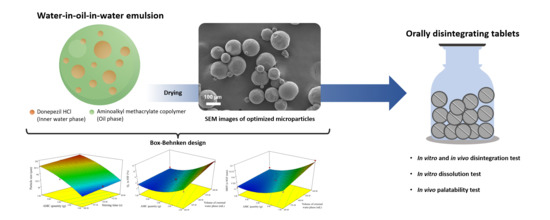
Optimized Taste-Masked Microparticles for Orally Disintegrating Tablets as a Promising Dosage Form for Alzheimer’s Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Identification of Factors Affecting Microparticle Preparation Using Quality Risk Assessment
2.3. Microparticle Preparation by DESE Technique
2.4. Optimization of DPH-Loaded Microparticles Using BBD
2.5. Characterization of DPH-Loaded Microparticles
2.5.1. Size Determination of Microparticles by Image Analysis
2.5.2. Morphology of Microparticles
2.5.3. Drug Content Measurement
2.5.4. Determination of Residual Solvent
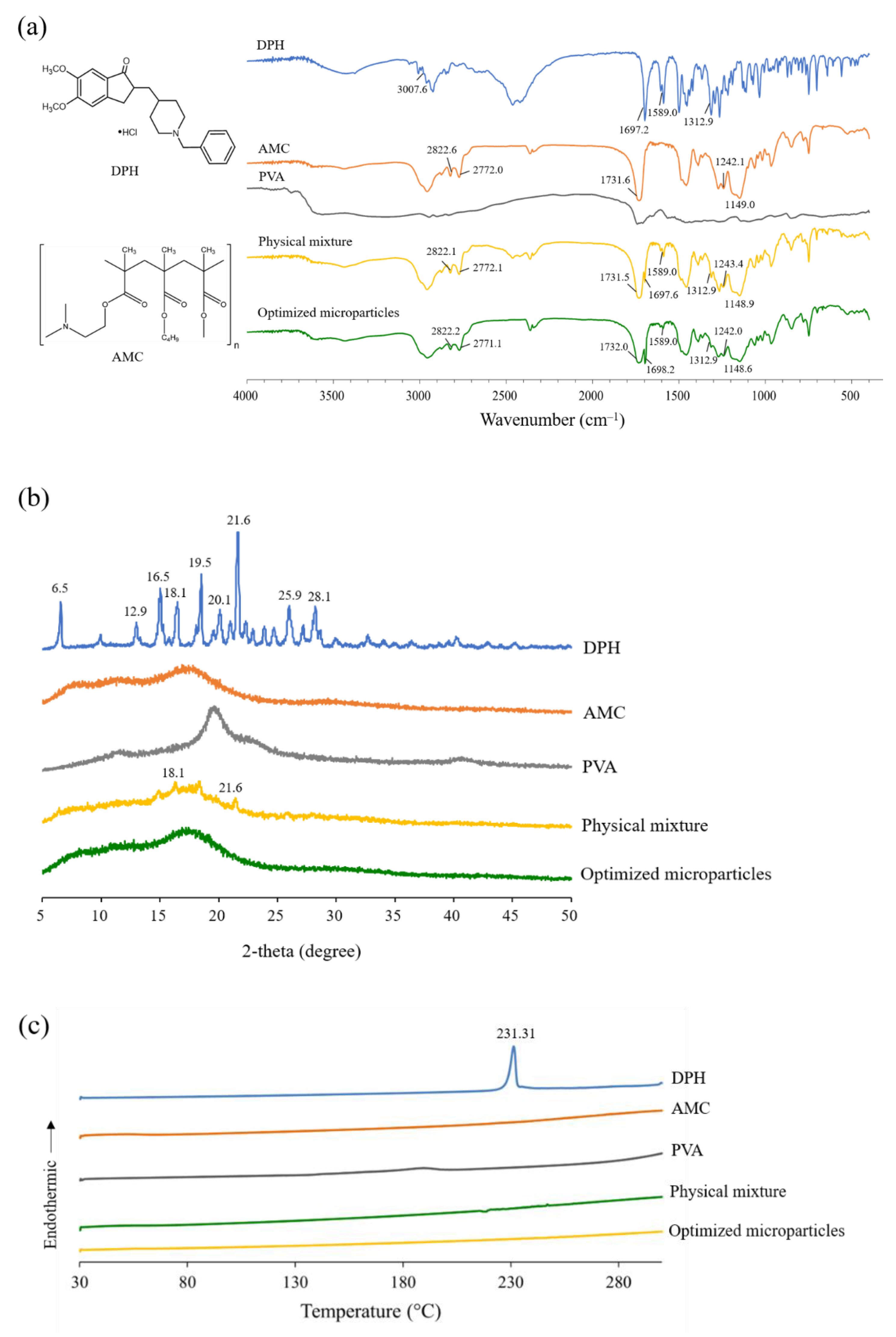
2.5.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.5.6. Powder X-ray Diffractometry (PXRD)
2.5.7. Differential Scanning Calorimetry (DSC) Measurement
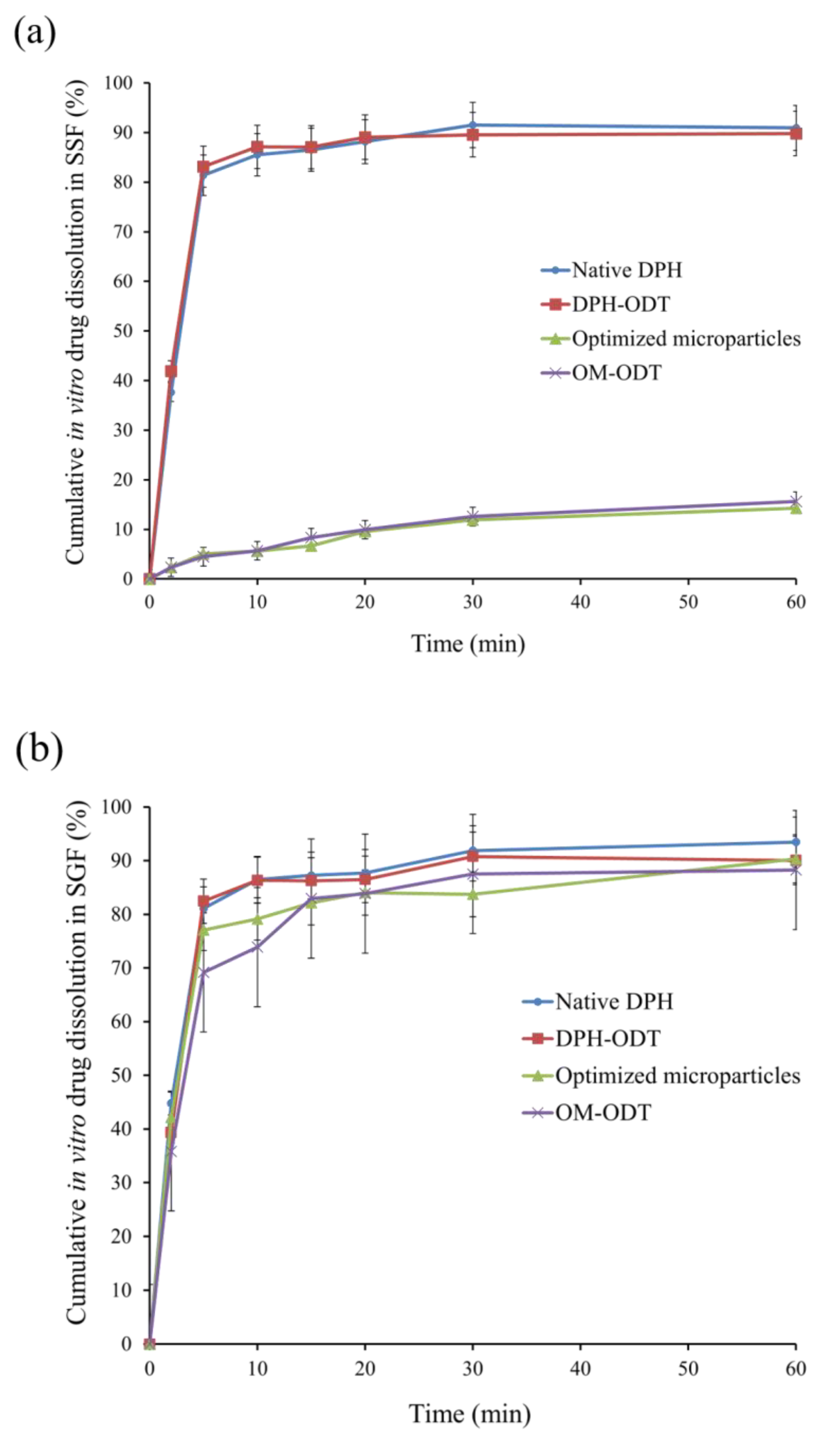
2.5.8. In vitro Dissolution Test of Microparticles
2.6. Preparation of ODTs
2.7. Characterization of ODTs
2.7.1. Thickness, and Hardness Measurement
2.7.2. Friability Test
2.7.3. In Vitro Disintegration Test
2.7.4. In Vitro Dissolution Test
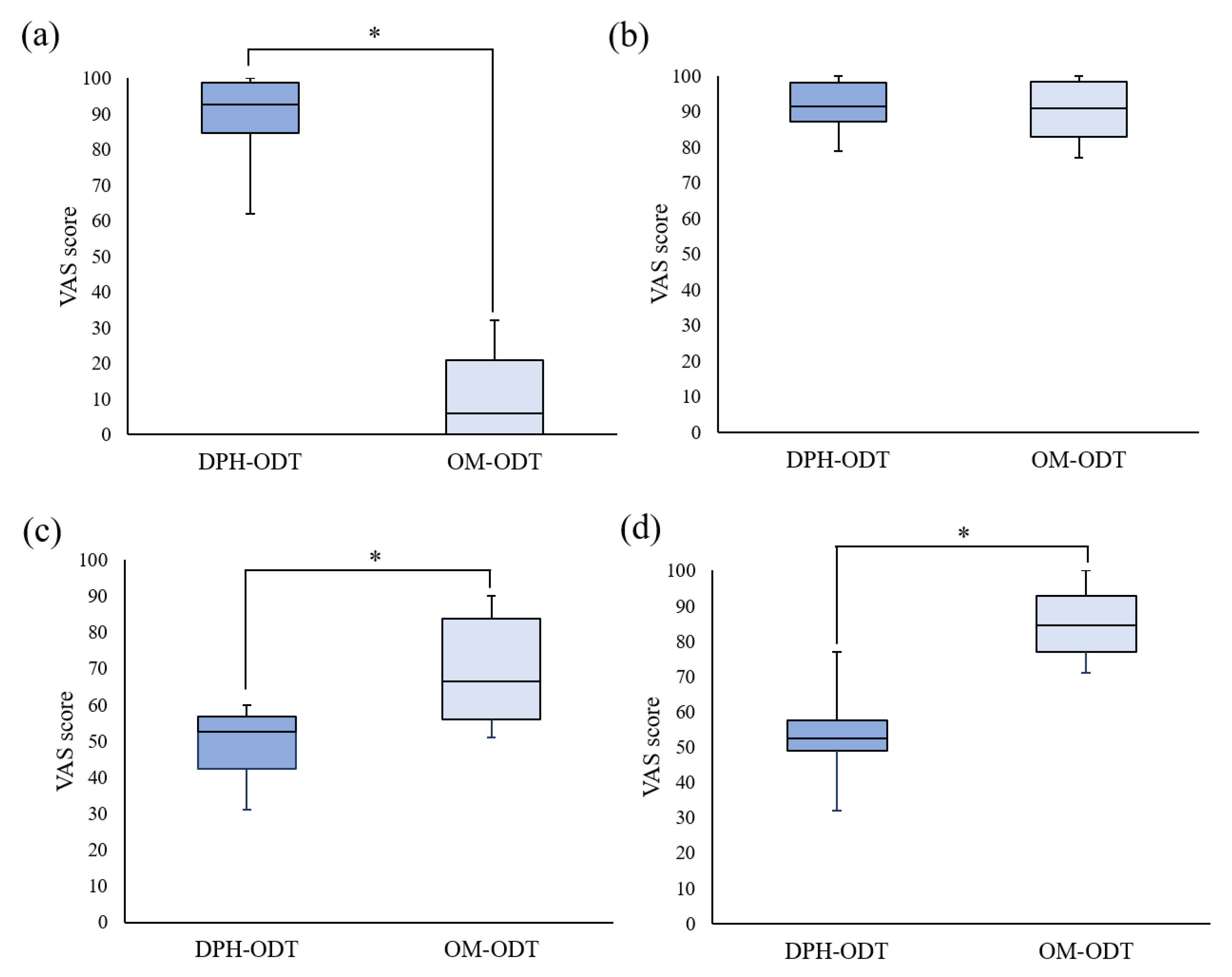
2.7.5. In Vivo Evaluation
Perception and Bitterness Threshold Determination
- I did not feel any difference between solution A and E
- I felt something, but I cannot differentiate the taste
- I felt a bitter taste
Palatability Test
3. Results and Discussion
3.1. Study on Factors Affecting Microparticle Preparation
3.2. Experimental Design
3.2.1. Effect of Independent Factors on Particle Size
3.2.2. Effect of Independent Factors on Q5 in SSF
3.2.3. Effect of Independent Factors on MDT in SGF
3.2.4. Validation of Mathematical Model
3.2.5. Optimization
3.3. Characterization of Optimized Microparticles
3.4. Preparation and Evaluation of ODTs
3.4.1. Physical Properties of ODTs
3.4.2. In Vitro Evaluation of ODTs
3.4.3. In Vivo Disintegration Test and Taste Evaluation of ODTs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Population Division of United Nations World Population Ageing. 2019. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf (accessed on 11 December 2020).
