A Review on Recent Advancement on Age-Related Hearing Loss: The Applications of Nanotechnology, Drug Pharmacology, and Biotechnology
Abstract
:1. Introduction
2. Methodology
3. Function of the Ear
4. Age-Related Hearing Loss
4.1. Pathophysiology of Age-Related Hearing Loss
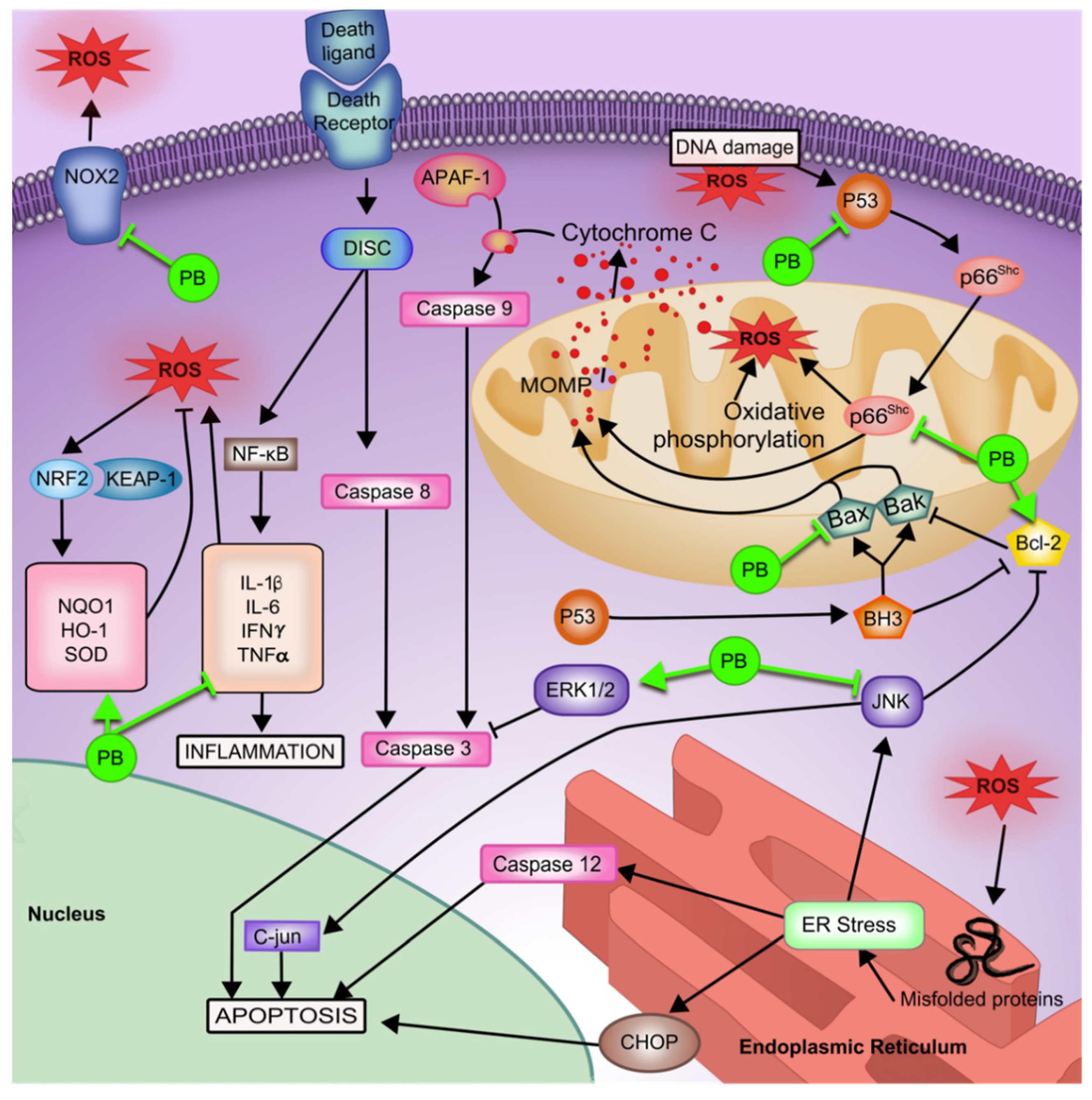
4.1.1. Oxidative Stress
4.1.2. Inflammation
4.1.3. Cellular Apoptosis
4.1.4. Autophagic Cell Protection
4.1.5. Recent Findings
5. Antioxidants
Potential Antioxidants for Use in ARHL Treatment
6. The Existing Barriers in Effective Treatment of Age-Related Hearing Loss
7. Local Drug Delivery of Antioxidants to Inner Ear
8. Nano and Micro Drug Carriers
9. Bile Acids
9.1. Bile Acid Synthesis, Metabolism and Microbial Crosstalk
9.2. Bile Acids in Drug Delivery
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haile, L.M.; Kamenov, K.; Briant, P.S.; Orji, A.U.; Steinmetz, J.D.; Abdoli, A.; Abdollahi, M.; Abu-Gharbieh, E.; Afshin, A.; Ahmed, H.; et al. Hearing loss prevalence and years lived with disability, 1990–2019: Findings from the Global Burden of Disease Study 2019. Lancet 2021, 397, 996–1009. [Google Scholar] [CrossRef]
- Tavanai, E.; Mohammadkhani, G. Role of antioxidants in prevention of age-related hearing loss: A review of literature. Eur. Arch. Otorhinolaryngol. 2017, 274, 1821–1834. [Google Scholar] [CrossRef]
- Pavlovic, N.; Golocorbin-Kon, S.; Ethanic, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile Acids and Their Derivatives as Potential Modifiers of Drug Release and Pharmacokinetic Profiles. Front. Pharmacol. 2018, 9, 1283. [Google Scholar] [CrossRef]
- Møller, A.; Mller, A.R. Hearing: Anatomy, Physiology, and Disorders of the Auditory System, 3rd ed.; Plural Publishing, Incorporated: San Diego, CA, USA, 2012. [Google Scholar]
- Costa, K.V.T.D.; Andrade, K.C.L.D.; Cavalcanti, M.E.D.; Frizzo, A.C.F.; Carnaúba, A.T.L.; Menezes, P.D.L. Hearing Loss at High Frequencies and Oxidative Stress: A New Paradigm for Different Etiologies. In An Excursus into Hearing Loss; Hatzopoulos, S., Ciorba, A., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Driver, E.C.; Kelley, M.W. Development of the cochlea. Development 2020, 147, dev162263. [Google Scholar] [CrossRef]
- Nin, F.; Hibino, H.; Doi, K.; Suzuki, T.; Hisa, Y.; Kurachi, Y. The endocochlear potential depends on two K+ diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc. Natl. Acad. Sci. USA 2008, 105, 1751–1756. [Google Scholar] [CrossRef] [Green Version]
- Anniko, M.; Wroblewski, R. Ionic environment of cochlear hair cells. Hear. Res. 1986, 22, 279–293. [Google Scholar] [CrossRef]
- Behrbohm, H.; Nawka, T.; Kaschke, O. Ear, Nose and Throat Diseases, 3rd ed.; Thieme Medical Publishers, Inc.: New York, NY, USA, 2009; pp. 1–21. [Google Scholar] [CrossRef]
- Fettiplace, R.; Kim, K.X. The physiology of mechanoelectrical transduction channels in hearing. Physiol. Rev. 2014, 94, 951–986. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, L.L.; Tucci, D.L. Hearing Loss in Adults. N. Engl. J. Med. 2017, 377, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- McDaid, D.; Park, A.L.; Chadha, S. Estimating the global costs of hearing loss. Int. J. Audiol. 2021, 60, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Gates, G.A.; Mills, J.H. Presbycusis. Lancet 2005, 366, 1111–1120. [Google Scholar] [CrossRef]
- Yang, C.H.; Schrepfer, T.; Schacht, J. Age-related hearing impairment and the triad of acquired hearing loss. Front. Cell Neurosci. 2015, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Van Eyken, E.; Van Camp, G.; Van Laer, L. The complexity of age-related hearing impairment: Contributing environmental and genetic factors. Audiol. Neurootol. 2007, 12, 345–358. [Google Scholar] [CrossRef]
- Rutherford, B.R.; Brewster, K.; Golub, J.S.; Kim, A.H.; Roose, S.P. Sensation and Psychiatry: Linking Age-Related Hearing Loss to Late-Life Depression and Cognitive Decline. Am. J. Psychiatry 2018, 175, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.R.; Metter, E.J.; O’Brien, R.J.; Resnick, S.M.; Zonderman, A.B.; Ferrucci, L. Hearing loss and incident dementia. Arch. Neurol. 2011, 68, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Ciorba, A.; Bianchini, C.; Pelucchi, S.; Pastore, A. The impact of hearing loss on the quality of life of elderly adults. Clin. Interv. Aging 2012, 7, 159–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawes, P.; Cruickshanks, K.J.; Moore, D.R.; Edmondson-Jones, M.; McCormack, A.; Fortnum, H.; Munro, K.J. Cigarette smoking, passive smoking, alcohol consumption, and hearing loss. J. Assoc. Res. Otolaryngol. 2014, 15, 663–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulop, T.; Larbi, A.; Khalil, A.; Cohen, A.A.; Witkowski, J.M. Are We Ill Because We Age? Front. Physiol. 2019, 10, 1508. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Vina, J.; Borras, C.; Gomez-Cabrera, M.C. A free radical theory of frailty. Free Radic. Biol. Med. 2018, 124, 358–363. [Google Scholar] [CrossRef]
- Menardo, J.; Tang, Y.; Ladrech, S.; Lenoir, M.; Casas, F.; Michel, C.; Bourien, J.; Ruel, J.; Rebillard, G.; Maurice, T.; et al. Oxidative stress, inflammation, and autophagic stress as the key mechanisms of premature age-related hearing loss in SAMP8 mouse Cochlea. Antioxid. Redox Signal. 2012, 16, 263–274. [Google Scholar] [CrossRef]
- Keithley, E.M. Pathology and mechanisms of cochlear aging. J. Neurosci. Res. 2020, 98, 1674–1684. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowl, M.R.; Dawson, S.J. Age-Related Hearing Loss. Cold Spring Harb. Perspect. Med. 2019, 9, a033217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fetoni, A.R.; Picciotti, P.M.; Paludetti, G.; Troiani, D. Pathogenesis of presbycusis in animal models: A review. Exp. Gerontol. 2011, 46, 413–425. [Google Scholar] [CrossRef]
