Glucuronides Hydrolysis by Intestinal Microbial β-Glucuronidases (GUS) Is Affected by Sampling, Enzyme Preparation, Buffer pH, and Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Feces Collection
2.3. Fecal Enzyme Preparation
2.4. pNPG Hydrolysis
2.5. Wogonoside Hydrolysis
2.6. Quantification of Wogonoside and Wogonin Using UPLC
2.7. Optimization of Incubation Buffer and MgCl2 Concentration
2.8. Kinetics of Wogonoside Hydrolysis
2.9. Statistical Analysis
3. Results
3.1. Confirmation of Wogonoside Metabolite Using UPLC
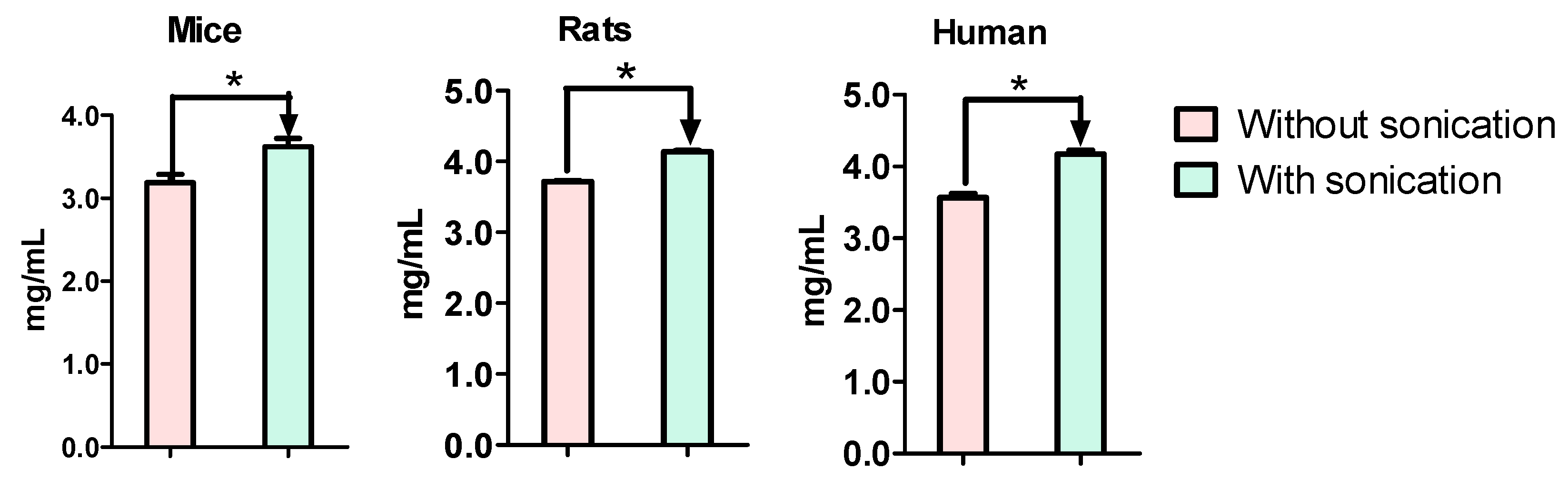
3.2. Total Protein Concentrations Were Different between Sonication and Suspension Preparations
3.3. Preparation Method Affected GUS Activity
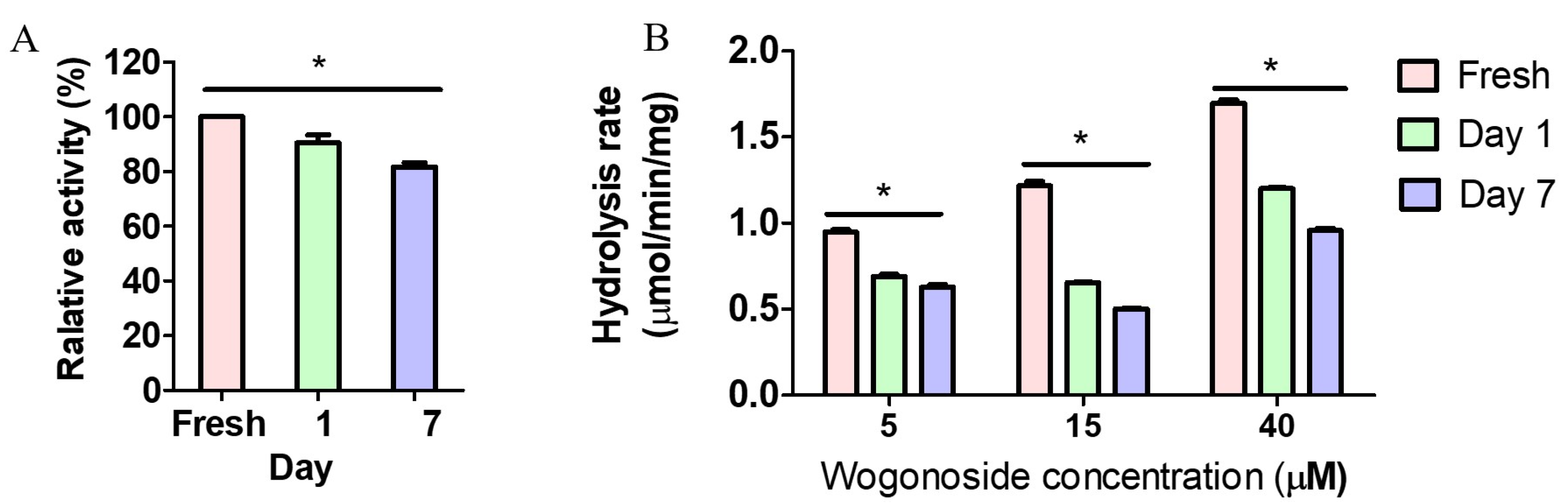
3.4. Feces Collection Time Affected Enzyme Activity
3.5. Magnesium Ions Affected Enzyme Activity
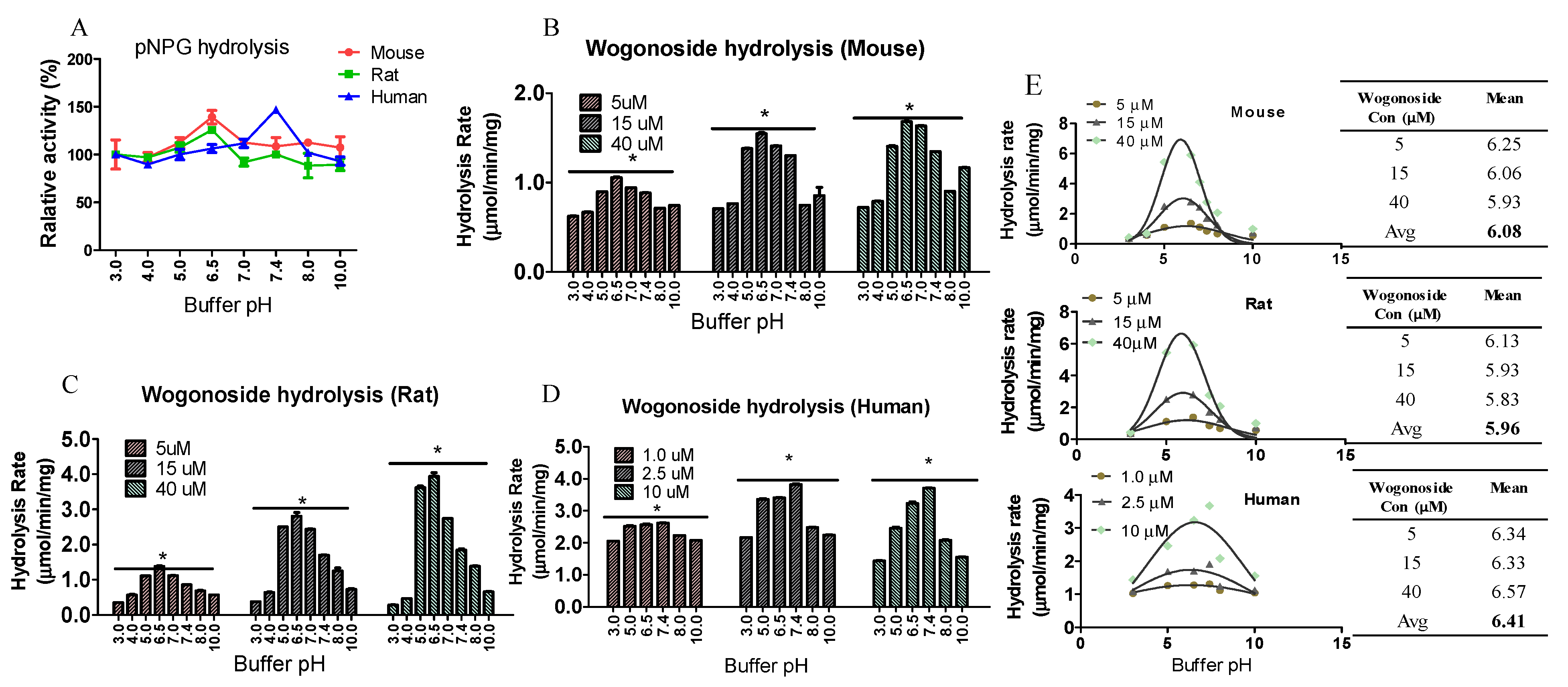
3.6. Buffer pH Value Affected Enzyme Activity
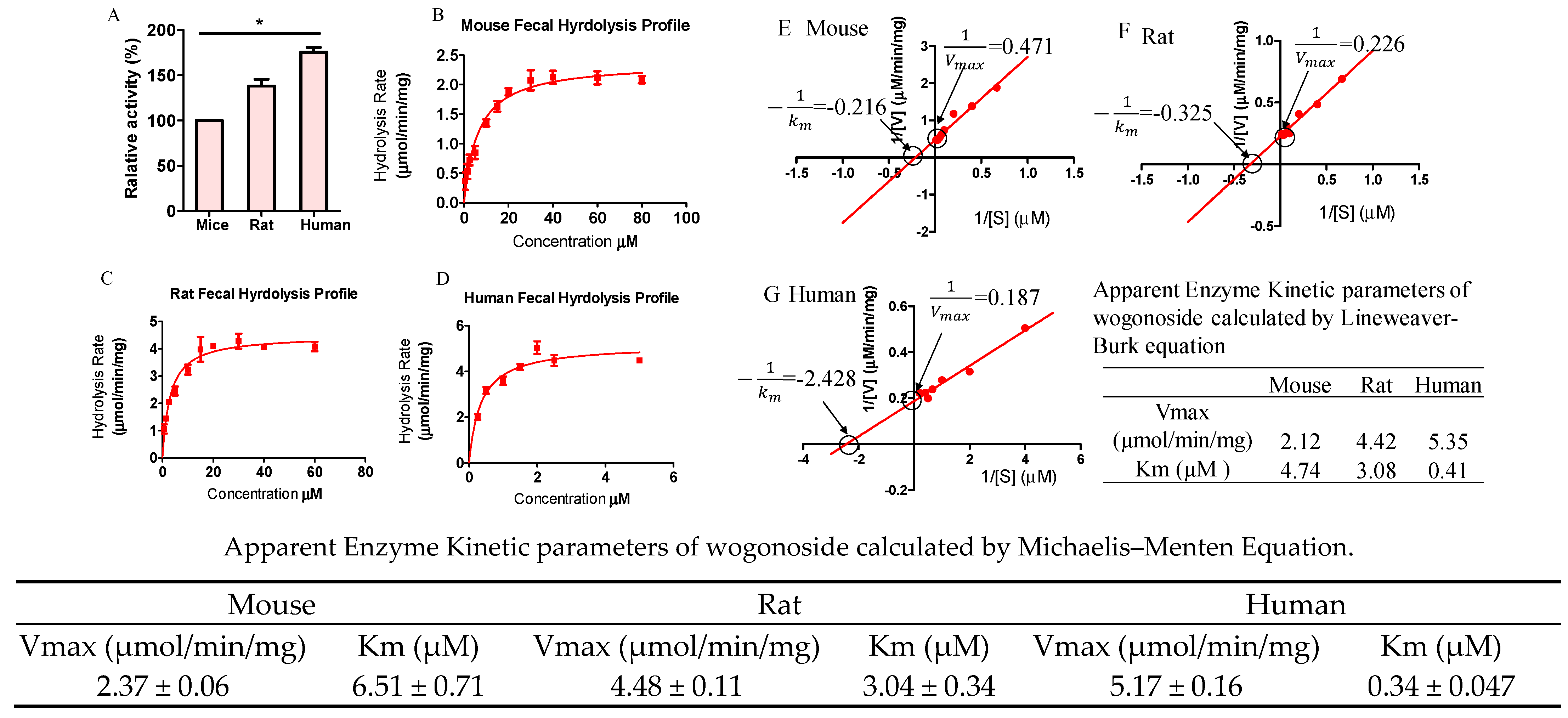
3.7. Hydrolysis Rates Are Different across Species
3.8. Hydrolysis Rates Are Highly Different Across Individuals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frick, J.S.; Autenrieth, I.B. The gut microflora and its variety of roles in health and disease. Curr. Top. Microbiol. Immunol. 2013, 358, 273–289. [Google Scholar]
- Tuohy, K.M.; Rouzaud, G.C.; Bruck, W.M.; Gibson, G.R. Modulation of the human gut microflora towards improved health using prebiotics—Assessment of efficacy. Curr. Pharm. Des. 2005, 11, 75–90. [Google Scholar] [CrossRef]
- Bird, A.R.; Brown, I.L.; Topping, D.L. Starches, resistant starches, the gut microflora and human health. Curr. Issues Intest. Microbiol. 2000, 1, 25–37. [Google Scholar]
- Mayo, B.; Vazquez, L.; Florez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Kim, J.S. Compound K derived from ginseng: Neuroprotection and cognitive improvement. Food Funct. 2016, 7, 4506–4515. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Yang, Y.Y.; Ouyang, D.S.; Yang, G.P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia 2015, 100, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef] [Green Version]
