Antimelanogenesis Effects of Leaf Extract and Phytochemicals from Ceylon Olive (Elaeocarpus serratus) in Zebrafish Model
Abstract
:1. Introduction
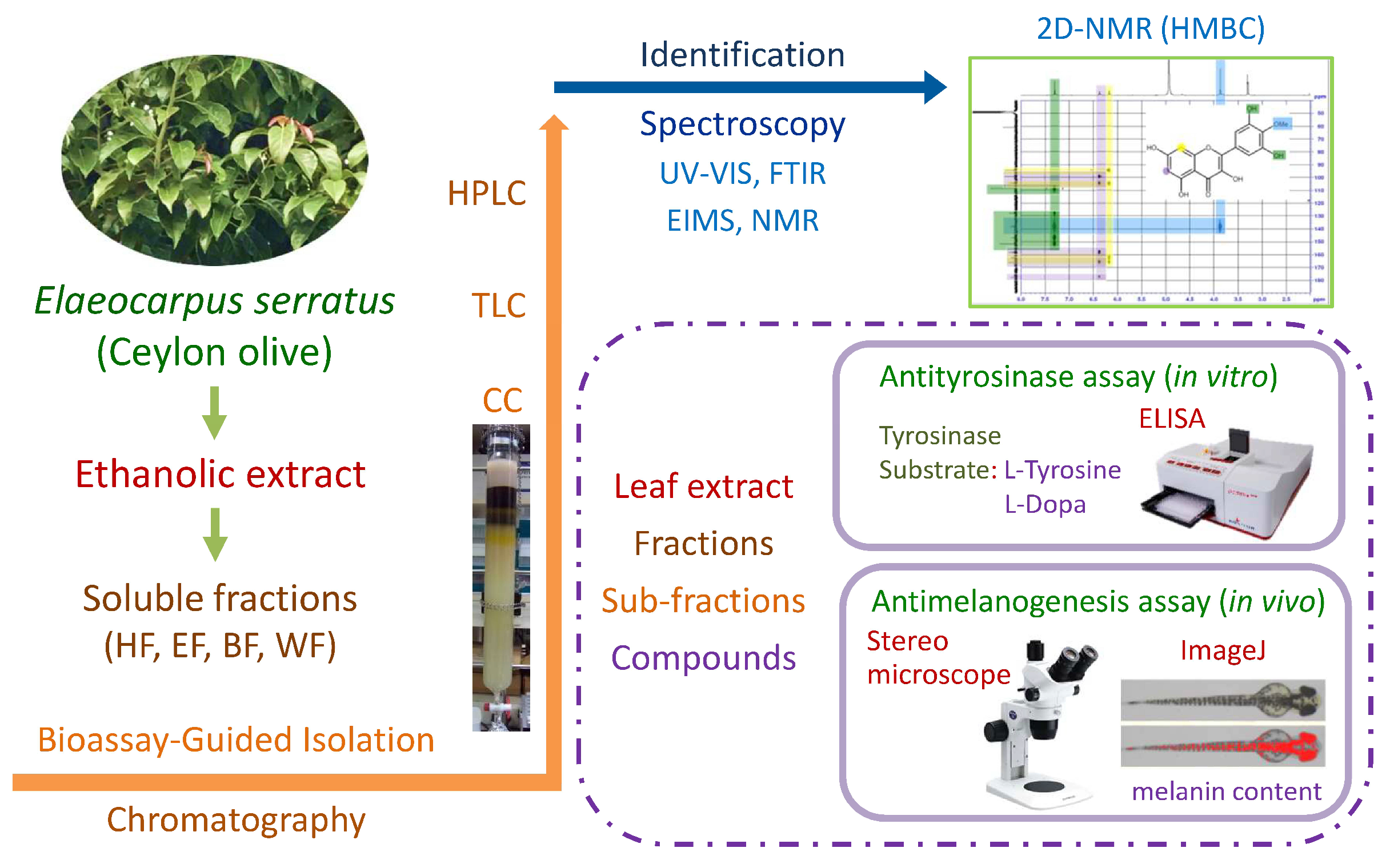
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Liquid–Liquid Partition
2.3. Column Chromatography and Thin Layer Chromatography
2.4. High-Performance Liquid Chromatography
2.5. Identification of Isolated Compounds
2.6. Antityrosinase Assay and Enzyme Kinetic Study
2.7. Evaluation of Antimelanogenesis Effect in Zebrafish
2.8. Statistical Analysis
3. Results and Discussion
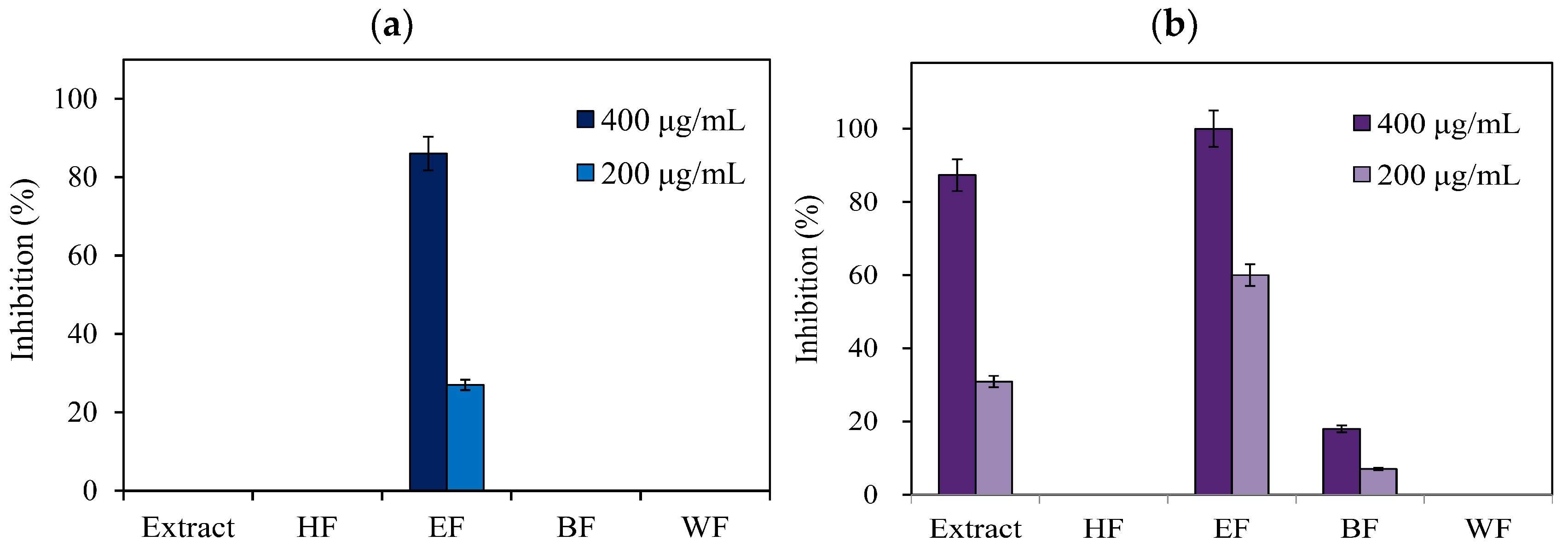
3.1. Antityrosinase Activity of E. serratus Leaf Extract and Its Fractions
3.2. Isolation and Identification of Compounds from Bioactive Subfractions
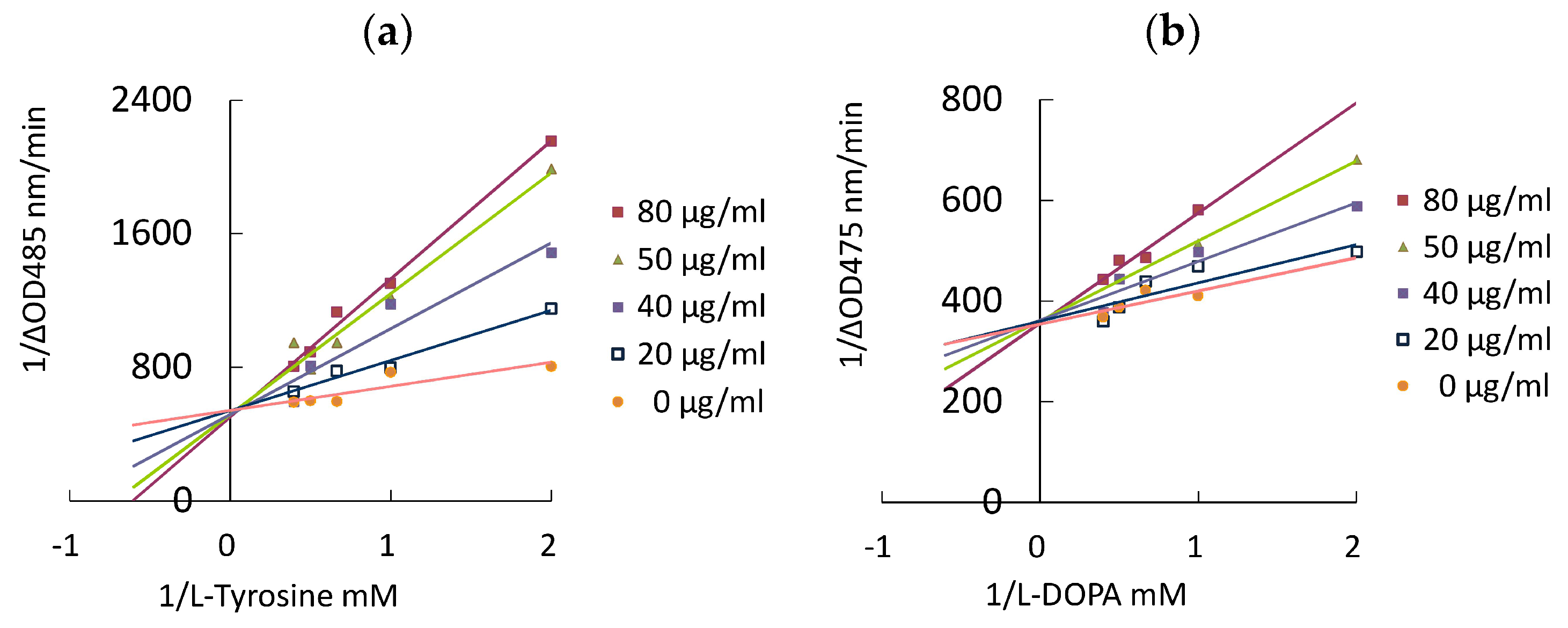
3.3. Antityrosinase Activity and Enzyme Kinetic Study of Isolated Compounds
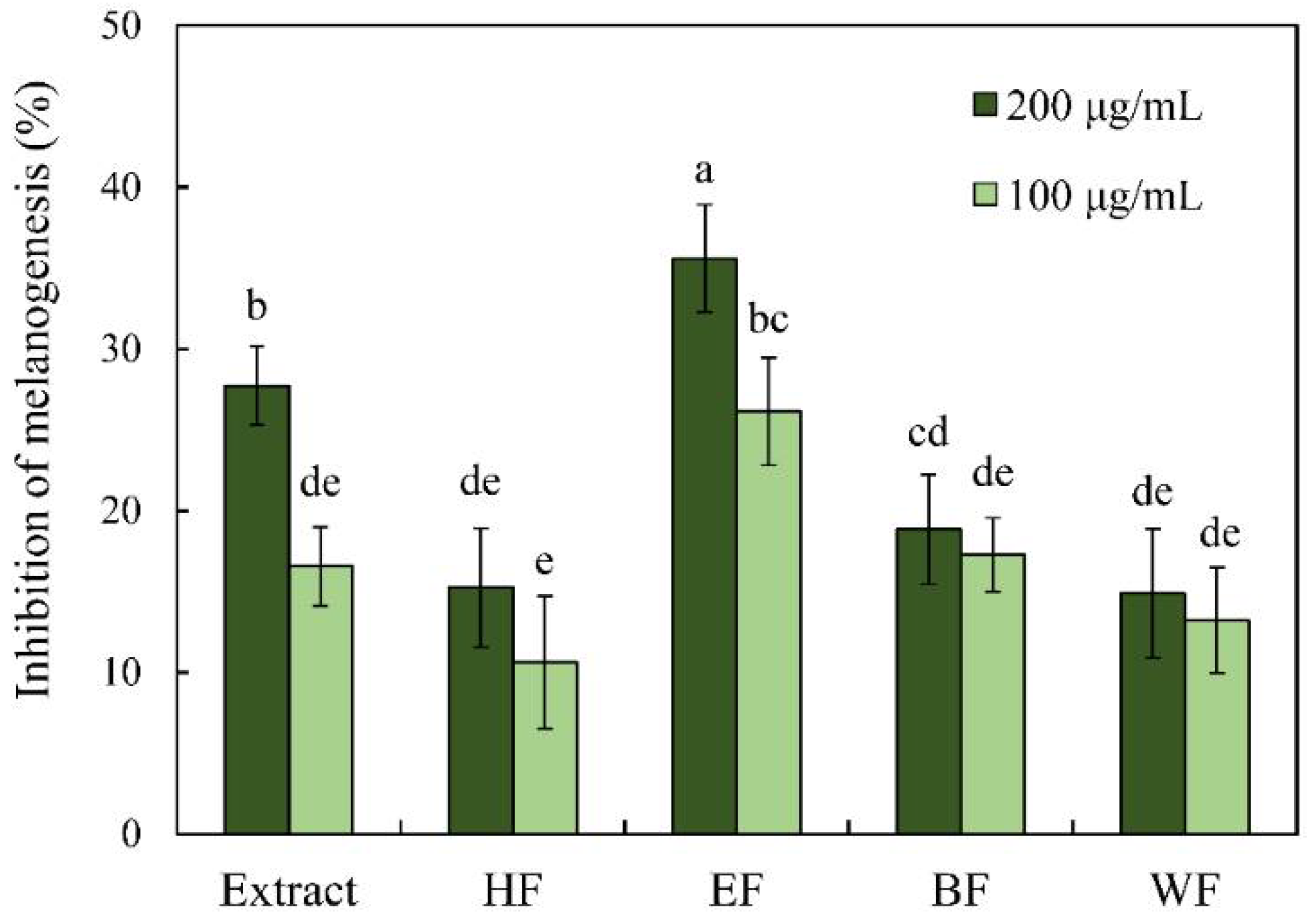
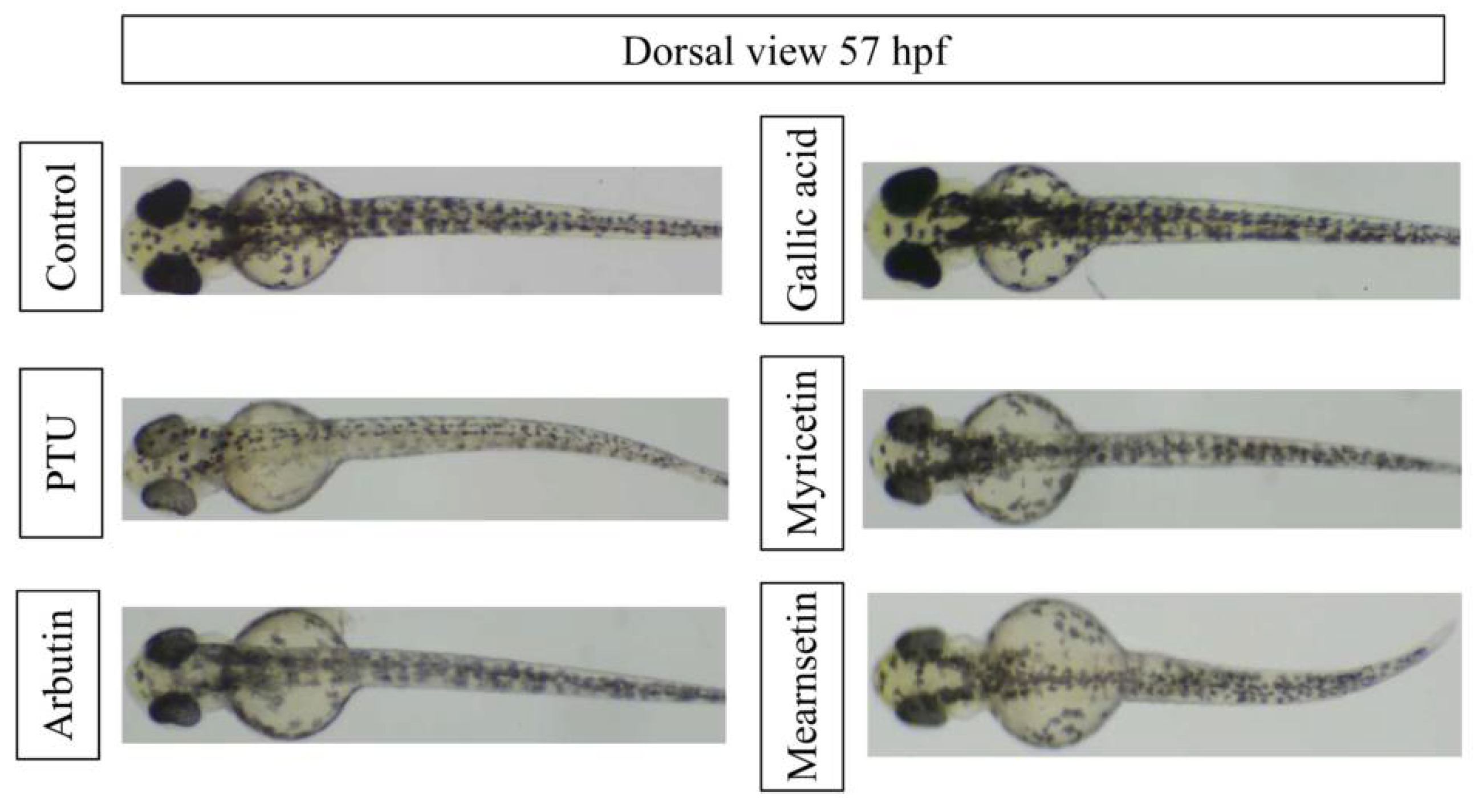
3.4. Antimelanogenesis Effects of Extract, Fractions, and Isolated Phytochemicals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Inhibitors of melanogenesis: A patent review (2009-2014). Expert Opin. Ther. Pat. 2015, 25, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Yardman-Frank, J.M.; Fisher, D.E. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp. Dermatol. 2021, 30, 560–561. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.A. Hydroxyanisole depigmentation: In-Vivo studies. J. Pathol. 1969, 97, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kumar, S.; Kim, S.H.; Seong, K.Y.; Lee, H.; Kim, C.; Jung, Y.S.; Yang, S.Y. Odorless glutathione microneedle patches for skin whitening. Pharmaceutics 2020, 12, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.K.; Sim, C.S.; Goh, C.L. Hydroquinone-induced exogenous ochronosis in Chinese—two case reports and a review. Int. J. Dermatol. 2008, 47, 639–640. [Google Scholar] [CrossRef]
