R.K.; Ali, M. Development and evaluation of acid-buffering bioadhesive vaginal tablet for mixed vaginal infections. AAPS PharmSciTech 2007, 8, 229–236. [Google Scholar] [CrossRef]
- Ceschel, G.C.; Maffei, P.; Borgia, S.L.; Ronchi, C.; Rossi, S. Development of a mucoadhesive dosage form for vaginal administration. Drug Dev. Ind. Pharm. 2001, 27, 541–547. [Google Scholar] [CrossRef]
- McConville, C.; Friend, D.R.; Clark, M.R.; Malcolm, K. Preformulation and development of a once-daily sustained-release tenofovir vaginal tablet tablet containing a single excipient. J. Pharm. Sci. 2013, 102, 1859–1868. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Cazorla-Luna, R.; Martín-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.-D. Optimization of tenofovir release from mucoadhesive vaginal tablets by polymer combination to prevent sexual transmission of HIV. Carbohydr. Polym. 2018, 179, 305–316. [Google Scholar] [CrossRef] [PubMed]
- McConville, C.; Major, I.; Devlin, B.; Brimer, A. Development of a multi-layered vaginal tablet containing dapivirine, levonorgestrel and acyclovir for use as a multipurpose prevention technology. Eur. J. Pharm. Biopharm. 2016, 104, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, R.; Teller, R.S.; Mesquita, P.M.M.; Herold, B.C.; Kiser, P.F. Osmotic pump tablets for delivery of antiretrovirals to the vaginal mucosa. Antivir. Res. 2013, 100, 255–258. [Google Scholar] [CrossRef] [Green Version]
- Perioli, L.; Ambrogi, V.; Pagano, C.; Massetti, E.; Rossi, C. New solid mucoadhesive systems for benzydamine vaginal administration. Colloids Surf. B Biointerfaces 2011, 84, 413–420. [Google Scholar] [CrossRef]
- Ekin, M.; Yaşar, L.; Savan, K.; Temur, M.; Uhri, M.; Gencer, I.; Kıvanç, E. The comparison of hyaluronic acid vaginal tablets with estradiol vaginal tablets in the treatment of atrophic vaginitis: A randomized controlled trial. Arch. Gynecol. Obstet. 2010, 283, 539–543. [Google Scholar] [CrossRef]
- Baloǧlu, E.; Özyazici, M.; Hizarcioǧlu, S.Y.; Karavana, H.A. An in vitro investigation for vaginal bioadhesive formulations: Bioadhesive properties and swelling states of polymer mixtures. II Farmaco 2003, 58, 391–396. [Google Scholar] [CrossRef]
- Hiorth, M.; Nilsen, S.; Tho, I. Bioadhesive mini-tablets for vaginal drug delivery. Pharmaceutics 2014, 6, 494–511. Available online: https://pubmed.ncbi.nlm.nih.gov/25166286/ (accessed on 21 March 2021). [CrossRef] [PubMed] [Green Version]
- Baffoe, C.S.; Nguyen, N.; Boyd, P.; Wang, W.; Morris, M.; McConville, C. Disulfiram-loaded immediate and extended release vaginal tablets for the localised treatment of cervical cancer. J. Pharm. Pharmacol. 2015, 67, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, S.; Costa, P.; Silva, J.; Teixeira, P. Effects of processing and storage on pediococcus pentosaceus SB83 in vaginal formulations: Lyophilized powder and tablets. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Nowak, J.; Laffleur, F.; Bernkop-Schnürch, A. Preactivated hyaluronic acid: A potential mucoadhesive polymer for vaginal delivery. Int. J. Pharm. 2015, 478, 383–389. [Google Scholar] [CrossRef]
- Thurman, A.; Clark, M.; Hurlburt, J.; Doncel, G. Intravaginal rings as delivery systems for microbicides and multipurpose prevention technologies. Int. J. Women’s Health 2013, 5, 695–708. Available online: /pmc/articles/PMC3808127/ (accessed on 24 April 2021). [CrossRef] [Green Version]
- Rochira, M.; Miglietta, M.R.; Richardson, J.L.; Ferrari, L.; Beccaro, M.; Benedetti, L. Novel vaginal delivery systems for calcitonin. II. Preparation and characterization of HYAFF® microspheres containing calcitonin. Int. J. Pharm. 1996, 144, 19–26. [Google Scholar] [CrossRef]
- Pliszczak, D.; Bourgeois, S.; Bordes, C.; Valour, J.-P.; Mazoyer, M.-A.; Orecchioni, A.; Nakache, E.; Lanteri, P. Improvement of an encapsulation process for the preparation of pro- and prebiotics-loaded bioadhesive microparticles by using experimental design. Eur. J. Pharm. Sci. 2011, 44, 83–92. [Google Scholar] [CrossRef]
- Maestrelli, F.; Jug, M.; Cirri, M.; Kosalec, I.; Mura, P. Characterization and microbiological evaluation of chitosan-alginate microspheres for cefixime vaginal administration. Carbohydr. Polym. 2018, 192, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, C.; Agrahari, V.; Murowchick, J.B.; Oyler, N.A.; Youan, B.B.C. Spray drying tenofovir loaded mucoadhesive and pH-sensitive microspheres intended for HIV prevention. Antivir. Res. 2013, 97, 334–346. [Google Scholar] [CrossRef] [Green Version]
- Albertini, B.; Passerini, N.; Di Sabatino, M.; Vitali, B.; Brigidi, P.; Rodriguez, L. Polymer-lipid based mucoadhesive microspheres prepared by spray-congealing for the vaginal delivery of econazole nitrate. Eur. J. Pharm. Sci. 2009, 36, 591–601. [Google Scholar] [CrossRef]
- Santiago, G.L.; Verstraelen, H.; Poelvoorde, N.; De Corte, S.; Claeys, G.; Trog, M.; De Backer, E.; Saerens, B.; Vervaet, C.; De Boeck, F.; et al. A pilot study evaluating the safety of vaginal administration of a multi-particulate pellet formulation. Eur. J. Pharm. Biopharm. 2009, 73, 399–403. [Google Scholar] [CrossRef]
