Development and Evaluation of a Human Skin Equivalent in a Semiautomatic Microfluidic Diffusion Chamber
Abstract
1. Introduction
2. Materials and Methods
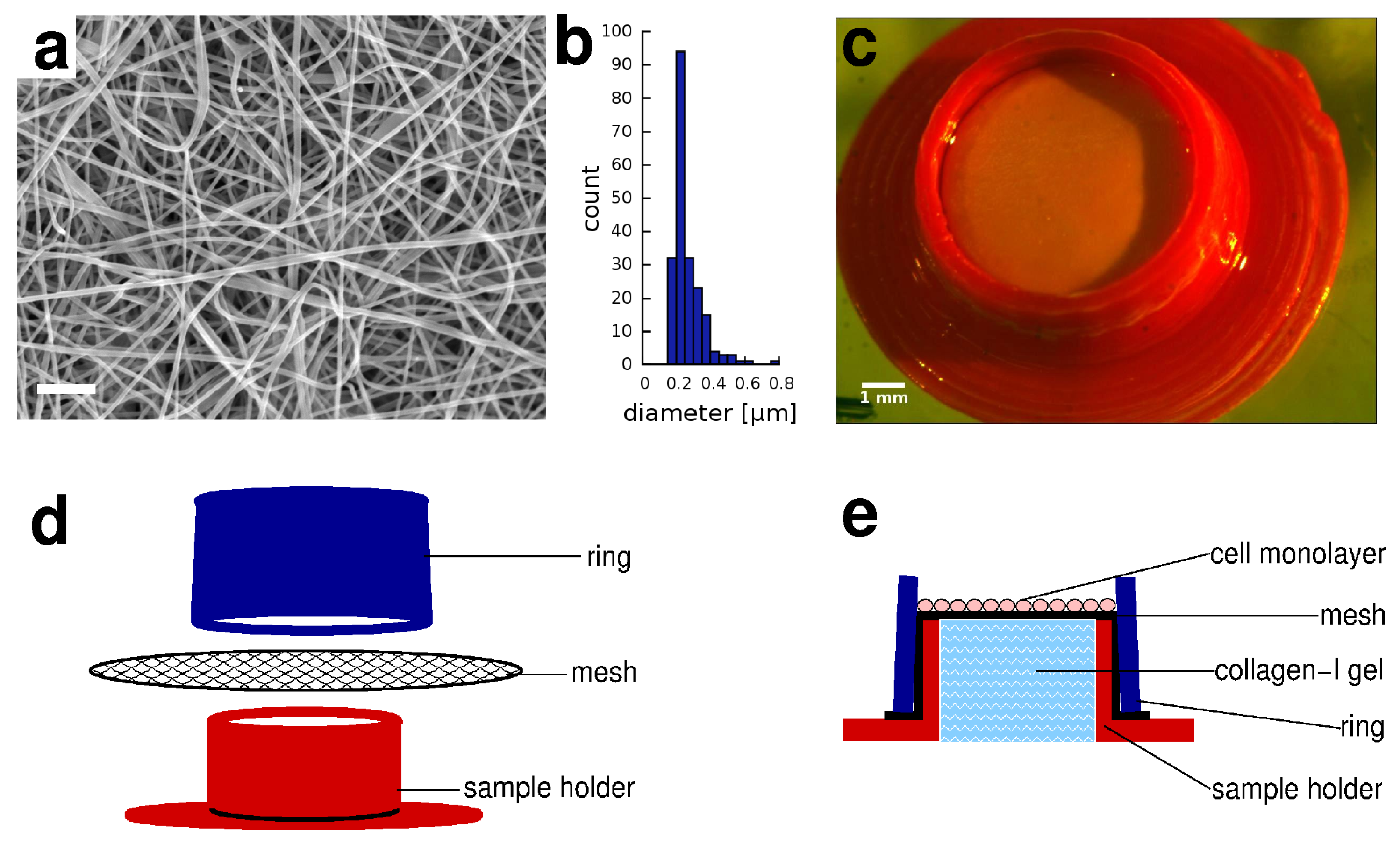
2.1. Electrospun Membranes
2.2. 3D Printed Sample Holders
2.3. Cell Culture
2.4. Cell Labeling and Viability
2.5. Histology
2.6. Microscopy
2.7. Human Skin Samples
2.8. Microfluidic Diffusion Cell Device
2.9. Spectrophotometry
2.10. Topical Formulation
3. Results
3.1. Skin Equivalent in a Sample Holder Device
3.2. Histology of Skin Equivalents
3.3. Transepithelial Transport Measurements
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Argoff, C.E. Topical analgesics in the management of acute and chronic pain. Mayo Clin. Proc. 2013, 88, 195–205. [Google Scholar] [CrossRef]
- Rasmussen, S.; Horkan, K.H.; Kotler, M. Pharmacokinetic Evaluation of Two Nicotine Patches in Smokers. Clin. Pharmacol. Drug Dev. 2018, 7, 506–512. [Google Scholar] [CrossRef]
- Arver, S.; Stief, C.; de la Rosette, J.; Jones, T.H.; Neijber, A.; Carrara, D. A new 2% testosterone gel formulation: A comparison with currently available topical preparations. Andrology 2018, 6, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Tamás, H.; Ambrus, R.; Szabóné, R.P. Investigation of permeability of intranasal formulations using Side-Bi-Side horizontal diffusion cell. Acta Pharm. Hung. 2015, 85, 19–28. [Google Scholar]
- Salmon, D.; Gilbert, E.; Gioia, B.; Haftek, M.; Pivot, C.; Verrier, B.; Pirot, F. New easy handling and sampling device for bioavailability screening of topical formulations. Eur. J. Dermatol. 2015, 25 (Suppl. 1), 23–29. [Google Scholar] [CrossRef]
- Ng, S.F.; Rouse, J.J.; Sanderson, F.D.; Meidan, V.; Eccleston, G.M. Validation of a static Franz diffusion cell system for in vitro permeation studies. AAPS PharmSciTech 2010, 11, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Addicks, W.J.; Flynn, G.L.; Weiner, N. Validation of a flow-through