- Islam, A.; Islam, M.S.; Uddin, M.N.; Hasan, M.M.I.; Akanda, M.R. The potential health benefits of the isoflavone glycoside genistin. Arch. Pharm. Res. 2020, 43, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Coldham, N.G.; Darby, C.; Hows, M.; King, L.J.; Zhang, A.Q.; Sauer, M.J. Comparative metabolism of genistin by human and rat gut microflora: Detection and identification of the end-products of metabolism. Xenobiotica 2002, 32, 45–62. [Google Scholar] [CrossRef]
- Tiribelli, C.; Ostrow, J.D. Intestinal flora and bilirubin. J. Hepatol. 2005, 42, 170–172. [Google Scholar] [CrossRef]
- Yang, G.; Ge, S.; Singh, R.; Basu, S.; Shatzer, K.; Zen, M.; Liu, J.; Tu, Y.; Zhang, C.; Wei, J.; et al. Glucuronidation: Driving factors and their impact on glucuronide disposition. Drug Metab. Rev. 2017, 49, 105–138. [Google Scholar] [CrossRef]
- Prijovich, Z.M.; Burnouf, P.A.; Chou, H.C.; Huang, P.T.; Chen, K.C.; Cheng, T.L.; Leu, Y.L.; Roffler, S.R. Synthesis and Antitumor Properties of BQC-Glucuronide, a Camptothecin Prodrug for Selective Tumor Activation. Mol. Pharm. 2016, 13, 1242–1250. [Google Scholar] [CrossRef]
- Nolen, H.W., 3rd; Friend, D.R. Menthol-beta-D-glucuronide: A potential prodrug for treatment of the irritable bowel syndrome. Pharm. Res. 1994, 11, 1707–1711. [Google Scholar] [CrossRef]
- Pellock, S.J.; Redinbo, M.R. Glucuronides in the gut: Sugar-driven symbioses between microbe and host. J. Biol. Chem. 2017, 292, 8569–8576. [Google Scholar] [CrossRef] [Green Version]