- Mehta, S.; De Beer, T.; Remon, J.P.; Vervaet, C. Effect of disintegrants on the properties of multiparticulate tablets comprising starch pellets and excipient granules. Int. J. Pharm. 2012, 422, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Hiorth, M.; Liereng, L.; Reinertsen, R.; Tho, I. Formulation of bioadhesive hexylaminolevulinate pellets intended for photodynamic therapy in the treatment of cervical cancer. Int. J. Pharm. 2013, 441, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Pinto Reis, C.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F.; Nanoencapsulation, I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 8–21. [Google Scholar] [CrossRef] [Green Version]
- Das Neves, J.; Sarmento, B. Precise engineering of dapivirine-loaded nanoparticles for the development of anti-HIV vaginal microbicides. Acta Biomater. 2015, 18, 77–87. [Google Scholar] [CrossRef]
- Gu, J.; Yang, S.; Ho, E.A. Biodegradable film for the targeted delivery of siRNA-loaded nanoparticles to vaginal immune cells. Mol. Pharm. 2015, 12, 2889–2903. [Google Scholar] [CrossRef]
- Yang, M.; Yu, T.; Wang, Y.Y.; Lai, S.K.; Zeng, Q.; Miao, B.; Hanes, J. Vaginal delivery of paclitaxel via nanoparticles with non-mucoadhesive surfaces suppresses cervical tumor growth. Adv. Healthc. Mater. 2014, 3, 1044–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, C.M.; Cardoso, J.F.; Perassoli, F.B.; Neto, A.S.D.O.; Pinto, L.M.; Marques, M.B.D.F.; Mussel, W.D.N.; Magalhães, J.; Moura, S.A.D.L.; Araújo, M.G.D.F.; et al. Amphotericin B-loaded eudragit RL100 nanoparticles coated with hyaluronic acid for the treatment of vulvovaginal candidiasis. Carbohydr. Polym. 2020, 230, 115608. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Reis, C.; Machado, A.; Barreiros, L.; Araújo, F.; Nunes, R.; Seabra, V.; Ferreira, D.; Segundo, M.; Sarmento, B.; das Neves, J. Nanoparticles-in-film for the combined vaginal delivery of anti-HIV microbicide drugs. J. Control. Release 2016, 243, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Basnet, P.; Acharya, G.; Škalko-Basnet, N. PEGylated liposomes for topical vaginal therapy improve delivery of interferon alpha. Eur. J. Pharm. Biopharm. 2017, 113, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Santos, S.S.; Lorenzoni, A.; Pegoraro, N.S.; Denardi, L.B.; Alves, S.H.; Schaffazick, S.R.; Cruz, L. Formulation and in vitro evaluation of coconut oil-core cationic nanocapsules intended for vaginal delivery of clotrimazole. Colloids Surf. B Biointerfaces 2014, 116, 270–276. [Google Scholar] [CrossRef]
- Varan, C.; Wickström, H.; Sandler, N.; Aktaş, Y.; Bilensoy, E. Inkjet printing of antiviral PCL nanoparticles and anticancer cyclodextrin inclusion complexes on bioadhesive film for cervical administration. Int. J. Pharm. 2017, 531, 701–713. [Google Scholar] [CrossRef]
- Machado, A.; Cunha-Reis, C.; Araújo, F.; Nunes, R.; Seabra, V.; Ferreira, D.; das Neves, J.; Sarmento, B. Development and in vivo safety assessment of tenofovir-loaded nanoparticles-in-film as a novel vaginal microbicide delivery system. Acta Biomater. 2016, 44, 332–340. [Google Scholar] [CrossRef]
- Sims, L.B.; Curtis, L.T.; Frieboes, H.B.; Steinbach-Rankins, J.M. Enhanced uptake and transport of PLGA-modified nanoparticles in cervical cancer. J. Nanobiotechnology 2016, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Maisel, K.; Reddy, M.; Xu, Q.; Chattopadhyay, S.; Cone, R.; Ensign, L.M.; Hanes, J. Nanoparticles coated with high molecular weight PEG penetrate mucus and provide uniform vaginal and colorectal distribution in vivo. Nanomedicine 2016, 11, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Lechanteur, A.; Furst, T.; Evrard, B.; Delvenne, P.; Piel, G.; Hubert, P. Promoting vaginal distribution of E7 and MCL-1 siRNA-silencing nanoparticles for cervical cancer treatment. Mol. Pharm. 2017, 14, 1706–1717. [Google Scholar] [CrossRef]
- Wang, X.; Fu, L.; Lin, W.; Zhang, W.; Pei, Q.; Zheng, X.; Liu, S.; Zhang, T.; Xie, Z. Vaginal delivery of mucus-penetrating organic nanoparticles for photothermal therapy against cervical intraepithelial neoplasia in mice. J. Mater. Chem. B 2019, 7, 4528–4537. [Google Scholar] [CrossRef]
- Frank, L.A.; Chaves, P.S.; D’Amore, C.M.; Contri, R.V.; Frank, A.G.; Beck, R.; Pohlmann, A.R.; Buffon, A.; Guterres, S.S. The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: Increasing penetration and adhesion of imiquimod in vaginal tissue. Eur. J. Pharm. Biopharm. 2017, 114, 202–212. [Google Scholar] [CrossRef]