- Chinnery, P.F. Mitochondrial disease in adults: What’s old and what’s new? EMBO Mol. Med. 2015, 7, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, K.O.; Klepper, K.; Saliba, J.; Friedman, R.A. Advances in understanding of presbycusis. J. Neurosci. Res. 2020, 98, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, X.; Hu, Y.; Sun, H.; He, Z.; Yuan, J.; Cai, H.; Sun, Y.; Huang, X.; Kong, W.; et al. Age-associated decline in Nrf2 signaling and associated mtDNA damage may be involved in the degeneration of the auditory cortex: Implications for central presbycusis. Int. J. Mol. Med. 2018, 42, 3371–3385. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; de la Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in Disease: Timing Is Everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, C.J.; Dodson, M.B.; Madhavan, L.; Zhang, D.D. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 2019, 134, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Chattopadhyay, A. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell Physiol. 2020, 235, 3119–3130. [Google Scholar] [CrossRef]
- Oishi, T.; Matsumaru, D.; Ota, N.; Kitamura, H.; Zhang, T.; Honkura, Y.; Katori, Y.; Motohashi, H. Activation of the NRF2 pathway in Keap1-knockdown mice attenuates progression of age-related hearing loss. NPJ Aging Mech. Dis. 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Vilchis-Landeros, M.M.; Matuz-Mares, D.; Vazquez-Meza, H. Regulation of Metabolic Processes by Hydrogen Peroxide Generated by NADPH Oxidases. Processes 2020, 8, 1424. [Google Scholar] [CrossRef]
- Paffenholz, R.; Bergstrom, R.A.; Pasutto, F.; Wabnitz, P.; Munroe, R.J.; Jagla, W.; Heinzmann, U.; Marquardt, A.; Bareiss, A.; Laufs, J.; et al. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004, 18, 486–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousset, F.; Nacher-Soler, G.; Coelho, M.; Ilmjarv, S.; Kokje, V.B.C.; Marteyn, A.; Cambet, Y.; Perny, M.; Roccio, M.; Jaquet, V.; et al. Redox activation of excitatory pathways in auditory neurons as mechanism of age-related hearing loss. Redox Biol. 2020, 30, 101434. [Google Scholar] [CrossRef]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Source of Chronic Inflammation in Aging. Front. Cardiovasc. Med. 2018, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Watson, N.; Ding, B.; Zhu, X.; Frisina, R.D. Chronic inflammation-inflammaging-in the ageing cochlea: A novel target for future presbycusis therapy. Ageing Res. Rev. 2017, 40, 142–148. [Google Scholar] [CrossRef]
- Warraich, U.E.; Hussain, F.; Kayani, H.U.R. Aging-Oxidative stress, antioxidants and computational modeling. Heliyon 2020, 6, e04107. [Google Scholar] [CrossRef]
- Neves, J.; Sousa-Victor, P. Regulation of inflammation as an anti-aging intervention. FEBS J. 2020, 287, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Krabbe, K.S.; Pedersen, M.; Bruunsgaard, H. Inflammatory mediators in the elderly. Exp. Gerontol. 2004, 39, 687–699. [Google Scholar] [CrossRef]
- Prasad, K.N.; Bondy, S.C. Increased oxidative stress, inflammation, and glutamate: Potential preventive and therapeutic targets for hearing disorders. Mech. Ageing Dev. 2020, 185, 111191. [Google Scholar] [CrossRef] [PubMed]
- Kanigur Sultuybek, G.; Soydas, T.; Yenmis, G. NF-kappaB as the mediator of metformin’s effect on ageing and ageing-related diseases. Clin. Exp. Pharmacol. Physiol. 2019, 46, 413–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, S.; Sahin, M.I.; Lewis, R.M.; Iyer, J.S.; Landegger, L.D.; Stankovic, K.M. Intracochlear Perfusion of Tumor Necrosis Factor-Alpha Induces Sensorineural Hearing Loss and Synaptic Degeneration in Guinea Pigs. Front. Neurol. 2019, 10, 1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lingappan, K. NF-kappaB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Du, Z.D.; Han, S.G.; Qu, T.F.; Guo, B.; Yu, S.K.; Wei, W.; Feng, S.; Liu, K.; Gong, S.S. Age-related insult of cochlear ribbon synapses: An early-onset contributor to d-galactose-induced aging in mice. Neurochem. Int. 2020, 133, 104649. [Google Scholar] [CrossRef]
- Morrill, S.; He, D.Z.Z. Apoptosis in inner ear sensory hair cells. J. Otol. 2017, 12, 151–164. [Google Scholar] [CrossRef]
- Wu, J.; Ye, J.; Kong, W.; Zhang, S.; Zheng, Y. Programmed cell death pathways in hearing loss: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2020, 53, e12915. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Yang, Q.; Liu, L.; Li, S.; Zhao, J.; Hu, J.; Liu, C.; Qian, D.; Gao, C. NADPH oxidase 2-dependent oxidative stress, mitochondrial damage and apoptosis in the ventral cochlear nucleus of d-galactose-induced aging rats. Neuroscience 2015, 286, 281–292. [Google Scholar] [CrossRef]
- Kong, W.J.; Wang, Y.; Wang, Q.; Hu, Y.J.; Han, Y.C.; Liu, J. The relation between d-galactose injection and mitochondrial DNA 4834 bp deletion mutation. Exp. Gerontol. 2006, 41, 628–634. [Google Scholar] [CrossRef]
- Zhong, Y.; Hu, Y.J.; Chen, B.; Peng, W.; Sun, Y.; Yang, Y.; Zhao, X.Y.; Fan, G.R.; Huang, X.; Kong, W.J. Mitochondrial transcription factor A overexpression and base excision repair deficiency in the inner ear of rats with d-galactose-induced aging. FEBS J. 2011, 278, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Yang, Y.; Hu, Y.; Sun, Y.; Zhang, S.; Peng, W.; Zhong, Y.; Huang, X.; Kong, W. A long-term high-fat diet increases oxidative stress, mitochondrial damage and apoptosis in the inner ear of d-galactose-induced aging rats. Hear. Res. 2012, 287, 15–24. [Google Scholar] [CrossRef]
- Someya, S.; Prolla, T.A. Mitochondrial oxidative damage and apoptosis in age-related hearing loss. Mech. Ageing Dev. 2010, 131, 480–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Someya, S.; Xu, J.; Kondo, K.; Ding, D.; Salvi, R.J.; Yamasoba, T.; Rabinovitch, P.S.; Weindruch, R.; Leeuwenburgh, C.; Tanokura, M.; et al. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc. Natl. Acad. Sci. USA 2009, 106, 19432–19437. [Google Scholar] [CrossRef] [Green Version]
- Ye, B.; Fan, C.; Shen, Y.; Wang, Q.; Hu, H.; Xiang, M. The Antioxidative Role of Autophagy in Hearing Loss. Front. Neurosci. 2018, 12, 1010. [Google Scholar] [CrossRef] [Green Version]