- Burdock, G.A.; Soni, M.G.; Carabin, I.G. Evaluation of health aspects of kojic acid in food. Regul. Toxicol. Pharmacol. 2001, 33, 80–101. [Google Scholar] [CrossRef]
- Cheng, S.L.; Huang, L.R.; Sheu, J.N.; Chen, S.T.; Sinchaikul, S.; Tsay, G.J. Toxicogenomics of kojic acid on gene expression profiling of A375 human malignant melanoma cells. Biol. Pharm. Bull. 2006, 29, 655–669. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.C.; Tseng, T.S.; Hsiao, N.W.; Lin, Y.L.; Wen, Z.H.; Tsai, C.C.; Lee, Y.C.; Lin, H.H.; Tsai, K.C. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci. Rep. 2015, 5, 7995. [Google Scholar] [CrossRef] [Green Version]
- Zolghadri, S.; Bahrami, A.; Khan, M.T.H.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [Green Version]
- Panichakul, T.; Rodboon, T.; Suwannalert, P.; Tripetch, C.; Rungruang, R.; Boohuad, N.; Youdee, P. Additive effect of a combination of Artocarpus lakoocha and Glycyrrhiza glabra extracts on tyrosinase inhibition in melanoma B16 cells. Pharmaceuticals 2020, 13, 310. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Seo, J.O.; Baek, S.H.; Kim, S.Y. Inhibitory effects of resveratrol on melanin synthesis in ultraviolet B-induced pigmentation in guinea pig skin. Biomol. Ther. 2014, 22, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, M.H.; Zhang, G.W.; Hu, X.; Xu, X.M.; Gong, D.M. Quercetin as a tyrosinase inhibitor: Inhibitory activity, conformational change and mechanism. Food Res. Int. 2017, 100, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.H.; Liu, W.Y.; Zhu, D.; Cao, Y.L.; Tang, A.F.; Gong, G.M.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef] [Green Version]