- Lucena, P.A.; Nascimento, T.L.; Gaeti, M.P.N.; De Ávila, R.I.; Mendes, L.P.; Vieira, M.S.; Fabrini, D.; Amaral, A.C.; Lima, E.M. In vivo vaginal fungal load reduction after treatment with itraconazole-loaded polycaprolactone-nanoparticles. J. Biomed. Nanotechnol. 2018, 14, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Marciello, M.; Rossi, S.; Caramella, C.; Remuñán-López, C. Freeze-dried cylinders carrying chitosan nanoparticles for vaginal peptide delivery. Carbohydr. Polym. 2017, 170, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Vigani, B.; Puccio, A.; Bonferoni, M.C.; Sandri, G.; Ferrari, F. Chitosan ascorbate nanoparticles for the vaginal delivery of antibiotic drugs in atrophic vaginitis. Mar. Drugs 2017, 15, 319. [Google Scholar] [CrossRef] [Green Version]
- Lalan, M.S.; Patel, V.N.; Misra, A. Polymers in vaginal drug delivery: Recent advancements. In Applications of Polymers in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 281–303. [Google Scholar]
- Yoo, J.-W.; Dharmala, K.; Lee, C.H. The physicodynamic properties of mucoadhesive polymeric films developed as female controlled drug delivery system. Int. J. Pharm. 2006, 309, 139–145. [Google Scholar] [CrossRef]
- Machado, R.M.; Palmeira-De-Oliveira, A.; de Oliveira, J.M.; Palmeira-De-Oliveira, R. Vaginal films for drug delivery. J. Pharm. Sci. 2013, 102, 2069–2081. [Google Scholar] [CrossRef]
- Rohan, L.C.; Sassi, A.B. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009, 11, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Akil, A.; Devlin, B.; Cost, M.; Rohan, L.C. Increased dapivirine tissue accumulation through vaginal film codelivery of dapivirine and tenofovir. Mol. Pharm. 2014, 11, 1533–1541. [Google Scholar] [CrossRef]
- Traore, Y.L.; Fumakia, M.; Gu, J.; A Ho, E. Dynamic mechanical behaviour of nanoparticle loaded biodegradable PVA films for vaginal drug delivery. J. Biomater. Appl. 2018, 32, 1119–1126. [Google Scholar] [CrossRef]
- Cautela, M.P.; Moshe, H.; Sosnik, A.; Sarmento, B.; Das Neves, J. Composite films for vaginal delivery of tenofovir disoproxil fumarate and emtricitabine. J. Pharm. Biopharm. 2019, 138, 3–10. [Google Scholar] [CrossRef]
- Vartak, R.; Patki, M.; Menon, S.; Jablonski, J.; Mediouni, S.; Fu, Y.; Valente, S.T.; Billack, B.; Patel, K. β-cyclodextrin polymer/Soluplus® encapsulated Ebselen ternary complex (EβpolySol) as a potential therapy for vaginal candidiasis and pre-exposure prophylactic for HIV. Int. J. Pharm. 2020, 589, 119863. [Google Scholar] [CrossRef]
- Politch, J.A.; Cu-Uvin, S.; Moench, T.R.; Tashima, K.T.; Marathe, J.G.; Guthrie, K.M.; Cabral, H.; Nyhuis, T.; Brennan, M.; Zeitlin, L.; et al. Safety, acceptability, and pharmacokinetics of a monoclonal antibody-based vaginal multipurpose prevention film (MB66): A Phase I randomized trial. PLoS Med. 2021, 18, e1003495. [Google Scholar] [CrossRef]
- Ham, A.S.; Rohan, L.C.; Boczar, A.; Yang, L.; Buckheit, K.W.; Buckheit, R.W. Vaginal film drug delivery of the pyrimidinedione IQP-0528 for the prevention of HIV infection. Pharm. Res. 2012, 29, 1897–1907. [Google Scholar] [CrossRef] [Green Version]
- Neurath, A.R.; Strick, N.; Li, Y.-Y. Water dispersible microbicidal cellulose acetate phthalate film. BMC Infect. Dis. 2003, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Kumar, L.; Reddy, M.S.; Shirodkar, R.K.; Pai, G.K.; Krishna, V.T.; Verma, R. Preparation and characterisation of fluconazole vaginal films for the treatment of vaginal Candidiasis. Indian J. Pharm. Sci. 2013, 75, 585–590. [Google Scholar]
- Gahlot, N.; Maheshwari, R.K. Formulation and development of vaginal films of poorly water soluble drug, metronidazole, using mixed solvency concept and their evaluations. J. Drug Deliv. Ther. 2018, 8, 41–48. [Google Scholar] [CrossRef]
- Zhang, W.; Parniak, M.A.; Sarafianos, S.G.; Cost, M.R.; Rohan, L.C. Development of a vaginal delivery film containing EFdA, a novel anti-HIV nucleoside reverse transcriptase inhibitor. Int. J. Pharm. 2014, 461, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Gong, T.; Zhang, W.; Parniak, M.A.; Graebing, P.W.; Moncla, B.; Gupta, P.; Empey, K.M.; Rohan, L.C. Preformulation and Vaginal Film Formulation Development of Microbicide Drug Candidate CSIC for HIV Prevention. J. Pharm. Innov. 2017, 12, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, M.; Shi, Y.; Gong, T.; Dezzutti, C.S.; Moncla, B.; Sarafianos, S.G.; Parniak, M.A.; Rohan, L.C. Vaginal microbicide film combinations of two reverse transcriptase inhibitors, EFdA and CSIC, for the prevention of HIV-1 sexual transmission. Pharm. Res. 2015, 32, 2960–2972. [Google Scholar] [CrossRef] [Green Version]
- Akil, A.; Parniak, M.A.; Dezzuitti, C.S.; Moncla, B.J.; Cost, M.R.; Li, M.; Rohan, L.C. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv. Transl. Res. 2011, 1, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.-W.; Acharya, G.; Lee, C.H. In vivo evaluation of vaginal films for mucosal delivery of nitric oxide. Biomaterials 2009, 30, 3978–3985. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bedoya, L.-M.; Peña, J.; Veiga, M.-D. Development of mucoadhesive vaginal films based on HPMC and zein as novel formulations to prevent sexual transmission of HIV. Int. J. Pharm. 2019, 570, 118643. [Google Scholar] [CrossRef]