- Zvěřová, M. Clinical aspects of Alzheimer’s disease. Clin. Biochem. 2019, 72, 3–6. [Google Scholar] [CrossRef]
- Sugimoto, H.; Ogura, H.; Arai, Y.; Iimura, Y.; Yamanishi, Y. Research and development of donepezil hydrochloride, a new type of acetylcholinesterase inhibitor. Jpn. J. Pharmacol. 2002, 89, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Alzheimer’s Association FDA-Approved Treatments for Alzheimer’s. Available online: https://www.alz.org/media/documents/fda-approved-treatments-alzheimers-ts.pdf (accessed on 10 December 2020).
- Affoo, R.H.; Foley, N.; Rosenbek, J.; Kevin Shoemaker, J.; Martin, R.E. Swallowing dysfunction and autonomic nervous system dysfunction in Alzheimer’s disease: A scoping review of the evidence. J. Am. Geriatr. Soc. 2013, 61, 2203–2213. [Google Scholar] [CrossRef]
- Sutthapitaksakul, L.; Dass, C.R.; Sriamornsak, P. Donepezil—An updated review of challenges in dosage form design. J. Drug Deliv. Sci. Technol. 2021, 63, 102549. [Google Scholar] [CrossRef]
- Moffat, A.; Osseltion, M.; Widdop, P. (Eds.) Clarke’s Analysis and Poisons, 4th ed.; Pharmaceutical Press: London, UK, 2011; ISBN 9780127059501. [Google Scholar]
- Li, M.; Rouaud, O.; Poncelet, D. Microencapsulation by solvent evaporation: State of the art for process engineering approaches. Int. J. Pharm. 2008, 363, 26–39. [Google Scholar] [CrossRef]
- Iqbal, M.; Zafar, N.; Fessi, H.; Elaissari, A. Double emulsion solvent evaporation techniques used for drug encapsulation. Int. J. Pharm. 2015, 496, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Hombreiro-Pérez, M.; Siepmann, J.; Zinutti, C.; Lamprecht, A.; Ubrich, N.; Hoffman, M.; Bodmeier, R.; Maincent, P. Non-degradable microparticles containing a hydrophilic and/or a lipophilic drug: Preparation, characterization and drug release modeling. J. Control Release 2003, 88, 413–428. [Google Scholar] [CrossRef]