- No, J.K.; Soung, D.Y.; Kim, Y.J.; Shim, K.H.; Jun, Y.S.; Rhee, S.H.; Yokozawa, T.; Chung, H.Y. Inhibition of tyrosinase by green tea components. Life Sci. 1999, 65, 241–246. [Google Scholar] [CrossRef]
- Park, H.Y.; Song, K.H.; Jung, P.M.; Kim, J.E.; Ro, H.; Kim, M.Y.; Ma, J.Y. Inhibitory effect of arctigenin from Fructus arctii extract on melanin synthesis via repression of tyrosinase expression. Evid. Based Complement. Alternat. Med. 2013, 2013, 965312. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.Y.; Cha, B.J.; Lee, Y.S.; Kim, G.S.; Noh, H.J.; Kim, S.Y.; Kang, H.C.; Kim, J.H.; Baek, N.I. The potential of minor ginsenosides isolated from the leaves of Panax ginseng as inhibitors of melanogenesis. Int. J. Mol. Sci. 2015, 16, 1677–1690. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.Y.; Lee, J.S.; Jeong, Y.T.; Byun, G.H.; Kim, J.H. Melanogenesis inhibition activity of floralginsenoside A from Panax ginseng berry. J. Ginseng Res. 2017, 41, 602–607. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, H.G.; Lee, Y.G.; Kim, J.H.; Lee, J.W.; Choi, B.R.; Jang, I.B.; Kim, G.S.; Baek, N.I. Isolation and quantification of ginsenoside rh23, a new anti-melanogenic compound from the leaves of Panax Ginseng. Molecules 2018, 23, 267. [Google Scholar] [CrossRef] [Green Version]
- Jayasinghe, L.; Amarasinghe, N.R.; Arundathie, B.G.; Rupasinghe, G.K.; Jayatilake, N.H.; Fujimoto, Y. Antioxidant flavonol glycosides from Elaeocarpus serratus and Filicium decipiens. Nat. Prod. Res. 2012, 26, 717–721. [Google Scholar] [CrossRef]
- Sharker, S.M.D.; Shahid, I.J. Assessment of antibacterial and cytotoxic activity of some locally used medicinal plants in Sundarban mangrove forest region. J. Pharm. Pharmacol. 2010, 4, 66–69. [Google Scholar]