- Gyotoku, T.; Aurelian, L.; Neurath, A.R. Cellulose acetate phthalate (CAP): An “inactive” pharmaceutical excipient with antiviral activity in the mouse model of genital herpesvirus infection. Antivir. Chem. Chemother. 1999, 10, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Zhao, Q.; Wallace, G.; Liu, S.; He, Y.; Shattock, R.; Neurath, A.R.; Jiang, B.S. Cellulose acetate 1,2-benzenedicarboxylate inhibits infection by cell-free and cell-associated primary HIV-1 isolates. AIDS Res. Hum. Retrovir. 2006, 22, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Regev, G.; Patel, S.K.; Moncla, B.J.; Twist, J.; Devlin, B.; Rohan, L.C. Novel Application of Hot Melt Extrusion for the Manufacturing of Vaginal Films Containing Microbicide Candidate Dapivirine. AAPS PharmSciTech 2019, 20, 239. [Google Scholar] [CrossRef]
- Machado, R.S.M.; Tomás, M.; Palmeira-De-Oliveira, A.; de Oliveira, J.M.; Palmeira-De-Oliveira, R. The vaginal sheet: An innovative form of vaginal film for the treatment of vaginal infections. Drug Dev. Ind. Pharm. 2020, 46, 135–145. [Google Scholar] [CrossRef]
- Garg, S.; Goldman, D.; Krumme, M.; Rohan, L.C.; Smoot, S.; Friend, D.R. Advances in development, scale-up and manufacturing of microbicide gels, films, and tablets. Antivir. Res. 2010, 88, S19–S29. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Cazorla-Luna, R.; Martín-Illana, A.; Galante, J.; Ruiz-Caro, R.; das Neves, J.; Veiga, M.-D. Design, fabrication and characterisation of drug-loaded vaginal films: State-of-the-art. J. Control. Release 2020, 327, 477–499. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Tambwekar, K.R.; Vermani, K.; Kandarapu, R.; Garg, A.; Waller, D.P.; Zaneveld, L.J. Development pharmaceutics of microbicide formulations. Part II: Formulation, evaluation, and challenges. AIDS Patient Care STDs 2003, 17, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Drumond, N.; van Riet-Nales, D.A.; Karapinar-Çarkit, F.; Stegemann, S. Patients’ appropriateness, acceptability, usability and preferences for pharmaceutical preparations: Results from a literature review on clinical evidence. Int. J. Pharm. 2017, 521, 294–305. [Google Scholar] [CrossRef]
- Ghosal, K.; Ranjan, A.; Bhowmik, B.B. A novel vaginal drug delivery system: Anti-HIV bioadhesive film containing abacavir. J. Mater. Sci. Mater. Med. 2014, 25, 1679–1689. [Google Scholar] [CrossRef]
- Bassi, P.; Kaur, G. Bioadhesive vaginal drug delivery of nystatin using a derivatized polymer: Development and characterization. Eur. J. Pharm. Biopharm. 2015, 96, 173–184. [Google Scholar] [CrossRef]
- Mishra, R.; Soni, K.; Mehta, T. Mucoadhesive vaginal film of fluconazole using cross-linked chitosan and pectin: In vitro and in vivo study. J. Therm. Anal. Calorim. 2017, 130, 1683–1695. [Google Scholar] [CrossRef]
- Dolci, L.S.; Albertini, B.; Di Filippo, M.F.; Bonvicini, F.; Passerini, N.; Panzavolta, S. Development and in vitro evaluation of mucoadhesive gelatin films for the vaginal delivery of econazole. Int. J. Pharm. 2020, 591, 119979. [Google Scholar] [CrossRef]
- Frankman, O.; Raabe, N.; A Ingemansson, C. Clinical Evaluation of C-Film, a Vaginal Contraceptive. J. Int. Med Res. 1975, 3, 292–296. [Google Scholar] [CrossRef]
- Hynes, J.S.; Sales, J.M.; Sheth, A.N.; Lathrop, E.; Haddad, L.B. Interest in multipurpose prevention technologies to prevent HIV/STIs and unintended pregnancy among young women in the United States. Contraception 2018, 97, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Schreier, J.; Sales, J.; Sheth, A.; Lathrop, E.; Haddad, L. Interest in multipurpose prevention technologies for protection against unintended pregnancy, human immunodeficiency virus and other sexually transmitted infections among women in the united states. Am. J. Obstet. Gynecol. 2017, 217, 731. [Google Scholar] [CrossRef]
- Fernandes, T.; Baxi, K.; Sawarkar, S.; Sarmento, B.; das Neves, J. Vaginal multipurpose prevention technologies: Promising approaches for enhancing women’s sexual and reproductive health. Expert Opin. Drug Deliv. 2020, 17, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.; Repka, S.; Mcginity, J. Bioadhesive Hot-Melt Extruded Film for Topical and Mucosal Adhesion Applications and Drug Delivery and Process for Preparation Thereof. U.S. Patent US6375963B1, 23 April 2002. [Google Scholar]
- Leon, T.; Gabel, P. Dissolvable Vaginal Deodorizing Films and Methods of Vaginal Deodorizing Utilizing Pliable Dissolvable Film. WO2004103232A1, 2 December 2004. [Google Scholar]
- Maniar, M.; Parandoosh, S. Ph-Responsive Film for Intravaginal Delivery of a Beneficial Agent. US20060018951A1, 4 April 2005. [Google Scholar]
- Yang, J.T.; Gao, H.M. Lactobacillus Drug Film. CN101199555A, 15 December 2006. [Google Scholar]
- Staab, R.J. Methods for Delivery of Medication Using a Dissolvable Device. US20130136784A1, 16 October 2009. [Google Scholar]
- Notario-Pérez, F.; Galante, J.; Martín-Illana, A.; Cazorla-Luna, R.; Sarmento, B.; Ruiz-Caro, R.; das Neves, J.; Veiga, M.D. Development of pH-sensitive vaginal films based on methacrylate copolymers for topical HIV-1 pre-exposure prophylaxis. Acta Biomater. 2021, 121, 316–327. [Google Scholar] [CrossRef]