- Pollet, R.M.; D’Agostino, E.H.; Walton, W.G.; Xu, Y.; Little, M.S.; Biernat, K.A.; Pellock, S.J.; Patterson, L.M.; Creekmore, B.C.; Isenberg, H.N.; et al. An Atlas of beta-Glucuronidases in the Human Intestinal Microbiome. Structure 2017, 25, 967–977.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellock, S.J.; Walton, W.G.; Ervin, S.M.; Torres-Rivera, D.; Creekmore, B.C.; Bergan, G.; Dunn, Z.D.; Li, B.; Tripathy, A.; Redinbo, M.R. Discovery and Characterization of FMN-Binding beta-Glucuronidases in the Human Gut Microbiome. J. Mol. Biol. 2019, 431, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Galeano-Castaneda, Y.; Urrea-Aguirre, P.; Piedrahita, S.; Bascunan, P.; Correa, M.M. Composition and structure of the culturable gut bacterial communities in Anopheles albimanus from Colombia. PLoS ONE 2019, 14, e0225833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, T.; Smith, D.L.; Yang, Z.; Gao, S.; Yin, T.; Jiang, Z.H.; You, M.; Gibbs, R.A.; Petrosino, J.F.; Hu, M. Bioactivity and bioavailability of ginsenosides are dependent on the glycosidase activities of the A/J mouse intestinal microbiome defined by pyrosequencing. Pharm. Res. 2013, 30, 836–846. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.R.; Hong, S.W.; Kim, Y.; Jang, S.E.; Kim, N.J.; Han, M.J.; Kim, D.H. Metabolic activities of ginseng and its constituents, ginsenoside rb1 and rg1, by human intestinal microflora. J. Ginseng Res. 2011, 35, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, H.K.; Hyun, Y.J.; Jang, S.E.; Han, M.J.; Lee, Y.S.; Kim, D.H. Development of fecal microbial enzyme mix for mutagenicity assay of natural products. J. Microbiol. Biotechnol. 2012, 22, 838–848. [Google Scholar] [CrossRef] [Green Version]
- Phruksawan, W.; Poapolathep, S.; Giorgi, M.; Imsilp, K.; Sakulthaew, C.; Owen, H.; Poapolathep, A. Toxicokinetic profile of fusarenon-X and its metabolite nivalenol in the goat (Capra hircus). Toxicon 2018, 153, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Zhu, C.; Wang, Z.; Luo, H.; Fu, R.; Zhao, X.; Zhao, X.; Jiang, L. Purification and characterization of a glucose-tolerant beta-glucosidase from black plum seed