- Kosztelnik, M.; Kurucz, A.; Papp, D.; Jones, E.; Sigmond, T.; Barna, J.; Traka, M.H.; Lorincz, T.; Szarka, A.; Banhegyi, G.; et al. Suppression of AMPK/aak-2 by NRF2/SKN-1 down-regulates autophagy during prolonged oxidative stress. FASEB J. 2019, 33, 2372–2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Iriarte Rodriguez, R.; Pulido, S.; Rodriguez-de la Rosa, L.; Magarinos, M.; Varela-Nieto, I. Age-regulated function of autophagy in the mouse inner ear. Hear. Res. 2015, 330, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Sun, Y.; Chen, S.; Zhou, X.; Wu, X.; Kong, W.; Kong, W. Impaired unfolded protein response in the degeneration of cochlea cells in a mouse model of age-related hearing loss. Exp. Gerontol. 2015, 70, 61–70. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kim, S.S.; Yeo, S.G. Impact of Endoplasmic Reticulum Stress in Otorhinolaryngologic Diseases. Int. J. Mol. Sci. 2020, 21, 4121. [Google Scholar] [CrossRef]
- Horikawa, C.; Kodama, S.; Tanaka, S.; Fujihara, K.; Hirasawa, R.; Yachi, Y.; Shimano, H.; Yamada, N.; Saito, K.; Sone, H. Diabetes and risk of hearing impairment in adults: A meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.Y.; Kim, Y.J.; Gil, E.S.; Kim, H.; Jang, J.H.; Choung, Y.H. Type 1 Diabetes Induces Hearing Loss: Functional and Histological Findings in An Akita Mouse Model. Biomedicines 2020, 8. [Google Scholar] [CrossRef]
- Parmar, S.M.; Khare, P.; Chaudhary, M. Evaluation of Effects of Diabetes Mellitus Type 2 and Hyperlipidemia on Hearing. Indian J. Otol. 2017, 23, 155–161. [Google Scholar] [CrossRef]
- Takechi, R.; Lam, V.; Brook, E.; Giles, C.; Fimognari, N.; Mooranian, A.; Al-Salami, H.; Coulson, S.H.; Nesbit, M.; Mamo, J.C.L. Blood-Brain Barrier Dysfunction Precedes Cognitive Decline and Neurodegeneration in Diabetic Insulin Resistant Mouse Model: An Implication for Causal Link. Front. Aging Neurosci. 2017, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Yadav, K.S. Etiology of Noise-Induced Hearing Loss (NIHL) and its Symptomatic Correlation with Audiometry Observations in Type II Diabetes. Indian J. Otolaryngol. Head Neck Surg. 2018, 70, 137–144. [Google Scholar] [CrossRef]
- Shen, Y.; Ye, B.; Chen, P.; Wang, Q.; Fan, C.; Shu, Y.; Xiang, M. Cognitive Decline, Dementia, Alzheimer’s Disease and Presbycusis: Examination of the Possible Molecular Mechanism. Front. Neurosci. 2018, 12, 394. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Zhang, F.; Kerbl-Knapp, J.; Akhmetshina, A.; Korbelius, M.; Kuentzel, K.B.; Vujic, N.; Horl, G.; Paar, M.; Kratky, D.; Steyrer, E.; et al. Tissue-Specific Landscape of Metabolic Dysregulation during Ageing. Biomolecules 2021, 11, 235. [Google Scholar] [CrossRef]
- Marie, A.; Larroze-Chicot, P.; Cosnier-Pucheu, S.; Gonzalez-Gonzalez, S. Senescence-accelerated mouse prone 8 (SAMP8) as a model of age-related hearing loss. Neurosci. Lett. 2017, 656, 138–143. [Google Scholar] [CrossRef]
- Akiguchi, I.; Pallas, M.; Budka, H.; Akiyama, H.; Ueno, M.; Han, J.; Yagi, H.; Nishikawa, T.; Chiba, Y.; Sugiyama, H.; et al. SAMP8 mice as a neuropathological model of accelerated brain aging and dementia: Toshio Takeda’s legacy and future directions. Neuropathology 2017, 37, 293–305. [Google Scholar] [CrossRef] [Green Version]
- Rhea, E.M.; Banks, W.A. The SAMP8 mouse for investigating memory and the role of insulin in the brain. Exp. Gerontol. 2017, 94, 64–68. [Google Scholar] [CrossRef]
- Mironczuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Pak, J.H.; Kim, Y.; Yi, J.; Chung, J.W. Antioxidant Therapy against Oxidative Damage of the Inner Ear: Protection and Preconditioning. Antioxidants 2020, 9, 1076. [Google Scholar] [CrossRef]
- Mbara, K.C.; Mofo Mato, P.E.; Driver, C.; Nzuza, S.; Mkhombo, N.T.; Gcwensa, S.K.; McObothi, E.N.; Owira, P.M. Metformin turns 62 in pharmacotherapy: Emergence of non-glycaemic effects and potential novel therapeutic applications. Eur. J. Pharmacol. 2021, 898, 173934. [Google Scholar] [CrossRef]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Role of metformin in various pathologies: State-of-the-art microcapsules for improving its pharmacokinetics. Ther. Deliv. 2020, 11, 733–753. [Google Scholar] [CrossRef]
- Cai, H.; Han, B.; Hu, Y.; Zhao, X.; He, Z.; Chen, X.; Sun, H.; Yuan, J.; Li, Y.; Yang, X.; et al. Metformin attenuates the Dgalactoseinduced aging process via the UPR through the AMPK/ERK1/2 signaling pathways. Int. J. Mol. Med. 2020, 45, 715–730. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhao, L.; Han, Y.; Liu, Y.; Chen, C.; Zhan, M.; Xiong, X.; Zhu, X.; Xiao, L.; Hu, C.; et al. Probucol ameliorates renal injury in diabetic nephropathy by inhibiting the expression of the redox enzyme p66Shc. Redox Biol. 2017, 13, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, Y.; He, P.; Li, D. Probucol inhibited Nox2 expression and attenuated podocyte injury in type 2 diabetic nephropathy of db/db mice. Biol. Pharm. Bull. 2013, 36, 1883–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Liu, C.; Chen, S.; Zhao, H.; Zhou, K.; Wang, W.; Yuan, Y.; Li, Z.; Guo, Y.; Shen, Z.; et al. Activation of the Nrf2/ARE signaling pathway by probucol contributes to inhibiting inflammation and neuronal apoptosis after spinal cord injury. Oncotarget 2017, 8, 52078–52093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Jiao, Z.; Liu, Y.; Chen, J.; Li, G.; Liu, T.; Tse, G.; Yuan, R. Probucol Protects Against Contrast-Induced Acute Kidney Injury via the Extracellular Signal-Regulated Kinases 1 and 2 (ERK1/2)/JNK-Caspase 3 Pathway in Diabetic Rats. Med. Sci. Monit. 2019, 25, 1038–1045. [Google Scholar] [CrossRef]
- Mir, H.A.; Ali, R.; Mushtaq, U.; Khanday, F.A. Structure-functional implications of longevity protein p66Shc in health and disease. Ageing Res. Rev. 2020, 63, 101139. [Google Scholar] [CrossRef]