- Vassallo, A.; Armentano, M.F.; Miglionico, R.; Caddeo, C.; Chirollo, C.; Gualtieri, M.J.; Ostuni, A.; Bisaccia, F.; Faraone, I.; Milella, L. Hura crepitans L. extract: Phytochemical characterization, antioxidant activity, and nanoformulation. Pharmaceutics 2020, 12, 553. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.L.; Wu, C.L.; Chang, S.T.; Huang, S.L.; Chang, H.T. Antioxidative lignans from phytochemical extract of Calocedrus formosana Florin. BioResources 2012, 7, 4122–4131. [Google Scholar]
- Li, W.H.; Chang, S.T.; Chang, S.C.; Chang, H.T. Isolation of antibacterial diterpenoids from Cryptomeria japonica bark. Nat. Prod. Res. 2008, 22, 1085–1093. [Google Scholar] [CrossRef]
- Starek, M.; Plenis, A.; Zagrobelna, M.; Dabrowska, M. Assessment of lipophilicity descriptors of selected NSAIDS obtained at different TLC stationary phases. Pharmaceutics 2021, 13, 440. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, D.; Granata, G.; Geraci, C.; Panico, A.; Siciliano, E.A.; Raciti, G.; Puglia, C. Carob seeds: Food waste or source of bioactive compounds? Pharmaceutics 2020, 12, 1090. [Google Scholar] [CrossRef]
- Wu, C.C.; Wu, C.L.; Huang, S.L.; Chang, H.T. Antifungal activity of liriodenine from Michelia formosana heartwood against wood-rotting fungi. Wood Sci. Technol. 2012, 46, 737–747. [Google Scholar] [CrossRef]
- Gutierrez-Macias, P.; Peralta-Cruz, J.; Borja-de-la-Rosa, A.; Barragan-Huerta, B.E. Peltomexicanin, a peltogynoid quinone methide from Peltogyne mexicana Martinez purple heartwood. Molecules 2016, 21, 186. [Google Scholar] [CrossRef] [Green Version]
- Nunes, A.; Marto, J.; Gonçalves, L.M.; Simões, S.; Félix, R.; Ascenso, A.; Lopes, F.; Ribeiro, H.M. Novel and modified neutrophil elastase inhibitor loaded in topical formulations for psoriasis management. Pharmaceutics 2020, 12, 358. [Google Scholar] [CrossRef] [Green Version]