- Cohen-Sela, E.; Chorny, M.; Koroukhov, N.; Danenberg, H.D.; Golomb, G. A new double emulsion solvent diffusion technique for encapsulating hydrophilic molecules in PLGA nanoparticles. J. Control Release 2009, 133, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Gaignaux, A.; Réeff, J.; Siepmann, F.; Siepmann, J.; De Vriese, C.; Goole, J.; Amighi, K. Development and evaluation of sustained-release clonidine-loaded PLGA microparticles. Int. J. Pharm. 2012, 437, 20–28. [Google Scholar] [CrossRef]
- Ding, S.; Serra, C.A.; Vandamme, T.F.; Yu, W.; Anton, N. Double emulsions prepared by two–step emulsification: History, state-of-the-art and perspective. J. Control Release 2019, 295, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Benjasirimongkol, P.; Piriyaprasarth, S.; Moribe, K.; Sriamornsak, P. Use of risk assessment and Plackett–Burman design for developing resveratrol spray-dried emulsions: A quality-by-design approach. AAPS PharmSciTech 2019, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Benjasirimongkol, P.; Piriyaprasarth, S.; Sriamornsak, P. Design and optimization of resveratrol-loaded porous calcium silicate powders for dissolution and photostability enhancement. Heliyon 2019, 5, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutthapitaksakul, L.; Sriamornsak, P. Effect of processing parameters on release profiles of donepezil hydrochloride-loaded microparticles. Key Eng. Mater. 2020, 859, 283–288. [Google Scholar] [CrossRef]
- Sutthapitaksakul, L.; Sriamornsak, P. Influence of process parameters on the characteristics of hydrophilic drug-loaded microparticles through double emulsion solvent evaporation technique. Key Eng. Mater. 2019, 819, 252–257. [Google Scholar] [CrossRef]
- Hoashi, Y.; Tozuka, Y.; Takeuchi, H. Development of a novel and simple method to evaluate disintegration of rapidly disintegrating tablets. Chem. Pharm. Bull. 2013, 61, 962–966. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Wan, X.; Luo, Z.; Liu, C.; Quan, P.; Cun, D.; Fang, L. A donepezil/cyclodextrin complexation orodispersible film: Effect of cyclodextrin on taste-masking based on dynamic process and in vivo drug absorption. Asian J. Pharm. Sci. 2019, 14, 183–192. [Google Scholar] [CrossRef]