diffusion cell for use in transdermal research. Pharm. Res. 1987, 4, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Ponmozhi, J.; Dhinakaran, S.; Varga-Medveczky, Z.; Fónagy, K.; Bors, L.A.; Iván, K.; Erdő, F. Development of Skin-On-A-Chip Platforms for Different Utilizations: Factors to Be Considered. Micromachines 2021, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Lukács, B.; Bajza, Á.; Kocsis, D.; Csorba, A.; Antal, I.; Iván, K.; Laki, A.J.; Erdő, F. Skin-on-a-Chip Device for Ex Vivo Monitoring of Transdermal Delivery of Drugs-Design, Fabrication, and Testing. Pharmaceutics 2019, 11, 445. [Google Scholar] [CrossRef]
- Bajza, Á.; Kocsis, D.; Berezvai, O.; Laki, A.J.; Lukács, B.; Imre, T.; Iván, K.; Szabó, P.; Erdő, F. Verification of P-Glycoprotein Function at the Dermal Barrier in Diffusion Cells and Dynamic “Skin-On-A-Chip” Microfluidic Device. Pharmaceutics 2020, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Alberti, M.; Dancik, Y.; Sriram, G.; Wu, B.; Teo, Y.L.; Feng, Z.; Bigliardi-Qi, M.; Wu, R.G.; Wang, Z.P.; Bigliardi, P.L. Multi-chamber microfluidic platform for high-precision skin permeation testing. Lab Chip 2017, 17, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Thomas, L.; Fusco, A.; Kalaoglu-Altan, O.I.; Basnett, P.; Cinelli, P.; Clerck, K.D.; Roy, I.; Donnarumma, G.; Coltelli, M.B.; et al. Electrosprayed Chitin Nanofibril/Electrospun Polyhydroxyalkanoate Fiber Mesh as Functional Nonwoven for Skin Application. J. Funct. Biomater. 2020, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Tarassoli, S.P.; Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Whitaker, S.; Doak, S.; Whitaker, I.S. Skin tissue engineering using 3D bioprinting: An evolving research field. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 615–623. [Google Scholar] [CrossRef]
- EU Directive 76/768/EEC. Available online: https://ec.europa.eu/growth/sectors/cosmetics/legislation_en (accessed on 1 June 2021).