- Silvestre, G.F.G.; Lucena, R.P.; Oliveira, G.D.; Pereira, H.N.; Dias, J.A.B.; Souza, I.A.; Alves, H.S. Anti-tumor and anti-inflammatory activity in vivo of Apodanthera congestiflora Cogn. (Cucurbitaceae). Pharmaceutics 2021, 13, 743. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Axiotis, E.; Petrakis, E.A.; Halabalaki, M.; Mitakou, S. Phytochemical profile and biological activity of endemic Sideritis sipylea Boiss. in North Aegean Greek islands. Molecules 2020, 25, 2022. [Google Scholar] [CrossRef]
- López, V.; Les, F.; Mevi, S.; Wandjou, J.G.N.; Cásedas, G.; Caprioli, G.; Maggi, F. Phytochemicals and enzyme inhibitory capacities of the methanolic extracts from the Italian apple cultivar Mela Rosa dei Monti Sibillini. Pharmaceuticals 2020, 13, 127. [Google Scholar] [CrossRef]
- Matsuura, R.; Ukeda, H.; Sawamura, M. Tyrosinase inhibitory activity of citrus essential oils. J. Agric. Food Chem. 2006, 54, 2309–2313. [Google Scholar] [CrossRef]
- Alam, M.B.; Seo, B.J.; Zhao, P.; Lee, S.H. Anti-melanogenic activities of Heracleum moellendorffii via ERK1/2-mediated MITF downregulation. Int. J. Mol. Sci. 2016, 17, 1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelis, A.; Hubert, J.; Aligiannis, N.; Michalea, R.; Abedini, A.; Nuzillard, J.M.; Gangloff, S.C.; Skaltsounis, A.L.; Renault, J.H. Bio-guided isolation of methanol-soluble metabolites of common spruce (Picea abies) bark by-products and investigation of their dermo-cosmetic properties. Molecules 2016, 21, 1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaita, E.; Lambrinidis, G.; Cheimonidi, C.; Agalou, A.; Beis, D.; Trougakos, I.; Mikros, E.; Skaltsounis, A.L.; Aligiannis, N. Anti-melanogenic properties of greek plants. A novel depigmenting agent from Morus alba wood. Molecules 2017, 22, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, D.K.; Pham, C.H.; Lee, I.S.; Jung, S.H.; Jeong, J.H.; Shin, H.S.; Yoo, H.M. Anti-melanogenesis activity of 6-O-isobutyrylbritannilactone from Inula britannica on B16F10 melanocytes and in vivo zebrafish models. Molecules 2020, 25, 3887. [Google Scholar] [CrossRef]
- Halaban, R.; Patton, R.S.; Cheng, E.; Svedine, S.; Trombetta, E.S.; Wahl, M.L.; Ariyan, S.; Hebert, D.N. Abnormal acidification of melanoma cells induces tyrosinase retention in the early secretory pathway. J. Biol. Chem. 2002, 277, 14821–14828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.H.; Yang, S.H.; Kim, D.S.; Kim, N.D.; Shin, H.J.; Liu, K. Novel quercetin derivative of 3,7-dioleylquercetin shows less toxicity and highly potent tyrosinase inhibition activity. Int. J. Mol. Sci. 2021, 22, 4264. [Google Scholar] [CrossRef] [PubMed]

| Specimen | IC50 (μg/mL) | |
|---|---|---|
| l-Tyrosine as the Substrate | l-DOPA as the Substrate | |
| Extract | – * | 267.87 ± 2.79 B |
| EF | 279.38 ± 3.06 a | 166.95 ± 2.38 C |
| E7 | 92.45 ± 2.06 d | 126.00 ± 3.43 D |
| E8 | 153.97 ± 4.51 b | 130.77 ± 0.80 D |
| E9 | 125.13 ± 5.01 c | 157.05 ± 1.20 C |
| E10 | 172.30 ± 2.53 b | 180.64 ± 6.61 C |
| Arbutin ** | 147.94 ± 1.64 b | 305.58 ± 1.28 A |
| ES1 | ES2 | ES3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Position | 13C | 1H | 13C | 1H | HMBC | 13C | 1H | HMBC |
| 1 | 122.0 | |||||||
| 2 | 110.6 | 7.07 (s) | 148.0 | 147.0 | ||||
| 3 | 146.4 | 136.9 | 138.1 | |||||
| 4 | 139.6 | 177.3 | 177.5 | |||||
| 5 | 146.4 | 162.5 | 104.5 | |||||
| 6 | 110.6 | 7.07 (s) | 99.2 | 6.17 (d, J = 1.95 Hz) | C-5, C-7, C-8, C-10 | 162.5 | 6.17 (d, J = 1.80 Hz) | C-5, C-7, C-8, C-10 |
| 7 | 170.4 | 165.6 | 99.4 | |||||
| 8 | 94.4 | 6.37 (d, J = 1.95 Hz) | C-6, C-7, C-9, C-10 | 166.2 | 6.37 (d, J = 1.80 Hz) | C-6, C-7, C-9, C-10 | ||