- Pisani, E.; Fattal, E.; Paris, J.; Ringard, C.; Rosilio, V.; Tsapis, N. Surfactant dependent morphology of polymeric capsules of perfluorooctyl bromide: Influence of polymer adsorption at the dichloromethane-water interface. J. Colloid Interface Sci. 2008, 326, 66–71. [Google Scholar] [CrossRef]
- Karataş, A.; Sonakin, Ö.Ö.; KiliÇarslan, M.; Baykara, T. Poly (ε-caprolactone) microparticles containing levobunolol HCl prepared by a multiple emulsion (W/O/W) solvent evaporation technique: Effects of some formulation parameters on microparticle characteristics. J. Microencapsul. 2009, 26, 63–74. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Tanaka, M.; Kishimoto, H.; Shiozawa, H.; Hasegawa, K.; Matsuyama, K.; Uchida, T. Preparation, characterization and taste-masking properties of polyvinylacetal diethylaminoacetate microspheres containing trimebutine. J. Pharm. Pharmacol. 2002, 54, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.H.; Kim, T.H.; Lee, S.; Chen, X.; Youn, Y.S.; Lee, K.C. PEGylated TNF-related apoptosis-inducing ligand (TRAIL) for effective tumor combination therapy. Biomaterials 2011, 32, 8529–8537. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian, E.; Vatanara, A.; Rouholamini Najafabadi, A.; Rouini, M.R.; Gilani, K.; Darabi, M. Preparation, characterization and optimization of sildenafil citrate loaded PLGA nanoparticles by statistical factorial design. DARU J. Pharm. Sci. 2013, 21, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bouriche, S.; Cózar-Bernal, M.J.; Rezgui, F.; Rabasco Álvarez, A.M.; González-Rodríguez, M.L. Optimization of preparation method by W/O/W emulsion for entrapping metformin hydrochloride into poly (lactic acid) microparticles using Box-Behnken design. J. Drug Deliv. Sci. Technol. 2019, 51, 419–429. [Google Scholar] [CrossRef]
- Pal, R. Rheology of simple and multiple emulsions. Curr. Opin. Colloid Interface Sci. 2011, 16, 41–60. [Google Scholar] [CrossRef]
- Alex, R.; Bodmeier, R. Encapsulation of water-soluble drugs by a modified solvent evaporation method. I. effect of process and formulation variables on drug entrapment. J. Microencapsul. 1990, 7, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Shimizu, M.; Kukizaki, M. Particle control of emulsion by membrane emulsification and its applications. Adv. Drug Deliv. Rev. 2000, 45, 47–56. [Google Scholar] [CrossRef]