- OECD. Test Guideline 427: Skin Absorption: In Vivo Method; OECD: Paris, France, 2004. [Google Scholar]
- OECD. Test Guideline 428: Skin Absorption: In Vitro Method; OECD: Paris, France, 2004. [Google Scholar]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies; OECD Series on Testing and Assessment; OECD: Paris, France, 2004. [Google Scholar]
- Kielhorn, J. International Programme on Chemical Safety Dermal Absorption; Environmental Health Criteria; WHO: Geneva, Swizerland, 2006. [Google Scholar]
- U.S. EPA. Dermal Exposure Assessment: A Summary of EPA Approaches; EPA/600/R-07/040F; U.S. EPA: Washington, DC, USA, 2007. [Google Scholar]
- Van der Schueren, L.; De Schoenmaker, B.; Kalaoglu-Altan, O.I.; De Clerck, K. An alternative solvent system for the steady state electrospinning of polycaprolactone. Eur. Polym. J. 2011, 47, 1256–1263. [Google Scholar] [CrossRef]
- Leach, M.K.; Feng, Z.Q.; Tuck, S.J.; Corey, J.M. Electrospinning fundamentals: Optimizing solution and apparatus parameters. J. Vis. Exp. 2011. [Google Scholar] [CrossRef]
- Alonso, C.; Martí, M.; Barba, C.; Lis, M.; Rubio, L.; Coderch, L. Skin penetration and antioxidant effect of cosmeto-textiles with gallic acid. J. Photochem. Photobiol. B 2016, 156, 50–55. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Hutmacher, D. Design, fabrication and characterization of PCL electrospun scaffolds. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef]
- Bölgen, N.; Menceloğlu, Y.Z.; Acatay, K.; Vargel, I.; Pişkin, E. In vitro and in vivo degradation of non-woven materials made of poly(epsilon-caprolactone) nanofibers prepared by electrospinning under different conditions. J. Biomater. Sci. Polym. Ed. 2005, 16, 1537–1555. [Google Scholar] [CrossRef]
- Rambhia, K.J.; Ma, P.X. Controlled drug release for tissue engineering. J. Control. Release 2015, 219, 119–128. [Google Scholar] [CrossRef]
- Ravi, P.R.; Vats, R.; Dalal, V.; Gadekar, N. Design, optimization and evaluation of poly-epsilon-caprolactone (PCL) based polymeric nanoparticles for oral delivery of lopinavir. Drug Dev. Ind. Pharm. 2015, 41, 131–140. [Google Scholar] [CrossRef]
- Zong, S.; Wang, X.; Yang, Y.; Wu, W.; Li, H.; Ma, Y.; Lin, W.; Sun, T.; Huang, Y.; Xie, Z.; et al. The use of cisplatin-loaded mucoadhesive nanofibers for local chemotherapy of cervical cancers in mice. Eur. J. Pharm. Biopharm. 2015, 93, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tan, R.S.; Wang, C.H. Biodegradable microparticles and fiber fabrics for sustained delivery of cisplatin to treat C6 glioma in vitro. J. Biomed. Mater. Res. A 2008, 85, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Li, L.; Yang, G.; Li, J.; Luo, C.; Gong, T.; Zhou, S. Controlled green tea polyphenols release from electrospun PCL/MWCNTs composite nanofibers. Int. J. Pharm. 2011, 421, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Yohe, S.T.; Herrera, V.L.M.; Colson, Y.L.; Grinstaff, M.W. 3D superhydrophobic electrospun meshes as reinforcement materials for sustained local drug delivery against colorectal cancer cells. J. Control. Release 2012, 162, 92–101. [Google Scholar] [CrossRef]
- Ignatova, M.G.; Manolova, N.E.; Toshkova, R.A.; Rashkov, I.B.; Gardeva, E.G.; Yossifova, L.S.; Alexandrov, M.T. Electrospun nanofibrous mats containing quaternized chitosan and polylactide with in vitro antitumor activity against HeLa cells. Biomacromolecules 2010, 11, 1633–1645. [Google Scholar] [CrossRef]
- Massella, D.; Ancona, A.; Garino, N.; Cauda, V.; Guan, J.; Salaun, F.; Barresi, A.A.; Ferri, A. Preparation of bio-functional textiles by surface functionalization of cellulose fabrics with caffeine loaded nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2018, 460, 012044. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, L.; Liang, Q.; Zhang, X.; Guan, H.; Xu, X.; Chen, X.; Jing, X. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J. Control. Release 2005, 105, 43–51. [Google Scholar] [CrossRef]
- Garg, K.; Bowlin, G.L. Electrospinning jets and nanofibrous structures. Biomicrofluidics 2011, 5, 13403. [Google Scholar] [CrossRef]