- Asiri, Y.A. Probucol attenuates cyclophosphamide-induced oxidative apoptosis, p53 and Bax signal expression in rat cardiac tissues. Oxid. Med. Cell Longev. 2010, 3, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Ai, S.; Chen, Z.; Li, C. Probucol promotes high glucose-induced proliferation and inhibits apoptosis by reducing reactive oxygen species generation in Muller cells. Int. Ophthalmol. 2019, 39, 2833–2842. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.L.; Zhang, K.Q.; Tian, T.; Zhang, H.; Fu, Q. Probucol improves erectile function via Activation of Nrf2 and coordinates the HO-1 / DDAH / PPAR-gamma/ eNOS pathways in streptozotocin-induced diabetic rats. Biochem. Biophys. Res. Commun. 2018, 507, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Y.; Cao, Y.; Wang, Q.; Anwaier, G.; Zhang, Q.; Qi, R. Mechanisms underlying the inhibitory effects of probucol on elastase-induced abdominal aortic aneurysm in mice. Br. J. Pharmacol. 2020, 177, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Foresti, R. Heme oxygenase-1 as a target for drug discovery. Antioxid. Redox. Signal. 2014, 20, 1810–1826. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhang, W.; Liu, B.; Gao, J.; Xiao, X.Q.; Zhang, F.; Zhou, H.M.; Wu, X.L.; Zhang, X. Probucol ameliorates the development of nonalcoholic steatohepatitis in rats fed high-fat diets. Dig. Dis. Sci. 2013, 58, 163–171. [Google Scholar] [CrossRef]
- Wagle, S.R.; Walker, D.; Kovacevic, B.; Gedawy, A.; Mikov, M.; Golocorbin-Kon, S.; Mooranian, A.; Al-Salami, H. Micro-Nano formulation of bile-gut delivery: Rheological, stability and cell survival, basal and maximum respiration studies. Sci. Rep. 2020, 10, 7715. [Google Scholar] [CrossRef]
- Wagle, S.R.; Kovacevic, B.; Walker, D.; Ionescu, C.M.; Jones, M.; Stojanovic, G.; Kojic, S.; Mooranian, A.; Al-Salami, H. Pharmacological and Advanced Cell Respiration Effects, Enhanced by Toxic Human-Bile Nano-Pharmaceuticals of Probucol Cell-Targeting Formulations. Pharmaceutics 2020, 12, 708. [Google Scholar] [CrossRef]
- Yamashita, S.; Matsuzawa, Y. Where are we with probucol: A new life for an old drug? Atherosclerosis 2009, 207, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Jaudoin, C.; Agnely, F.; Nguyen, Y.; Ferrary, E.; Bochot, A. Nanocarriers for drug delivery to the inner ear: Physicochemical key parameters, biodistribution, safety and efficacy. Int. J. Pharm. 2021, 592, 120038. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Rana, I.; Choi, H.I.; Lee, C.H.; Baek, S.W.; Lim, C.W.; Khan, N.; Arif, S.T.; Sahar, N.U.; Alvi, A.M.; et al. Potential and Applications of Nanocarriers for Efficient Delivery of Biopharmaceuticals. Pharmaceutics 2020, 12, 1184. [Google Scholar] [CrossRef]
- Glueckert, R.; Johnson Chacko, L.; Rask-Andersen, H.; Liu, W.; Handschuh, S.; Schrott-Fischer, A. Anatomical basis of drug delivery to the inner ear. Hear. Res. 2018, 368, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, M.; Smyth, H.; Zhang, F. Otic drug delivery systems: Formulation principles and recent developments. Drug Dev. Ind. Pharm. 2018, 44, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, S.; Abbott, N.J.; Shi, X.; Steyger, P.S.; Dabdoub, A. Delivery of therapeutics to the inner ear: The challenge of the blood-labyrinth barrier. Sci. Transl. Med. 2019, 11, eaao0935. [Google Scholar] [CrossRef]
- El Kechai, N.; Agnely, F.; Mamelle, E.; Nguyen, Y.; Ferrary, E.; Bochot, A. Recent advances in local drug delivery to the inner ear. Int. J. Pharm. 2015, 494, 83–101. [Google Scholar] [CrossRef]
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Hollingsworth, D.B.; Kelley, D.M.; Kmucha, S.T.; Moonis, G.; et al. Clinical Practice Guideline: Sudden Hearing Loss (Update). Otolaryngol. Head Neck Surg. 2019, 161, S1–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goycoolea, M.V. Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol. 2001, 121, 437–447. [Google Scholar] [CrossRef]
- Sahni, R.S.; Paparella, M.M.; Schachern, P.A.; Goycoolea, M.V.; Le, C.T. Thickness of the human round window membrane in different forms of otitis media. Arch. Otolaryngol. Head Neck Surg. 1987, 113, 630–634. [Google Scholar] [CrossRef]
- Schachern, P.A.; Paparella, M.M.; Goycoolea, M.V.; Duvall, A.J., 3rd; Choo, Y.B. The permeability of the round window membrane during otitis media. Arch. Otolaryngol. Head Neck Surg. 1987, 113, 625–629. [Google Scholar] [CrossRef]
- Luers, J.C.; Huttenbrink, K.B.; Beutner, D. Surgical anatomy of the round window-Implications for cochlear implantation. Clin. Otolaryngol. 2018, 43, 417–424. [Google Scholar] [CrossRef]
- Zou, J.; Sood, R.; Ranjan, S.; Poe, D.; Ramadan, U.A.; Pyykko, I.; Kinnunen, P.K. Size-dependent passage of liposome nanocarriers with preserved posttransport integrity across the middle-inner ear barriers in rats. Otol. Neurotol. 2012, 33, 666–673. [Google Scholar] [CrossRef]
- Cai, H.; Liang, Z.; Huang, W.; Wen, L.; Chen, G. Engineering PLGA nano-based systems through understanding the influence of nanoparticle properties and cell-penetrating peptides for cochlear drug delivery. Int. J. Pharm. 2017, 532, 55–65. [Google Scholar] [CrossRef]
- Liu, H.; Chen, S.; Zhou, Y.; Che, X.; Bao, Z.; Li, S.; Xu, J. The effect of surface charge of glycerol monooleate-based nanoparticles on the round window membrane permeability and cochlear distribution. J. Drug. Target. 2013, 21, 846–854. [Google Scholar] [CrossRef]
- Goycoolea, M.V.; Muchow, D.; Martinez, G.C.; Aguila, P.B.; Goycoolea, H.G.; Goycoolea, C.V.; Schachern, P.; Knight, W. Permeability of the human round-window membrane to cationic ferritin. Arch. Otolaryngol. Head Neck Surg. 1988, 114, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dellamary, L.; Fernandez, R.; Ye, Q.; LeBel, C.; Piu, F. Principles of inner ear sustained release following intratympanic administration. Laryngoscope 2011, 121, 385–391. [Google Scholar] [CrossRef]