and its structural changes in ionic liquids. Food Chem. 2019, 274, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Boonkaew, B.; Udompaisarn, S.; Arthan, D.; Somana, J. Expression and characterization of a recombinant stevioside hydrolyzing beta-glycosidase from Enterococcus casseliflavus. Protein Expr. Purif. 2019, 163, 105449. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.L.; Sharma, N.; Kumar Singh, A.; Singh Sodhi, S.; Zhang, J.J.; Mongre, R.K.; Ghosh, M.; Kim, N.; Ho Park, Y.; Kee Jeong, D. Anti-tumor activity of wogonin, an extract from Scutellaria baicalensis, through regulating different signaling pathways. Chin. J. Nat. Med. 2017, 15, 15–40. [Google Scholar] [CrossRef]
- Tai, M.C.; Tsang, S.Y.; Chang, L.Y.; Xue, H. Therapeutic potential of wogonin: A naturally occurring. flavonoid. CNS Drug Rev. 2005, 11, 141–150. [Google Scholar] [CrossRef]
- Wei, B.; Wang, P.P.; Yan, Z.X.; Yan, R. Characteristics and molecular determinants of a highly selective and efficient glycyrrhizin-hydrolyzing beta-glucuronidase from Staphylococcus pasteuri 3I10. Appl. Microbiol. Biotechnol. 2018, 102, 9193–9205. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Chadha, B.S.; Kumar, B.A.; Saini, H.S. Purification and characterization of two endoglucanases from Melanocarpus sp. MTCC 3922. Bioresour. Technol. 2007, 98, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Biernat, K.A.; Pellock, S.J.; Bhatt, A.P.; Bivins, M.M.; Walton, W.G.; Tran, B.N.T.; Wei, L.; Snider, M.C.; Cesmat, A.P.; Tripathy, A.; et al. Structure, function, and inhibition of drug reactivating human gut microbial beta-glucuronidases. Sci. Rep. 2019, 9, 825. [Google Scholar] [CrossRef] [Green Version]