| 9 | 158.2 | 94.5 | ||||||
| 10 | 104.5 | 158.3 | ||||||
| 1′ | 123.1 | 128.0 | ||||||
| 2′/6′ | 108.5 | 7.33 (s) | C-2, C-1′, C-3′/C-5′, C-4′ | 108.6 | 7.30(s) | C-2, C-1′, C-3′/C-5′, C-4′ | ||
| 3′/5′ | 146.7 | 151.7 | ||||||
| 4′ | 137.4 | 138.5 | ||||||
| 7′ | 60.8 | 3.87(s) | C-4′ | |||||
| Specimen | Content (mg/g) | ||
|---|---|---|---|
| Gallic Acid | Myricetin | Mearnsetin | |
| EF | 59.72 ± 1.08 | 45.21 ± 0.19 | 22.66 ± 0.30 |
| E7 | - * | - | 433.38 ± 3.02 |
| E8 | 417.64 ± 8.04 | 132.83 ± 1.56 | 136.48 ± 3.33 |
| E9 | 235.04 ± 7.08 | 406.41 ± 0.41 | 50.62 ± 1.94 |
| E10 | - | 336.41 ± 12.12 | - |
| Specimen | IC50 | |
|---|---|---|
| l-Tyrosine as the Substrate | l-DOPA as the Substrate | |
| Gallic acid | 92.40 ± 4.12 *,b (0.54 ± 0.02) ** | 106.91 ± 2.19 C (0.63 ± 0.01) |
| Myricetin | 74.20 ± 7.10 c (0.23 ± 0.02) | 190.78 ± 2.09 B (0.60 ± 0.01) |
| Mearnsetin | 56.57 ± 1.26 d (0.17 ± 0.01) | 118.92 ± 2.91 C (0.36 ± 0.01) |
| Arbutin *** | 147.94 ± 1.64 a (0.54 ± 0.01) | 305.58 ± 1.28 A (1.12 ± 0.01) |
| Compound | Substrate | Kinetic Parameter | Concentration (μg/mL) | Potential | Inhibition Type | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 40 | 50 | 80 | 100 | |||||
| Gallic acid | l-Tyrosine | Vmax | 0.0011 | 0.0012 | 0.0012 | 0.0012 | 0.0011 | ― | Competitive |
| Km | 0.0484 | 0.1458 | 0.3003 | 0.7299 | 0.9054 | ↑ | |||
| l-DOPA | Vmax | 0.0035 | 0.0034 | 0.0034 | 0.0034 | 0.0035 | ― | Competitive | |
| Km | 0.0733 | 0.1592 | 0.2788 | 0.4385 | 0.9462 | ↑ | |||
| Myricetin | l-Tyrosine | Vmax | 0.0012 | 0.0010 | 0.0009 | 0.0008 | 0.0007 | ↓ | Mixed |
| Km | 0.1415 | 0.2997 | 0.3741 | 0.3813 | 0.4225 | ↑ | |||
| l-DOPA | Vmax | 0.0035 | 0.0028 | 0.0025 | 0.0023 | 0.0020 | ↓ | Mixed | |
| Km | 0.1422 | 0.2877 | 0.3132 | 0.3920 | 0.4068 | ↑ | |||