- Mikulec, A.A.; Hartsock, J.J.; Salt, A.N. Permeability of the round window membrane is influenced by the composition of applied drug solutions and by common surgical procedures. Otol. Neurotol. 2008, 29, 1020–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szeto, B.; Chiang, H.; Valentini, C.; Yu, M.; Kysar, J.W.; Lalwani, A.K. Inner ear delivery: Challenges and opportunities. Laryngoscope Investig. Otolaryngol. 2020, 5, 122–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathnam, C.; Chueng, S.D.; Ying, Y.M.; Lee, K.B.; Kwan, K. Developments in Bio-Inspired Nanomaterials for Therapeutic Delivery to Treat Hearing Loss. Front. Cell Neurosci. 2019, 13, 493. [Google Scholar] [CrossRef]
- Patel, J.; Szczupak, M.; Rajguru, S.; Balaban, C.; Hoffer, M.E. Inner Ear Therapeutics: An Overview of Middle Ear Delivery. Front. Cell Neurosci. 2019, 13, 261. [Google Scholar] [CrossRef] [Green Version]
- Mittal, R.; Pena, S.A.; Zhu, A.; Eshraghi, N.; Fesharaki, A.; Horesh, E.J.; Mittal, J.; Eshraghi, A.A. Nanoparticle-based drug delivery in the inner ear: Current challenges, limitations and opportunities. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1312–1320. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xu, Y.; Cao, W.; Xie, S.; Wen, L.; Chen, G. Understanding the translocation mechanism of PLGA nanoparticles across round window membrane into the inner ear: A guideline for inner ear drug delivery based on nanomedicine. Int. J. Nanomed. 2018, 13, 479–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverstein, H.; Thompson, J.; Rosenberg, S.I.; Brown, N.; Light, J. Silverstein MicroWick. Otolaryngol. Clin. N. Am. 2004, 37, 1019–1034. [Google Scholar] [CrossRef]
- Hill, A.; Geissler, S.; Meyring, M.; Hecht, S.; Weigandt, M.; Mader, K. In vitro-in vivo evaluation of nanosuspension release from subcutaneously implantable osmotic pumps. Int. J. Pharm. 2013, 451, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Breyer, S.; Geissler, S.; Mier, W.; Haberkorn, U.; Weigandt, M.; Mader, K. How do in-vitro release profiles of nanosuspensions from Alzet(R) pumps correspond to the in-vivo situation? A case study on radiolabeled fenofibrate. J. Control. Release 2013, 168, 77–87. [Google Scholar] [CrossRef] [PubMed]
- El Kechai, N.; Mamelle, E.; Nguyen, Y.; Huang, N.; Nicolas, V.; Chaminade, P.; Yen-Nicolay, S.; Gueutin, C.; Granger, B.; Ferrary, E.; et al. Hyaluronic acid liposomal gel sustains delivery of a corticoid to the inner ear. J. Control. Release 2016, 226, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Long, W.; Liang, Z.; Wen, L.; Yang, F.; Chen, G. A novel vehicle for local protein delivery to the inner ear: Injectable and biodegradable thermosensitive hydrogel loaded with PLGA nanoparticles. Drug Dev. Ind. Pharm. 2018, 44, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Engmer Berglin, C.; Videhult Pierre, P.; Ekborn, A.; Bramer, T.; Edsman, K.; Hultcrantz, M.; Laurell, G. Local treatment of the inner ear: A study of three different polymers aimed for middle ear administration. Acta. Otolaryngol. 2015, 135, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Havenith, S.; Versnel, H.; Agterberg, M.J.; de Groot, J.C.; Sedee, R.J.; Grolman, W.; Klis, S.F. Spiral ganglion cell survival after round window membrane application of brain-derived neurotrophic factor using gelfoam as carrier. Hear. Res. 2011, 272, 168–177. [Google Scholar] [CrossRef]
- Silverstein, H.; Arruda, J.; Rosenberg, S.I.; Deems, D.; Hester, T.O. Direct round window membrane application of gentamicin in the treatment of Meniere’s disease. Otolaryngol. Head Neck Surg. 1999, 120, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Kurioka, T.; Kaitsuka, T.; Tomizawa, K.; Matsunobu, T.; Hakim, F.; Mizutari, K.; Miwa, T.; Yamada, T.; Ise, M.; et al. Protein transduction therapy into cochleae via the round window niche in guinea pigs. Mol. Ther. Methods Clin. Dev. 2016, 3, 16055. [Google Scholar] [CrossRef] [PubMed]
- Mader, K.; Lehner, E.; Liebau, A.; Plontke, S.K. Controlled drug release to the inner ear: Concepts, materials, mechanisms, and performance. Hear. Res. 2018, 368, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Ding, S.; Cai, H.; Wang, J.; Wen, L.; Yang, F.; Chen, G. Nanomedicine strategy for optimizing delivery to outer hair cells by surface-modified poly(lactic/glycolic acid) nanoparticles with hydrophilic molecules. Int. J. Nanomed. 2016, 11, 5959–5969. [Google Scholar] [CrossRef] [Green Version]
- Lajud, S.A.; Nagda, D.A.; Qiao, P.; Tanaka, N.; Civantos, A.; Gu, R.; Cheng, Z.; Tsourkas, A.; O’Malley, B.W., Jr.; Li, D. A novel chitosan-hydrogel-based nanoparticle delivery system for local inner ear application. Otol. Neurotol. 2015, 36, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Microparticles, microcapsules and microspheres: A review of recent developments and prospects for oral delivery of insulin. Int. J. Pharm. 2018, 537, 223–244. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Al-Salami, H. Eudragit®-based microcapsules of probucol with a gut-bacterial processed secondary bile acid. Ther. Deliv. 2018, 9, 811–821. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Influence of Biotechnological Processes, Speed of Formulation Flow and Cellular Concurrent Stream-Integration on Insulin Production from β-cells as a Result of Co-Encapsulation with a Highly Lipophilic Bile Acid. Cell. Mol. Bioeng. 2018, 11, 65–75. [Google Scholar] [CrossRef]
- Mamo, J.C.; Lam, V.; Al-Salami, H.; Brook, E.; Mooranian, A.; Nesbit, M.; Graneri, L.; D’Alonzo, Z.; Fimognari, N.; Stephenson, A.; et al. Sodium alginate capsulation increased brain delivery of probucol and suppressed neuroinflammation and neurodegeneration. Ther. Deliv. 2018, 9, 703–709. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Pritz, C.O.; Dudas, J.; Rask-Andersen, H.; Schrott-Fischer, A.; Glueckert, R. Nanomedicine strategies for drug delivery to the ear. Nanomedicine 2013, 8, 1155–1172. [Google Scholar] [CrossRef]