- Siewert, M.; Dressman, J.; Brown, C.K.; Shah, V.P. FIP/AAPS guidelines for dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech 2003, 65, 129–134. [Google Scholar]
- Sohi, H.; Sultana, Y.; Khar, R.K. Taste masking technologies in oral pharmaceuticals: Recent developments and approaches. Drug Dev. Ind. Pharm. 2004, 30, 429–448. [Google Scholar] [CrossRef] [Green Version]
- Aserin, A. Multiple Emulsions: Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 0470209259. [Google Scholar]
- Nakano, Y.; Maeda, A.; Uchida, S.; Namiki, N. Preparation and evaluation of unpleasant taste-masked pioglitazone orally disintegrating tablets. Int. J. Pharm. 2013, 446, 160–165. [Google Scholar] [CrossRef]
- Kimura, S.I.; Uchida, S.; Kanada, K.; Namiki, N. Effect of granule properties on rough mouth feel and palatability of orally disintegrating tablets. Int. J. Pharm. 2015, 484, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Burapapadh, K.; Takeuchi, H.; Sriamornsak, P. Novel pectin-based nanoparticles prepared from nanoemulsion templates for improving in vitro dissolution and in vivo absorption of poorly water-soluble drug. Eur. J. Pharm. Biopharm. 2012, 82, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Weerapol, Y.; Cheewatanakornkool, K.; Sriamornsak, P. Impact of gastric pH and dietary fiber on calcium availability of various calcium salts. Silpakorn Univ. Sci. Technol. J. 2010, 4, 15–23. [Google Scholar] [CrossRef]
- Yan, Y.D.; Woo, J.S.; Kang, J.H.; Yong, C.S.; Choi, H.-G. Preparation and evaluation of taste-masked donepezil hydrochloride orally disintegrating tablets. Biol. Pharm. Bull. 2010, 33, 1364–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zainuddin, R.; Kulkarni, A.; Chavan, H.; Patil, G.; Zaheer, Z. Taste masking of donepezil hydrochloride using different ion exchange resins—A comparative study. Indones. J. Pharm. 2013, 24, 107–115. [Google Scholar]
- Huanbutta, K.; Yunsir, A.; Sriamornsak, P.; Sangnim, T. Development and in vitro/in vivo evaluation of tamarind seed gum-based oral disintegrating tablets after fabrication by freeze drying. J. Drug Deliv. Sci. Technol. 2019, 54, 101298. [Google Scholar] [CrossRef]