- Griesser, J.; Hetényi, G.; Bernkop-Schnürch, A. Thiolated hyaluronic acid as versatile mucoadhesive polymer: From the chemistry behind to product developments-What are the capabilities? Polymers 2018, 10, 243. Available online: www.mdpi.com/journal/polymers (accessed on 20 May 2021). [CrossRef] [Green Version]
- Cook, M.T.; Brown, M. Polymeric gels for intravaginal drug delivery. J. Control. Release 2018, 270, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Borin, M.T. Systemic absorption of clindamycin following intra vaginal application of clindamycin phosphate 1% cream. J. Clin. Pharmacol. 1990, 30, 33–38. Available online: https://accp1.onlinelibrary.wiley.com/doi/full/10.1002/j.1552-4604.1990.tb03435.x (accessed on 20 May 2021). [CrossRef]
- Gong, E.; Matthews, B.; McCarthy, T.; Chu, J.; Holan, G.; Raff, J.; Sacks, S. Evaluation of dendrimer SPL7013, a lead microbicide candidate against herpes simplex viruses. Antivir. Res. 2005, 68, 139–146. [Google Scholar] [CrossRef]
- Friedl, H.E.; Dünnhaupt, S.; Waldner, C.; Bernkop-Schnürch, A. Preactivated thiomers for vaginal drug delivery vehicles. Biomaterials 2013, 34, 7811–7818. [Google Scholar] [CrossRef]
- Podaralla, S.; Alt, C.; Shankar, G.N. Formulation development and evaluation of innovative two-polymer (SR-2P) bioadhesive vaginal gel. AAPS PharmSciTech 2014, 15, 928–938. [Google Scholar] [CrossRef] [Green Version]
- D’Cruz, O.J.; Uckun, F.M. Gel-microemulsions as vaginal spermicides and intravaginal drug delivery vehicles. Contraception 2001, 64, 113–123. [Google Scholar] [CrossRef]
- Kejdušová, M.; Vysloužil, J.; Kubová, K.; Celer, V.; Krásna, M.; Pechová, A.; Vyskočilová, V.; Košťál, V. Antimicrobial properties of microparticles based on carmellose cross-linked by Cu2+ ions. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pradines, B.; Bories, C.; Vauthier, C.; Ponchel, G.; Loiseau, P.M.; Bouchemal, K. Drug-free chitosan coated poly(isobutylcyanoacrylate) nanoparticles are active against Trichomonas vaginalis and non-toxic towards pig vaginal mucosa. Pharm. Res. 2014, 32, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Luganini, A.; Nicoletto, S.F.; Pizzuto, L.; Pirri, G.; Giuliani, A.; Landolfo, S.; Gribaudo, G. Inhibition of herpes simplex virus type 1 and type 2 infections by peptide-derivatized dendrimers. Agents Chemother. 2011, 55, 3231–3239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navath, R.S.; Menjoge, A.R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Injectable PAMAM dendrimer-PEG hydrogels for the treatment of genital infections: Formulation and in vitro and in vivo evaluation. Mol. Pharm. 2011, 8, 1209–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.T.; Shin, B.K.; Garripelli, V.K.; Kim, J.K.; Davaa, E.; Jo, S.; Park, J.S. A thermosensitive vaginal gel formulation with HPγCD for the pH-dependent release and solubilization of amphotericin B. Eur. J. Pharm. Sci. 2010, 41, 399–406. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Salmazi, R.; Bernegossi, J.; Ramos, M.A.D.S.; Bauab, T.; Chorilli, M. A Curcumin-loaded liquid crystal precursor mucoadhesive system for the treatment of vaginal candidiasis. Int. J. Nanomed. 2015, 10, 4815–4824. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Sun, K.; Mu, H.; Tang, M.; Liang, R.; Wang, A.; Liu, W. pH and temperature dual-sensitive liposome gel based on novel cleavable mPEG-Hz-CHEMS polymeric vaginal delivery system. Int. J. Nanomed. 2012, 7, 2621–2630. [Google Scholar] [CrossRef] [Green Version]
- Blum, J.S.; Weller, C.E.; Booth, C.J.; Babar, I.A.; Liang, X.; Slack, F.; Saltzman, W.M. Prevention of K-Ras- and Pten-mediated intravaginal tumors by treatment with camptothecin-loaded PLGA nanoparticles. Drug Deliv. Transl. Res. 2011, 1, 383–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigucci, F.; Abruzzo, A.; Vitali, B.; Saladini, B.; Cerchiara, T.; Gallucci, M.C.; Luppi, B. Vaginal inserts based on chitosan and carboxymethylcellulose complexes for local delivery of chlorhexidine: Preparation, characterization and antimicrobial activity. Int. J. Pharm. 2015, 478, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Bigucci, F.; Cerchiara, T.; Saladini, B.; Gallucci, M.; Cruciani, F.; Vitali, B.; Luppi, B. Chitosan/alginate complexes for vaginal delivery of chlorhexidine digluconate. Carbohydr. Polym. 2013, 91, 651–658. [Google Scholar] [CrossRef]
- Zong, S.; Wang, X.; Yang, Y.; Wu, W.; Li, H.; Ma, Y.; Lin, W.; Sun, T.; Huang, Y.; Xie, Z.; et al. The use of cisplatin-loaded mucoadhesive nanofibers for local chemotherapy of cervical cancers in mice. Eur. J. Pharm. Biopharm. 2015, 93, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Dobaria, N.; Mashru, R. Design and in vitro evaluation of a novel bioadhesive vaginal drug delivery system for clindamycin phosphate. Pharm. Dev. Technol. 2009, 15, 405–414. [Google Scholar] [CrossRef]