- Badee, J.; Qiu, N.; Parrott, N.; Collier, A.C.; Schmidt, S.; Fowler, S. Optimization of Experimental Conditions of Automated Glucuronidation Assays in Human Liver Microsomes Using a Cocktail Approach and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Drug Metab. Dispos. 2019, 47, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut microbial beta-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 2019, 294, 18586–18599. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Pellock, S.J.; Biernat, K.A.; Walton, W.G.; Wallace, B.D.; Creekmore, B.C.; Letertre, M.M.; Swann, J.R.; Wilson, I.D.; Roques, J.R.; et al. Targeted inhibition of gut bacterial beta-glucuronidase activity enhances anticancer drug efficacy. Proc. Natl. Acad. Sci. USA 2020, 117, 7374–7381. [Google Scholar] [CrossRef] [Green Version]
- Kinouchi, T.; Nishifuji, K.; Ohnishi, Y. In vitro intestinal microflora-mediated metabolism of biliary metabolites from 1-nitropyrene-treated rats. Microbiol. Immunol. 1987, 31, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wei, B.; Yan, R. Amoxapine Demonstrates Incomplete Inhibition of beta-Glucuronidase Activity from Human Gut Microbiota. SLAS Discov. 2018, 23, 76–83. [Google Scholar]
- Cowan, J.A. Structural and catalytic chemistry of magnesium-dependent enzymes. Biometals 2002, 15, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Yoo, P.; Lee, T.; Klopf, W.; Takao, D. The role of pH in the glucuronidation of raloxifene, mycophenolic acid and ezetimibe. Mol. Pharm. 2009, 6, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Basit, A.W.; Murdan, S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J. Pharm. Pharmacol. 2008, 60, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan Med. Bull. 1999, 46, 183–196. [Google Scholar]
- Fang, Y.; Gao, J.; Wang, T.; Tian, X.; Gao, N.; Zhou, J.; Zhang, H.F.; Wen, Q.; Jin, H.; Xing, Y.R.; et al. Intraindividual Variation and Correlation of Cytochrome P450 Activities in Human Liver Microsomes. Mol. Pharm. 2018, 15, 5312–5318. [Google Scholar] [CrossRef]