- Huanbutta, K.; Sriamornsak, P.; Singh, I.; Sangnim, T. Manufacture of 2D-printed precision drug-loaded orodispersible film prepared from tamarind seed gum substrate. Appl. Sci. 2021, 11, 5852. [Google Scholar] [CrossRef]





| Ingredients | Quantity (mg/tablet) | |
|---|---|---|
| DPH-ODT | OM-ODT | |
| DPH | 5 | - |
| Optimized microparticles | - | 28.5 |
| Mannitol | 90 | 90 |
| Spray-dried lactose monohydrate | 72 | 48.5 |
| Microcrystalline cellulose | 21 | 21 |
| Crospovidone | 8 | 8 |
| PVP K-30 | 2 | 2 |
| Magnesium stearate | 2 | 2 |
| Factors | CQAs | |
|---|---|---|
| Particle Size | Drug Dissolution | |
| Formulation factors: | ||
| Drug amount | Medium | Low |
| Organic solvent amount | Low | Low |
| Polymer amount | High | High |
| Stabilizer concentration | High | High |
| pH of external water phase | Low | Low |
| Volume of internal phase | Medium | Medium |
| Volume of oil phase | Medium | Low |
| Volume of external water phase | High | High |
| Operating conditions: | ||
| Ultrasonication time in the first emulsification | Medium | Low |
| Stirring time in the second emulsification | High | High |
| Stirring rate in the second emulsification | Medium | Low |
| Parameter | Level | |||||
| Low (−1) | Medium (0) | High (+1) | ||||
| Independent factors | ||||||
| X1: AMC amount (g) | 3 | 5 | 7 | |||
| X2: Stirring time (s) | 90 | 135 | 180 | |||
| X3: Volume of outer water phase (mL) | 350 | 400 | 450 | |||
| Responses | ||||||
| Y1: Particle size (µm) | ||||||
| Y2: Q5 in SSF (%) | ||||||
| Y3: MDT in SGF (min) | ||||||
| Standard Run Order | Independent Factors | Experimental Values of Responses | ||||
| X1 | X2 | X3 | Y1 | Y2 | Y3 | |
| 1 | 3 | 90 | 400 | 116.24 | 14.23 | 5.15 |
| 2 | 7 | 90 | 400 | 247.73 | 5.41 | 9.86 |
| 3 | 3 | 180 | 400 | 82.50 | 11.71 | 5.27 |
| 4 | 7 | 180 | 400 | 213.02 | 3.64 | 10.52 |
| 5 | 3 | 135 | 350 | 113.82 | 13.90 | 4.34 |
| 6 | 7 | 135 | 350 | 226.01 | 5.32 | 7.56 |
| 7 | 3 | 135 | 450 | 99.31 | 8.21 | 6.32 |