- Chang, J.Y.; Oh, Y.-K.; Choi, H.-G.; Kim, Y.B.; Kim, C.-K. Rheological evaluation of thermosensitive and mucoadhesive vaginal gels in physiological conditions. Int. J. Pharm. 2002, 241, 155–163. [Google Scholar] [CrossRef]
- Mishra, R.; Joshi, P.; Mehta, T. Formulation, development and characterization of mucoadhesive film for treatment of vaginal candidiasis. Int. J. Pharm. Investig. 2016, 6, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Cu, Y.; Booth, C.J.; Saltzman, W.M. In vivo distribution of surface-modified PLGA nanoparticles following intravaginal delivery. J. Control. Release 2011, 156, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Das Neves, J.; Araújo, F.; Andrade, F.; Amiji, M.; Bahia, M.F.; Sarmento, B. Biodistribution and pharmacokinetics of Dapivirine-loaded nanoparticles after vaginal delivery in mice. Pharm. Res. 2014, 31, 1834–1845. [Google Scholar] [CrossRef]
- Ensign, L.M.; Hoen, T.E.; Maisel, K.; Cone, R.A.; Hanes, J.S. Enhanced vaginal drug delivery through the use of hypotonic formulations that induce fluid uptake. Biomaterials 2013, 34, 6922–6929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parodi, B.; Russo, E.; Caviglioli, G.; Baldassari, S.; Gaglianone, N.; Schito, A.M.; Cafaggi, S. A chitosan lactate/poloxamer 407-based matrix containing Eudragit RS microparticles for vaginal delivery of econazole: Design and in vitro evaluation. Drug Dev. Ind. Pharm. 2013, 39, 1911–1920. [Google Scholar] [CrossRef]
- Derby, N.; Lal, M.; Aravantinou, M.; Kizima, L.; Barnable, P.; Rodriguez, A.; Lai, M.; Wesenberg, A.; Ugaonkar, S.; Levendosky, K.; et al. Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Zhang, J.; Martin, A.; Kelley, K.; McNicholl, J.M.; Buckheit, R.W., Jr.; Smith, J.M.; Ham, A.S. Safety and pharmacokinetics of quick-dissolving polymeric vaginal films delivering the antiretroviral IQP-0528 for preexposure prophylaxis. Antimicrob. Agents Chemother. 2016, 60, 4140–4150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahalingam, A.; Smith, E.; Fabian, J.; Damian, F.R.; Peters, J.J.; Clark, M.R.; Friend, D.R.; Katz, D.F.; Kiser, P.F. Design of a semisolid vaginal microbicide gel by relating composition to properties and performance. Pharm. Res. 2010, 27, 2478–2491. [Google Scholar] [CrossRef]
- Dobaria, N.B.; Badhan, A.C.; Mashru, R.C. A novel itraconazole bioadhesive film for vaginal delivery: Design, optimization, and physicodynamic characterization. AAPS PharmSciTech 2009, 10, 951–959. [Google Scholar] [CrossRef] [Green Version]
- Karavana, S.Y.; Rençbe, S.; Şenyiğit, Z.A.; Baloğlu, E. A New In-Situ Gel Formulation of Itraconazole for Vaginal Administration. Pharmacol. Pharm. 2012, 3, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Mirza, M.A.; Ahmad, S.; Mallick, N.; Manzoor, N.; Talegaonkar, S.; Iqbal, Z. Development of a novel synergistic thermosensitive gel for vaginal candidiasis: An in vitro, in vivo evaluation. Colloids Surf. B Biointerfaces 2013, 103, 275–282. [Google Scholar] [CrossRef]
- Małolepsza-Jarmołowska, K. Studies on gynecological hydrophilic lactic acid preparations part 8: Use of chitosan as lactic acid carrier in intravaginal tablets. Acta Pol. Pharm. Drug Res. 2007, 64, 69–72. [Google Scholar]
- Bouchemal, K.; Frelichowska, J.; Martin, L.; Moal, V.L.-L.; Le Grand, R.; Dereuddre-Bosquet, N.; Djabourov, M.; Aka-Any-Grah, A.; Koffi, A.; Ponchel, G. Note on the formulation of thermosensitive and mucoadhesive vaginal hydrogels containing the miniCD4 M48U1 as anti-HIV-1 microbicide. Int. J. Pharm. 2013, 454, 649–652. [Google Scholar] [CrossRef]
- Forbes, C.J.; McCoy, C.F.; Murphy, D.J.; Woolfson, A.D.; Moore, J.P.; Evans, A.; Shattock, R.J.; Malcolm, R.K. Modified silicone elastomer vaginal gels for sustained release of antiretroviral HIV microbicides. J. Pharm. Sci. 2014, 103, 1422–1432. [Google Scholar] [CrossRef] [Green Version]
- Jalil, A.; Asim, M.H.; Le, N.-M.N.; Laffleur, F.; Matuszczak, B.; Tribus, M.; Bernkop–Schnürch, A. S-protected gellan gum: Decisive approach towards mucoadhesive antimicrobial vaginal films. Int. J. Biol. Macromol. 2019, 130, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Perioli, L.; Ambrogi, V.; Venezia, L.; Pagano, C.; Ricci, M.; Rossi, C. Chitosan and a modified chitosan as agents to improve performances of mucoadhesive vaginal gels. Colloids Surf. B Biointerfaces 2008, 66, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.-S.A.; Ismail, S.; Fetih, G.; Shaaban, O.; Hassanein, K.; Ellah, N.A. Development and characterization of thermosensitive pluronic-based metronidazole in situ gelling formulations for vaginal application. Acta Pharm. 2012, 62, 59–70. [Google Scholar] [CrossRef]
- El-Kamel, A.; Sokar, M.; Naggar, V.; Al Gamal, S. Chitosan and sodium alginate-based bioadhesive vaginal tablets. Vol. 4, AAPS PharmSci. AAPS PharmSci 2002, 4, 224–230. [Google Scholar] [CrossRef]