- Jones, M.; Walker, D.; Ionescu, C.M.; Kovacevic, B.; Wagle, S.R.; Mooranian, A.; Brown, D.; Al-Salami, H. Microencapsulation of Coenzyme Q10 and bile acids using ionic gelation vibrational jet flow technology for oral delivery. Ther. Deliv. 2020, 11, 791–805. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Takechi, R.; Al-Sallami, H.; Mikov, M.; Goločorbin-Kon, S.; Kovacevic, B.; Arfuso, F.; Al-Salami, H. Probucol-poly(meth)acrylate-bile acid nanoparticles increase IL-10, and primary bile acids in prediabetic mice. Ther. Deliv. 2019, 10, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Zamani, N.; Luna, G.; Al-Sallami, H.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Kovacevic, B.; Al-Salami, H. Bile acid-polymer-probucol microparticles: Protective effect on pancreatic β-cells and decrease in type 1 diabetes development in a murine model. Pharm. Dev. Technol. 2019, 24, 1272–1277. [Google Scholar] [CrossRef]
- Mathavan, S.; Ionescu, C.M.; Kovacevic, B.; Mikov, M.; Golocorbin-Kon, S.; Mooranian, A.; Dass, C.R.; Al-Salami, H. Formulation buoyancy of nanoencapsulated gliclazide using primary, conjugated and deconjugated bile acids. Ther. Deliv. 2019, 10, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chao, T.; Brant, J.; O’Malley, B., Jr.; Tsourkas, A.; Li, D. Advances in nano-based inner ear delivery systems for the treatment of sensorineural hearing loss. Adv. Drug Deliv. Rev. 2017, 108, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory bowel disease: Clinical aspects and treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negrulj, R.; Mooranian, A.; Al-Salami, H. Potentials and Limitations of Bile Acids in Type 2 Diabetes Mellitus: Applications of Microencapsulation as a Novel Oral Delivery System. J. Endocrinol. Diabetes Mellit. 2013, 1, 49–59. [Google Scholar]
- Ethanic, M.; Stanimirov, B.; Pavlovic, N.; Golocorbin-Kon, S.; Al-Salami, H.; Stankov, K.; Mikov, M. Pharmacological Applications of Bile Acids and Their Derivatives in the Treatment of Metabolic Syndrome. Front. Pharmacol. 2018, 9, 1382. [Google Scholar] [CrossRef]
- Woodhams, L.; Al-Salami, H. The roles of bile acids and applications of microencapsulation technology in treating Type 1 diabetes mellitus. Ther. Deliv. 2017, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, M.C.; Cautela, J.; Galantini, L. Physiology and Physical Chemistry of Bile Acids. Int. J. Mol. Sci. 2021, 22, 1780. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Mikov, M.; Golocorbin-Kon, S.; Arfuso, F.; Al-Salami, H. Novel chenodeoxycholic acid-sodium alginate matrix in the microencapsulation of the potential antidiabetic drug, probucol. An in vitro study. J. Microencapsul. 2015, 32, 589–597. [Google Scholar] [CrossRef]
- Mooranian, A.; Raj Wagle, S.; Kovacevic, B.; Takechi, R.; Mamo, J.; Lam, V.; Watts, G.F.; Mikov, M.; Golocorbin-Kon, S.; Stojanovic, G.; et al. Bile acid bio-nanoencapsulation improved drug targeted-delivery and pharmacological effects via cellular flux: 6-months diabetes preclinical study. Sci. Rep. 2020, 10, 106. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Mikov, M.; Golocorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Kovacevic, B.; Al-Salami, H. Bio Micro-Nano Technologies of Antioxidants Optimised Their Pharmacological and Cellular Effects, ex vivo, in Pancreatic beta-Cells. Nanotechnol. Sci. Appl. 2020, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. Multicompartmental, multilayered probucol microcapsules for diabetes mellitus: Formulation characterization and effects on production of insulin and inflammation in a pancreatic beta-cell line. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1642–1653. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Zamani, N.; Mikov, M.; Golocorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Al-Salami, H. Novel nano-encapsulation of probucol in microgels: Scanning electron micrograph characterizations, buoyancy profiling, and antioxidant assay analyses. Artif. Cells Nanomed. Biotechnol. 2018, 46, S741–S747. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Zamani, N.; Mikov, M.; Golocorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Kovacevic, B.; Al-Salami, H. A second-generation micro/nano capsules of an endogenous primary un-metabolised bile acid, stabilized by Eudragit-alginate complex with antioxidant compounds. Saudi. Pharm. J. 2020, 28, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The influence of stabilized deconjugated ursodeoxycholic acid on polymer-hydrogel system of transplantable NIT-1 cells. Pharm. Res. 2016, 33, 1182–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooranian, A.; Negrulj, R.; Mathavan, S.; Martinez, J.; Sciarretta, J.; Chen-Tan, N.; Mukkur, T.K.; Mikov, M.; Lalic-Popovic, M.; Stojancevic, M.; et al. An advanced microencapsulated system: A platform for optimized oral delivery of antidiabetic drug-bile acid formulations. Pharm. Dev. Technol. 2015, 20, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. The effect of a tertiary bile acid, taurocholic acid, on the morphology and physical characteristics of microencapsulated probucol: Potential applications in diabetes: A characterization study. Drug Deliv. Transl. Res. 2015, 5, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Al-Sallami, H.S.; Fang, Z.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Arfuso, F.; Al-Salami, H. Release and swelling studies of an innovative antidiabetic-bile acid microencapsulated formulation, as a novel targeted therapy for diabetes treatment. J. Microencapsul. 2015, 32, 151–156. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Sallami, H.; Fang, Z.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Watts, G.; Matthews, V.; Arfuso, F.; et al. Probucol Release from Novel Multicompartmental Microcapsules for the Oral Targeted Delivery in Type 2 Diabetes. AAPS PharmSciTech 2015, 16, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Negrulj, R.; Mathavan, S.; Martinez, J.; Sciarretta, J.; Chen-Tan, N.; Mukkur, T.K.; Mikov, M.; Lalic-Popovic, M.; Stojancevic, M.; et al. Stability and Release Kinetics of an Advanced Gliclazide-Cholic Acid Formulation: The Use of Artificial-Cell Microencapsulation in Slow Release Targeted Oral Delivery of Antidiabetics. J. Pharm. Innov. 2014, 9, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acid Biology, Pathophysiology, and Therapeutics. Clin. Liver Dis. 2020, 15, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acids as Metabolic Regulators and Nutrient Sensors. Annu. Rev. Nutr. 2019, 39, 175–200. [Google Scholar] [CrossRef]