| 8 | 7 | 135 | 450 | 228.61 | 4.79 | 12.67 |
| 9 | 5 | 90 | 350 | 194.25 | 6.50 | 5.78 |
| 10 | 5 | 180 | 350 | 157.52 | 7.57 | 6.03 |
| 11 | 5 | 90 | 450 | 186.28 | 5.45 | 7.54 |
| 12 | 5 | 180 | 450 | 174.72 | 4.93 | 7.11 |
| 13 | 5 | 135 | 400 | 184.82 | 5.46 | 5.17 |
| 14 | 5 | 135 | 400 | 187.53 | 6.20 | 5.67 |
| 15 | 5 | 135 | 400 | 193.62 | 5.54 | 6.14 |
| 16 | 5 | 135 | 400 | 185.23 | 4.35 | 7.78 |
| 17 | 5 | 135 | 400 | 183.72 | 5.47 | 6.03 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value, prob > F |
|---|---|---|---|---|---|
| Y1 | |||||
| Model | 34,641.40 | 3 | 11,547.13 | 236.38 | <0.0001 |
| X1 | 31,689.03 | 1 | 31,689.03 | 648.72 | <0.0001 |
| X2 | 1703.53 | 1 | 1703.53 | 34.87 | <0.0001 |
| X12 | 1248.84 | 1 | 1248.84 | 25.57 | 0.0002 |
| Residual | 635.04 | 13 | 48.85 | ||
| Lack of fit | 572.29 | 9 | 63.59 | 4.05 | 0.0954 |
| Pure error | 62.75 | 4 | 15.69 | ||
| Cor total | 35,276.44 | 16 | |||
| Regression coefficient: R2 = 0.9820, adjusted R2 = 0.9778, predicted R2 = 0.9693 | |||||
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value, prob > F |
|---|---|---|---|---|---|
| Y2 | |||||
| Model | 153.73 | 4 | 38.43 | 35.25 | <0.0001 |
| X1 | 104.33 | 1 | 104.33 | 95.69 | <0.0001 |
| X3 | 12.28 | 1 | 12.28 | 11.26 | 0.0057 |
| X1X3 | 6.66 | 1 | 6.66 | 6.11 | 0.0294 |
| X12 | 30.47 | 1 | 30.47 | 27.95 | 0.0002 |
| Residual | 13.08 | 12 | 1.09 | ||
| Lack of fit | 11.31 | 8 | 1.41 | 3.19 | 0.1384 |
| Pure error | 1.77 | 4 | 0.44 | ||
| Cor total | 166.82 | 16 | |||
| Regression coefficient: R2 = 0.9216, adjusted R2 = 0.8954, predicted R2 = 0.7923 | |||||
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value, prob > F |
|---|---|---|---|---|---|
| Y3 | |||||
| Model | 72.92 | 4 | 18.23 | 45.27 | <0.0001 |
| X1 | 47.68 | 1 | 47.68 | 118.39 | <0.0001 |
| X3 | 12.33 | 1 | 12.33 | 30.61 | 0.0001 |
| X1X3 | 2.45 | 1 | 2.45 | 6.08 | 0.0297 |
| X12 | 10.47 | 1 | 10.47 | 26.00 | 0.0003 |
| Residual | 4.83 | 12 | 0.40 | ||
| Lack of fit | 4.26 | 8 | 0.53 | 3.71 | 0.1104 |
| Pure error | 0.57 | 4 | 0.14 | ||
| Cor total | 77.76 | 16 | |||