- Perioli, L.; Ambrogi, V.; Pagano, C.; Scuota, S.; Rossi, C. FG90 chitosan as a new polymer for metronidazole mucoadhesive tablets for vaginal administration. Int. J. Pharm. 2009, 377, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Planinšek, O.; Škalko-Basnet, N.; Tho, I. Tablets of pre-liposomes govern in situ formation of liposomes: Concept and potential of the novel drug delivery system. Eur. J. Pharm. Biopharm. 2014, 88, 443–454. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Samuel, P.; Uckun, F.M. Conceival, a novel noncontraceptive vaginal vehicle for lipophilic microbicides. AAPS PharmSciTech 2005, 6, E56–E64. [Google Scholar] [CrossRef] [Green Version]
- Friedland, B.A.; Hoesley, C.J.; Plagianos, M.; Hoskin, E.; Zhang, S.; Teleshova, N.; Alami, M.; Novak, L.; Kleinbeck, K.R.; Katzen, L.L.; et al. First-in-human trial of MIV-150 and zinc acetate coformulated in a carrageenan gel: Safety, pharmacokinetics, acceptability, adherence, and pharmacodynamics. J. Acquir. Immune Defic. Syndr. 2016, 73, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Kenney, J.; Singer, R.; Derby, N.; Aravantinou, M.; Abraham, C.J.; Menon, R.; Seidor, S.; Zhang, S.; Gettie, A.; Blanchard, J.F.; et al. A single dose of a MIV-150/zinc acetate gel provides 24h of protection against vaginal simian human immunodeficiency virus reverse transcriptase infection, with more limited protection rectally 8-24h after gel use. AIDS Res. Hum. Retrovir. 2012, 28, 1476–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, C.; Woodrow, K.A. Electrospun solid dispersions of maraviroc for rapid intravaginal preexposure prophylaxis of HIV. Antimicrob. Agents Chemother. 2014, 58, 4855–4865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevher, E.; Şensoy, D.; Zloh, M.; Mülazımoğlu, L. Preparation and characterisation of natamycin: γ-cyclodextrin inclusion complex and its evaluation in vaginal mucoadhesive formulations. J. Pharm. Sci. 2008, 97, 4319–4335. [Google Scholar] [CrossRef] [PubMed]
- Hombach, J.; Palmberger, T.F.; Bernkop-Schnürch, A. Development and in vitro evaluation of a mucoadhesive vaginal delivery system for nystatin. J. Pharm. Sci. 2009, 98, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Martín-Villena, M.; Fernández-Campos, F.; Calpena-Campmany, A.; DE Februaryrer, N.B.; Ruiz-Martínez, M.; Clares-Naveros, B. Novel microparticulate systems for the vaginal delivery of nystatin: Development and characterization. Carbohydr. Polym. 2013, 94, 1–11. [Google Scholar] [CrossRef]
- Kuo-Haller, P.; Cu, Y.; Blum, J.; Appleton, J.A.; Saltzman, W.M. Vaccine delivery by polymeric vehicles in the mouse reproductive tract induces sustained local and systemic immunity. Mol. Pharm. 2010, 7, 1585–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talwar, G.; Dar, S.A.; Rai, M.K.; Reddy, K.; Mitra, D.; Kulkarni, S.V.; Doncel, G.F.; Buck, C.; Schiller, J.T.; Muralidhar, S.; et al. A novel polyherbal microbicide with inhibitory effect on bacterial, fungal and viral genital pathogens. Int. J. Antimicrob. Agents 2008, 32, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Almomen, A.; Cho, S.; Yang, C.-H.; Aliyah, A.; Jarboe, E.A.; Peterson, C.M.; Huh, K.M.; Janát-Amsbury, M.M. Thermosensitive progesterone hydrogel: A safe and effective new formulation for vaginal application. Pharm. Res. 2015, 32, 2266–2279. [Google Scholar] [CrossRef] [Green Version]
- Campaña-Seoane, M.; Peleteiro, A.; Laguna, R.; Otero-Espinar, F.J. Bioadhesive emulsions for control release of progesterone resistant to vaginal fluids clearance. Int. J. Pharm. 2014, 477, 495–505. [Google Scholar] [CrossRef]
- Tasdighi, E.; Azar, Z.J.; Mortazavi, S.A. Development and In-vitro evaluation of a contraceptive vagino-adhesive propranolol hydrochloride gel. Iran. J. Pharm. Res. 2012, 11, 13–26. [Google Scholar]
- Teller, R.S.; Rastogi, R.; Johnson, T.J.; Blair, M.J.; Hitchcock, R.W.; Kiser, P.F. Intravaginal flux controlled pump for sustained release of macromolecules. Pharm. Res. 2014, 31, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Shibata, A.; Goede, M.; Sanford, B.; La Bruzzo, K.; Belshan, M.; Destache, C.J. Development and evaluation of a thermosensitive vaginal gel containing raltegravir+efavirenz loaded nanoparticles for HIV prophylaxis. Antivir. Res. 2012, 96, 430–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, E.; Chen, Y.; Dash, A.; Sayre, C.; Davies, N.; Gu, K.; Yang, S. Novel intravaginal nanomedicine for the targeted delivery of saquinavir to CD+ immune cells. Int. J. Nanomed. 2013, 8, 2847–2858. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Patel, J. Mucoadhesive microemulsion based prolonged release vaginal gel for anti-fungal drug. J. Pharm. Tech. Res. 2012, 2. [Google Scholar]
- Patel, A. Development and evaluation of mucoadhesive vaginal tablet of sertaconazole for vaginal candidiasis. Int. J. PharmTech Res. 2011, 3, 2175–2182. [Google Scholar]