- Balakrishnan, P.B.; Gardella, L.; Forouharshad, M.; Pellegrino, T.; Monticelli, O. Star poly(epsilon-caprolactone)-based electrospun fibers as biocompatible scaffold for doxorubicin with prolonged drug release activity. Colloids Surf. B Biointerfaces 2018, 161, 488–496. [Google Scholar] [CrossRef]
- Michel, M.; Auger, F.A.; Germain, L. Anchored skin equivalent cultured in vitro: A new tool for percutaneous absorption studies. In Vitro Cell. Dev. Biol. Anim. 1993, 29, 834–837. [Google Scholar] [CrossRef]
- Auger, F.A.; Pouliot, R.; Tremblay, N.; Guignard, R.; Noël, P.; Juhasz, J.; Germain, L.; Goulet, F. Multistep production of bioengineered skin substitutes: Sequential modulation of culture conditions. In Vitro Cell. Dev. Biol. Anim. 2000, 36, 96–103. [Google Scholar] [CrossRef]
- Augustine, R. Skin bioprinting: A novel approach for creating artificial skin from synthetic and natural building blocks. Prog. Biomater. 2018, 7, 77–92. [Google Scholar] [CrossRef]
- Van Kogelenberg, S.; Yue, Z.; Dinoro, J.N.; Baker, C.S.; Wallace, G.G. Three-Dimensional Printing and Cell Therapy for Wound Repair. Adv. Wound Care 2018, 7, 145–155. [Google Scholar] [CrossRef]
- Cubo, N.; Garcia, M.; Cañizo, J.F.D.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2016, 9, 015006. [Google Scholar] [CrossRef]
- Michel, M.; Germain, L.; Bélanger, P.M.; Auger, F.A. Functional evaluation of anchored skin equivalent cultured in vitro: Percutaneous absorption studies and lipid analysis. Pharm. Res. 1995, 12, 455–458. [Google Scholar] [CrossRef]
- Labouta, H.I.; Thude, S.; Schneider, M. Setup for investigating gold nanoparticle penetration through reconstructed skin and comparison to published human skin data. J. Biomed. Opt. 2013, 18, 061218. [Google Scholar] [CrossRef]
- Augustine, R.; Dominic, E.A.; Reju, I.; Kaimal, B.; Kalarikkal, N.; Thomas, S. Electrospun poly(epsilon-caprolactone)-based skin substitutes: In vivo evaluation of wound healing and the mechanism of cell proliferation. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1445–1454. [Google Scholar] [CrossRef]
- Augustine, R.; Nethi, S.K.; Kalarikkal, N.; Thomas, S.; Patra, C.R. Electrospun polycaprolactone (PCL) scaffolds embedded with europium hydroxide nanorods (EHNs) with enhanced vascularization and cell proliferation for tissue engineering applications. J. Mater. Chem. B 2017, 5, 4660–4672. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, X.; Yildirimer, L.; Lang, Q.; Lin, Z.Y.W.; Zheng, R.; Zhang, Y.; Cui, W.; Annabi, N.; Khademhosseini, A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017, 49, 66–77. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef]
- Luo, L.; Lane, M.E. Topical and transdermal delivery of caffeine. Int. J. Pharm. 2015, 490, 155–164. [Google Scholar] [CrossRef]
- Abaci, H.E.; Guo, Z.; Doucet, Y.; Jacków, J.; Christiano, A. Next generation human skin constructs as advanced tools for drug development. Exp. Biol. Med. 2017, 242, 1657–1668. [Google Scholar] [CrossRef]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tárnoki-Zách, J.; Mehes, E.; Varga-Medveczky, Z.; Isai, D.G.; Barany, N.; Bugyik, E.; Revesz, Z.; Paku, S.; Erdo, F.; Czirok, A. Development and Evaluation of a Human Skin Equivalent in a Semiautomatic Microfluidic Diffusion Chamber. Pharmaceutics 2021, 13, 910. https://doi.org/10.3390/pharmaceutics13060910
Tárnoki-Zách J, Mehes E, Varga-Medveczky Z, Isai DG, Barany N, Bugyik E, Revesz Z, Paku S, Erdo F, Czirok A. Development and Evaluation of a Human Skin Equivalent in a Semiautomatic Microfluidic Diffusion Chamber. Pharmaceutics. 2021; 13(6):910. https://doi.org/10.3390/pharmaceutics13060910
Chicago/Turabian StyleTárnoki-Zách, Júlia, Elod Mehes, Zsófia Varga-Medveczky, Dona Greta Isai, Nandor Barany, Edina Bugyik, Zsolt Revesz, Sándor Paku, Franciska Erdo, and Andras Czirok. 2021. "Development and Evaluation of a Human Skin Equivalent in a Semiautomatic Microfluidic Diffusion Chamber" Pharmaceutics 13, no. 6: 910. https://doi.org/10.3390/pharmaceutics13060910
APA StyleTárnoki-Zách, J., Mehes, E., Varga-Medveczky, Z., Isai, D. G., Barany, N., Bugyik, E., Revesz, Z., Paku, S., Erdo, F., & Czirok, A. (2021). Development and Evaluation of a Human Skin Equivalent in a Semiautomatic Microfluidic Diffusion Chamber. Pharmaceutics, 13(6), 910. https://doi.org/10.3390/pharmaceutics13060910