- Lalic-Popovic, M.; Vasovic, V.; Milijasevic, B.; Golocorbin-Kon, S.; Al-Salami, H.; Mikov, M. Deoxycholic Acid as a Modifier of the Permeation of Gliclazide through the Blood Brain Barrier of a Rat. J. Diabetes Res. 2013, 2013, 598603. [Google Scholar] [CrossRef] [PubMed]
- Ethanic, M.; Stanimirov, B.; Pavlovic, N.; Vukmirovic, S.; Lazic, J.; Al-Salami, H.; Mikov, M. Transport and Biotransformation of Gliclazide and the Effect of Deoxycholic Acid in a Probiotic Bacteria Model. Front. Pharmacol. 2019, 10, 1083. [Google Scholar] [CrossRef]
- Kim, K.; Yoon, I.; Chun, I.; Lee, N.; Kim, T.; Gwak, H.S. Effects of bile salts on the lovastatin pharmacokinetics following oral administration to rats. Drug Deliv. 2011, 18, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.S.; Moses, A.C.; Silver, R.D.; Flier, J.S.; Carey, M.C. Nasal absorption of insulin: Enhancement by hydrophobic bile salts. Proc. Natl. Acad. Sci. USA 1985, 82, 7419–7423. [Google Scholar] [CrossRef] [Green Version]
- Negrulj, R.; Mooranian, A.; Chen-Tan, N.; Al-Sallami, H.S.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Watts, G.F.; Arfuso, F.; Al-Salami, H. Swelling, mechanical strength, and release properties of probucol microcapsules with and without a bile acid, and their potential oral delivery in diabetes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Watts, G.F.; Arfuso, F.; Al-Salami, H. An optimized probucol microencapsulated formulation integrating a secondary bile acid (deoxycholic acid) as a permeation enhancer. Drug Des. Devel. Ther. 2014, 8, 1673–1683. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Al-Sallami, H.S.; Fang, Z.; Mukkur, T.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Arfuso, F.; et al. Novel artificial cell microencapsulation of a complex gliclazide-deoxycholic bile acid formulation: A characterization study. Drug Des. Devel. Ther. 2014, 8, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Wagle, S.R.; Kovacevic, B.; Walker, D.; Ionescu, C.M.; Shah, U.; Stojanovic, G.; Kojic, S.; Mooranian, A.; Al-Salami, H. Alginate-based drug oral targeting using bio-micro/nano encapsulation technologies. Expert Opin. Drug Deliv. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Zamani, N.; Kovacevic, B.; Ionescu, C.M.; Luna, G.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Kojic, S.; Al-Salami, H. Pharmacological effects of secondary bile acid microparticles in diabetic murine model. Curr. Diabetes Rev. 2020. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Takechi, R.; Luna, G.; Mikov, M.; Goločorbin-Kon, S.; Elnashar, M.; Arfuso, F.; Al-Salami, H. An in vivo pharmacological study: Variation in tissue-accumulation for the drug probucol as the result of targeted microtechnology and matrix-acrylic acid optimization and stabilization techniques. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Zamani, N.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Al-Salami, H. Stability and biological testing of taurine-conjugated bile acid antioxidant microcapsules for diabetes treatment. Ther. Deliv. 2019, 10, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Zamani, N.; Takechi, R.; Al-Sallami, H.; Mikov, M.; Goločorbin-Kon, S.; Kovacevic, B.; Arfuso, F.; Al-Salami, H. Pharmacological effects of nanoencapsulation of human-based dosing of probucol on ratio of secondary to primary bile acids in gut, during induction and progression of type 1 diabetes. Artif. Cells Nanomed. Biotechnol 2018, 46, S748–S754. [Google Scholar] [CrossRef]
- Mooranian, A.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. The effect of molecular weights of microencapsulating polymers on viability of mouse-cloned pancreatic β-cells: Biomaterials, osmotic forces and potential applications in diabetes treatment. Pharm. Dev. Technol. 2018, 23, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Electrokinetic potential-stabilization by bile acid-microencapsulating formulation of pancreatic β-cells cultured in high ratio poly-L-ornithine-gel hydrogel colloidal dispersion: Applications in cell-biomaterials, tissue engineering and biotechnological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1156–1162. [Google Scholar] [CrossRef] [Green Version]
- Al-Salami, H.; Mamo, J.C.; Mooranian, A.; Negrulj, R.; Lam, V.; Elahy, M.; Takechi, R. Long-Term Supplementation of Microencapsulated ursodeoxycholic Acid Prevents Hypertension in a Mouse Model of Insulin Resistance. Exp. Clin. Endocrinol. Diabetes 2017, 125, 28–32. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Biological Assessments of Encapsulated Pancreatic β-Cells: Their Potential Transplantation in Diabetes. Cell. Mol. Bioeng. 2016, 9, 530–537. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Fakhoury, M.; Arfuso, F.; Jones, F.; Al-Salami, H. Advanced bile acid-based multi-compartmental microencapsulated pancreatic beta-cells integrating a polyelectrolyte-bile acid formulation, for diabetes treatment. Artif. CellsNanomed. Biotechnol. 2016, 44, 588–595. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. Characterization of a novel bile acid-based delivery platform for microencapsulated pancreatic beta-cells. Artif. Cells Nanomed. Biotechnol. 2016, 44, 194–200. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H.; Morahan, G.; Jamieson, E. Designing anti-diabetic β-cells microcapsules using polystyrenic sulfonate, polyallylamine, and a tertiary bile acid: Morphology, bioenergetics, and cytokine analysis. Biotechnol. Prog. 2016, 32, 501–509. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The incorporation of water-soluble gel matrix into bile acid-based microcapsules for the delivery of viable β-cells of the pancreas, in diabetes treatment: Biocompatibility and functionality studies. Drug Deliv. Transl. Res. 2016, 6, 17–23. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. Viability and topographical analysis of microencapsulated β-cells exposed to a biotransformed tertiary bile acid: An ex vivo study. Int. J. Nano Biomater. 2016, 6, 74–82. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Mamo, J.; Al-Sallami, H.; Al-Salami, H. The biological effects of the hypolipidaemic drug probucol microcapsules fed daily for 4 weeks, to an insulin-resistant mouse model: Potential hypoglycaemic and anti-inflammatory effects. Drug Deliv. Transl. Res. 2018, 8, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.; Serafim, C.; Rijo, P.; Reis, C.P. Bile acids and bile acid derivatives: Use in drug delivery systems and as therapeutic agents. Expert Opin. Drug Deliv. 2016, 13, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Xiong, H.; Lin, P.; Lai, L.; Yang, H.; Liu, Y.; Huang, Q.; Chen, S.; Ye, Y.; Sun, Y.; et al. Activation of miR-34a impairs autophagic flux and promotes cochlear cell death via repressing ATG9A: Implications for age-related hearing loss. Cell Death Dis. 2017, 8, e3079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooranian, A.; Zamani, N.; Ionescu, C.M.; Takechi, R.; Luna, G.; Mikov, M.; Golocorbin-Kon, S.; Kovacevic, B.; Al-Salami, H. Oral gavage of nano-encapsulated conjugated acrylic acid-bile acid formulation in type 1 diabetes altered pharmacological profile of bile acids, and improved glycaemia and suppressed inflammation. Pharmacol. Rep. 2020, 72, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Gvoic, M.; Vukmirovic, S.; Al-Salami, H.; Mooranian, A.; Mikov, M.; Stankov, K. Bile acids as novel enhancers of CNS targeting antitumor drugs: A comprehensive review. Pharm. Dev. Technol. 2021, 1–17. [Google Scholar] [CrossRef]
- Mathavan, S.; Ionescu, C.M.; Kovacevic, B.; Mikov, M.; Golocorbin-Kon, S.; Mooranian, A.; Dass, C.R.; Al-Salami, H. Histological effects of pharmacologically active human bile acid nano/micro-particles in Type-1 diabetes. Ther. Deliv. 2020, 11, 157–171. [Google Scholar] [CrossRef]
- Maksimovic, V.; Pavlovic-Popovic, Z.; Vukmirovic, S.; Cvejic, J.; Mooranian, A.; Al-Salami, H.; Mikov, M.; Golocorbin-Kon, S. Molecular mechanism of action and pharmacokinetic properties of methotrexate. Mol. Biol. Rep. 2020, 47, 4699–4708. [Google Scholar] [CrossRef] [PubMed]
- Kecman, S.; Škrbić, R.; Badnjevic Cengic, A.; Mooranian, A.; Al-Salami, H.; Mikov, M.; Golocorbin-Kon, S. Potentials of human bile acids and their salts in pharmaceutical nano delivery and formulations adjuvants. Technol. Health Care 2020, 28, 325–335. [Google Scholar] [CrossRef]
- Jovic, J.; Milijasevic, B.; Vukmirovic, S.; Vasovic, V.; Mikov, M.; Mooranian, A.; Al-Salami, H.; Golocorbin-Kon, S. Pharmacokinetic and Drug Absorption Profiles of the Anti-Hyperglycaemic Agent Gliclazide in Oral Tissue-Targeted Microcapsules in Rats. Scr. Med. 2020, 51, 15–20. [Google Scholar] [CrossRef]
- Zangerolamo, L.; Vettorazzi, J.F.; Rosa, L.R.O.; Carneiro, E.M.; Barbosa, H.C.L. The bile acid TUDCA and neurodegenerative disorders: An overview. Life Sci. 2021, 272, 119252. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, M.; Yuan, J.; Li, B.; Entenman, S.; Yu, H.; Zheng, Q.Y. Tauroursodeoxycholic acid prevents hearing loss and hair cell death in Cdh23(erl/erl) mice. Neuroscience 2016, 316, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Berger, E.; Haller, D. Structure-function analysis of the tertiary bile acid TUDCA for the resolution of endoplasmic reticulum stress in intestinal epithelial cells. Biochem. Biophys. Res. Commun. 2011, 409, 610–615. [Google Scholar] [CrossRef]
- Balandraud-Pieri, N.; Queneau, P.E.; Caroli-Bosc, F.X.; Bertault-Peres, P.; Montet, A.M.; Durand, A.; Montet, J.C. Effects of tauroursodeoxycholate solutions on cyclosporin A bioavailability in rats. Drug Metab. Dispos. 1997, 25, 912–916. [Google Scholar] [PubMed]
- Yao, B.; He, J.; Yin, X.; Shi, Y.; Wan, J.; Tian, Z. The protective effect of lithocholic acid on the intestinal epithelial barrier is mediated by the vitamin D receptor via a SIRT1/Nrf2 and NF-kappaB dependent mechanism in Caco-2 cells. Toxicol. Lett. 2019, 316, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Buki, B.; Junger, H.; Zhang, Y.; Lundberg, Y.W. The Price of Immune Responses and the Role of Vitamin D in the Inner Ear. Otol. Neurotol. 2019, 40, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Minasyan, A.; Keisala, T.; Zhang, Y.; Wang, J.H.; Lou, Y.R.; Kalueff, A.; Pyykko, I.; Tuohimaa, P. Progressive hearing loss in mice with a mutated vitamin D receptor gene. Audiol. Neurootol. 2008, 13, 219–230. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chester, J.; Johnston, E.; Walker, D.; Jones, M.; Ionescu, C.M.; Wagle, S.R.; Kovacevic, B.; Brown, D.; Mikov, M.; Mooranian, A.; et al. A Review on Recent Advancement on Age-Related Hearing Loss: The Applications of Nanotechnology, Drug Pharmacology, and Biotechnology. Pharmaceutics 2021, 13, 1041. https://doi.org/10.3390/pharmaceutics13071041
Chester J, Johnston E, Walker D, Jones M, Ionescu CM, Wagle SR, Kovacevic B, Brown D, Mikov M, Mooranian A, et al. A Review on Recent Advancement on Age-Related Hearing Loss: The Applications of Nanotechnology, Drug Pharmacology, and Biotechnology. Pharmaceutics. 2021; 13(7):1041. https://doi.org/10.3390/pharmaceutics13071041
Chicago/Turabian StyleChester, Jacqueline, Edan Johnston, Daniel Walker, Melissa Jones, Corina Mihaela Ionescu, Susbin Raj Wagle, Božica Kovacevic, Daniel Brown, Momir Mikov, Armin Mooranian, and et al. 2021. "A Review on Recent Advancement on Age-Related Hearing Loss: The Applications of Nanotechnology, Drug Pharmacology, and Biotechnology" Pharmaceutics 13, no. 7: 1041. https://doi.org/10.3390/pharmaceutics13071041