| Mearnsetin | l-Tyrosine | Vmax | 0.0018 | 0.0018 | 0.0019 | 0.0019 | 0.0020 | ― | Competitive |
| Km | 0.2673 | 0.5567 | 0.9953 | 1.3976 | 1.6487 | ↑ | |||
| l-DOPA | Vmax | 0.0028 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | ― | Competitive | |
| Km | 0.1862 | 0.2114 | 0.3217 | 0.4399 | 0.6136 | ↑ | |||
| Specimen | IC50 (μM) |
|---|---|
| Gallic acid | ― ** |
| Myricetin | 349.96 ± 42.67 a |
| Mearnsetin | 121.01 ± 0.96 b |
| PTU * | 26.29 ± 4.17 c |
| Arbutin * | 323.69 ± 19.77 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Liu, I.-H.; Huang, X.-Z.; Chen, H.-J.; Chang, S.-T.; Chang, M.-L.; Ho, Y.-T.; Chang, H.-T. Antimelanogenesis Effects of Leaf Extract and Phytochemicals from Ceylon Olive (Elaeocarpus serratus) in Zebrafish Model. Pharmaceutics 2021, 13, 1059. https://doi.org/10.3390/pharmaceutics13071059
Huang C-Y, Liu I-H, Huang X-Z, Chen H-J, Chang S-T, Chang M-L, Ho Y-T, Chang H-T. Antimelanogenesis Effects of Leaf Extract and Phytochemicals from Ceylon Olive (Elaeocarpus serratus) in Zebrafish Model. Pharmaceutics. 2021; 13(7):1059. https://doi.org/10.3390/pharmaceutics13071059
Chicago/Turabian StyleHuang, Chi-Ya, I-Hsuan Liu, Xiang-Zhe Huang, Hui-Jen Chen, Shang-Tzen Chang, Mei-Ling Chang, Yu-Tung Ho, and Hui-Ting Chang. 2021. "Antimelanogenesis Effects of Leaf Extract and Phytochemicals from Ceylon Olive (Elaeocarpus serratus) in Zebrafish Model" Pharmaceutics 13, no. 7: 1059. https://doi.org/10.3390/pharmaceutics13071059
APA StyleHuang, C.-Y., Liu, I.-H., Huang, X.-Z., Chen, H.-J., Chang, S.-T., Chang, M.-L., Ho, Y.-T., & Chang, H.-T. (2021). Antimelanogenesis Effects of Leaf Extract and Phytochemicals from Ceylon Olive (Elaeocarpus serratus) in Zebrafish Model. Pharmaceutics, 13(7), 1059. https://doi.org/10.3390/pharmaceutics13071059