| Regression coefficient: R2 = 0.9379, adjusted R2 = 0.9171, predicted R2 = 0.8217 | |||||
| Independent Factors | |||
| No. | AMC Amount (g) | Stirring Time (s) | Volume of Water Phase (mL) |
| F1 | 5.5 | 120 | 350 |
| F2 | 4.8 | 160 | 380 |
| F3 | 5.2 | 100 | 430 |
| Responses | |||
| No. | Actual value | Predicted value | RMSE |
| Particle size (µm) | |||
| F1 | 209.29 | 202.60 | 11.63 |
| F2 | 159.20 | 168.50 | |
| F3 | 217.11 | 200.55 | |
| Q5 in SSF (%) | |||
| F1 | 5.51 | 5.90 | 0.50 |
| F2 | 6.97 | 6.65 | |
| F3 | 5.43 | 4.72 | |
| MDT in SGF (min) | |||
| F1 | 6.49 | 5.41 | 1.06 |
| F2 | 6.62 | 5.45 | |
| F3 | 8.10 | 7.19 | |
| Formulation | Thickness (mm) ± SD | Hardness (N) ± SD | In Vitro Disintegration Time (s) ± SD | Friability (%) | In Vivo Disintegration Time (s) ± SD |
|---|---|---|---|---|---|
| DPH-ODTs | 2.18 ± 0.02 | 38.0 ± 2.8 | 13.0 ± 0.8 | 0.90 | 22.6 ± 3.4 |
| OM-ODTs | 2.34 ± 0.05 | 42.0 ± 0.8 | 14.0 ± 1.2 | 0.76 | 18.8 ± 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutthapitaksakul, L.; Thanawuth, K.; Dass, C.R.; Sriamornsak, P. Optimized Taste-Masked Microparticles for Orally Disintegrating Tablets as a Promising Dosage Form for Alzheimer’s Disease Patients. Pharmaceutics 2021, 13, 1046. https://doi.org/10.3390/pharmaceutics13071046
Sutthapitaksakul L, Thanawuth K, Dass CR, Sriamornsak P. Optimized Taste-Masked Microparticles for Orally Disintegrating Tablets as a Promising Dosage Form for Alzheimer’s Disease Patients. Pharmaceutics. 2021; 13(7):1046. https://doi.org/10.3390/pharmaceutics13071046
Chicago/Turabian StyleSutthapitaksakul, Lalinthip, Kasitpong Thanawuth, Crispin R. Dass, and Pornsak Sriamornsak. 2021. "Optimized Taste-Masked Microparticles for Orally Disintegrating Tablets as a Promising Dosage Form for Alzheimer’s Disease Patients" Pharmaceutics 13, no. 7: 1046. https://doi.org/10.3390/pharmaceutics13071046
APA StyleSutthapitaksakul, L., Thanawuth, K., Dass, C. R., & Sriamornsak, P. (2021). Optimized Taste-Masked Microparticles for Orally Disintegrating Tablets as a Promising Dosage Form for Alzheimer’s Disease Patients. Pharmaceutics, 13(7), 1046. https://doi.org/10.3390/pharmaceutics13071046