- Mumper, R.J.; Bell, M.A.; Worthen, D.R.; Cone, R.A.; Lewis, G.R.; Paull, J.; Moench, T.R. Formulating a sulfonated antiviral dendrimer in a vaginal microbicidal gel having dual mechanisms of action. Drug Dev. Ind. Pharm. 2009, 35, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Hunter, S.K.; Andracki, M.E.; Krieg, A.M. Biodegradable microspheres containing group B streptococcus vaccine: Immune response in mice. Am. J. Obstet. Gynecol. 2001, 185, 1174–1179. [Google Scholar] [CrossRef]
- Mas, N.; Galiana, I.; Hurtado, S.; Mondragón, L.; Bernardos, A.; Sancenón, F.; Murguía, J.R. Enhanced antifungal efficacy of tebuconazole using gated pH-driven mesoporous nanoparticles. Int. J. Nanomed. 2014, 9, 2597–2606. [Google Scholar]
- Cazorla-Luna, R.; Notario-Pérez, F.; Martín-Illana, A.; Bedoya, L.-M.; Tamayo, A.; Rubio, J.; Ruiz-Caro, R.; Veiga, M.-D. Development and in vitro/ ex vivo characterization of vaginal mucoadhesive bilayer films based on ethylcellulose and biopolymers for vaginal sustained release of tenofovir. Biomacromolecules 2020, 21, 2309–2319. [Google Scholar] [CrossRef]
- Meng, J.; Sturgis, T.F.; Youan, B.-B.C. Engineering tenofovir loaded chitosan nanoparticles to maximize microbicide mucoadhesion. Eur. J. Pharm. Sci. 2011, 44, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Zhang, T.; Agrahari, V.; Ezoulin, M.J.; Youan, B.-B.C. Comparative biophysical properties of tenofovir-loaded, thiolated and nonthiolated chitosan nanoparticles intended for HIV prevention. Nanomedicine 2014, 9, 1595–1612. [Google Scholar] [CrossRef] [Green Version]
- Agrahari, V.; Zhang, C.; Zhang, T.; Li, W.; Gounev, T.K.; Oyler, N.A.; Youan, B.-B.C. Hyaluronidase-sensitive nanoparticle templates for triggered release of HIV/AIDS microbicide in vitro. AAPS J. 2013, 16, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alukda, D.; Sturgis, T.; Youan, B.C. Formulation of tenofovir-loaded functionalized solid lipid nanoparticles intended for HIV prevention. J. Pharm. Sci. 2011, 100, 3345–3356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, M.R.; Peet, M.M.; Davis, S.; Doncel, G.F.; Friend, D.R. Evaluation of rapidly disintegrating vaginal tablets of tenofovir, emtricitabine and their combination for HIV-1 prevention. Pharmaceutics 2014, 6, 616–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepúlveda-Crespo, D.; Serramía, M.J.; Sánchez-Rodríguez, J.; Lorente, R.; Gómez, R.; De La Mata, F.J.; Jimenez, J.L.; Muñoz-Fernández, M.Á. Broad-spectrum Anti-HIV-1 activity of anionic carbosilane dendrimers and synergy in combination with maraviroc and tenofovir as topical microbicide. AIDS Res. Hum. Retrovir. 2014, 30, A144. [Google Scholar] [CrossRef]
- Akil, A.; Agashe, H.; Dezzutti, C.S.; Moncla, B.J.; Hillier, S.L.; Devlin, B.; Shi, Y.; Uranker, K.; Rohan, L.C. Formulation and characterization of polymeric films containing combinations of antiretrovirals (ARVs) for HIV prevention. Pharm. Res. 2014, 32, 458–468. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Sturgis, T.F.; Youan, B.B.C. PH-responsive nanoparticles releasing tenofovir intended for the prevention of HIV transmission. Eur. J. Pharm. Biopharm. 2011, 79, 526–536. [Google Scholar] [CrossRef] [Green Version]
- Calvo, N.L.; Svetaz, L.A.; Alvarez, V.A.; Quiroga, A.D.; Lamas, M.C.; Leonardi, D. Chitosan-hydroxypropyl methylcellulose tioconazole films: A promising alternative dosage form for the treatment of vaginal candidiasis. Int. J. Pharm. 2019, 556, 181–191. [Google Scholar] [CrossRef]
- Grammen, C.; Mooter, G.V.D.; Appeltans, B.; Michiels, J.; Crucitti, T.; Ariën, K.K.; Augustyns, K.; Augustijns, P.; Brouwers, J. Development and characterization of a solid dispersion film for the vaginal application of the anti-HIV microbicide UAMC01398. Int. J. Pharm. 2014, 475, 238–244. [Google Scholar] [CrossRef]
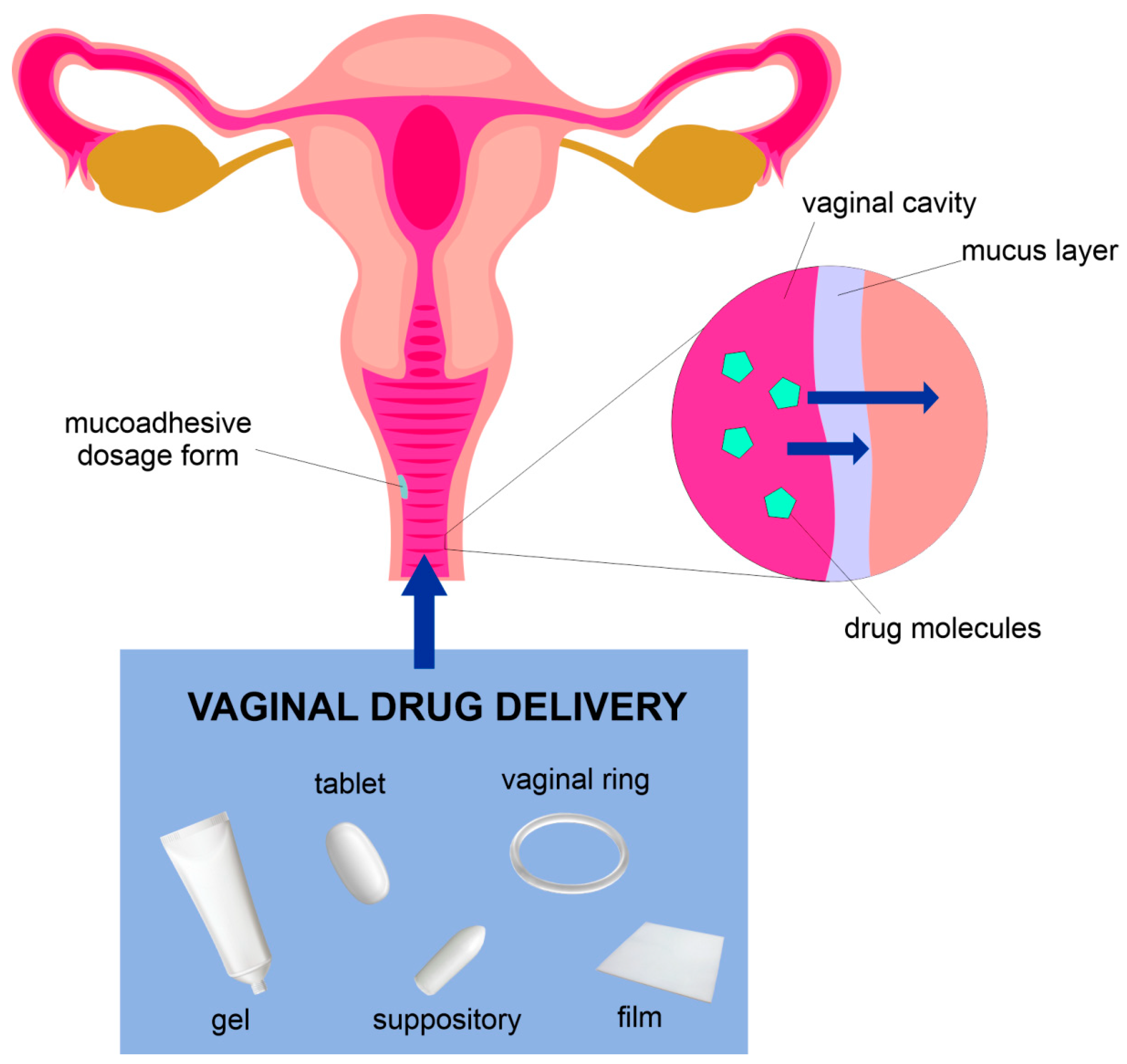





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osmałek, T.; Froelich, A.; Jadach, B.; Tatarek, A.; Gadziński, P.; Falana, A.; Gralińska, K.; Ekert, M.; Puri, V.; Wrotyńska-Barczyńska, J.; et al. Recent Advances in Polymer-Based Vaginal Drug Delivery Systems. Pharmaceutics 2021, 13, 884. https://doi.org/10.3390/pharmaceutics13060884
Osmałek T, Froelich A, Jadach B, Tatarek A, Gadziński P, Falana A, Gralińska K, Ekert M, Puri V, Wrotyńska-Barczyńska J, et al. Recent Advances in Polymer-Based Vaginal Drug Delivery Systems. Pharmaceutics. 2021; 13(6):884. https://doi.org/10.3390/pharmaceutics13060884
Chicago/Turabian StyleOsmałek, Tomasz, Anna Froelich, Barbara Jadach, Adam Tatarek, Piotr Gadziński, Aleksandra Falana, Kinga Gralińska, Michał Ekert, Vinam Puri, Joanna Wrotyńska-Barczyńska, and et al. 2021. "Recent Advances in Polymer-Based Vaginal Drug Delivery Systems" Pharmaceutics 13, no. 6: 884. https://doi.org/10.3390/pharmaceutics13060884
APA StyleOsmałek, T., Froelich, A., Jadach, B., Tatarek, A., Gadziński, P., Falana, A., Gralińska, K., Ekert, M., Puri, V., Wrotyńska-Barczyńska, J., & Michniak-Kohn, B. (2021). Recent Advances in Polymer-Based Vaginal Drug Delivery Systems. Pharmaceutics, 13(6), 884. https://doi.org/10.3390/pharmaceutics13060884