- Den Braver-Sewradj, S.P.; den Braver, M.W.; van Dijk, M.; Zhang, Y.; Dekker, S.J.; Wijaya, L.; Vermeulen, N.P.E.; Richert, L.; Commandeur, J.N.M.; Vos, J.C. Inter-individual Variability in Activity of the Major Drug Metabolizing Enzymes in Liver Homogenates of 20 Individuals. Curr. Drug Metab. 2018, 19, 370–381. [Google Scholar] [CrossRef]
- Toutain, P.L.; Ferran, A.; Bousquet-Melou, A. Species differences in pharmacokinetics and pharmacodynamics. Handb. Exp. Pharmacol. 2010, 19–48. [Google Scholar] [CrossRef]
- Creekmore, B.C.; Gray, J.H.; Walton, W.G.; Biernat, K.A.; Little, M.S.; Xu, Y.; Liu, J.; Gharaibeh, R.Z.; Redinbo, M.R. Mouse Gut Microbiome-Encoded beta-Glucuronidases Identified Using Metagenome Analysis Guided by Protein Structure. mSystems 2019, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugenholtz, F.; de Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, B.W.; Campbell, W.L.; Franklin, W.; Delclos, K.B.; Cerniglia, C.E. Metabolism of 6-nitrochrysene by intestinal microflora. Appl. Environ. Microbiol. 1988, 54, 197–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebuzoeme, C.; Etim, I.; Ikimi, A.; Song, J.; Du, T.; Hu, M.; Liang, D.; Gao, S. Glucuronides Hydrolysis by Intestinal Microbial β-Glucuronidases (GUS) Is Affected by Sampling, Enzyme Preparation, Buffer pH, and Species. Pharmaceutics 2021, 13, 1043. https://doi.org/10.3390/pharmaceutics13071043
Ebuzoeme C, Etim I, Ikimi A, Song J, Du T, Hu M, Liang D, Gao S. Glucuronides Hydrolysis by Intestinal Microbial β-Glucuronidases (GUS) Is Affected by Sampling, Enzyme Preparation, Buffer pH, and Species. Pharmaceutics. 2021; 13(7):1043. https://doi.org/10.3390/pharmaceutics13071043
Chicago/Turabian StyleEbuzoeme, Christabel, Imoh Etim, Autumn Ikimi, Jamie Song, Ting Du, Ming Hu, Dong Liang, and Song Gao. 2021. "Glucuronides Hydrolysis by Intestinal Microbial β-Glucuronidases (GUS) Is Affected by Sampling, Enzyme Preparation, Buffer pH, and Species" Pharmaceutics 13, no. 7: 1043. https://doi.org/10.3390/pharmaceutics13071043





