1. Introduction
Oral delivery of active pharmaceutical ingredients has been the predominately preferred route of administration due to its simplicity and capacity to induce topical and systemic administration while promoting patient compliance [
1]. Among the different types of diseases, gastrointestinal diseases present a unique target for oral drug delivery, as it can achieve a direct and topical drug presence in the intestinal tissues without the need for or the side effects associated with systemic absorption or circulation. Inflammatory Bowel Diseases (IBD), such as Crohn’s disease and colitis, as well as intestinal and colorectal cancers (CRC), are prominent examples of gastrointestinal diseases. Briefly, IBD is frequently observed in western countries and it is estimated that approximately 1.3% of adults are diagnosed with either Crohn’s or ulcerative colitis in the US alone [
2]. Intestinal and CRC are a leading cause of cancer-related mortality and morbidity worldwide, accounting for the third-highest incidence and death rate in men and women [
3]. Despite the recent advent of novel approaches, the current standard of care therapies can have low specificity, which correlates to significant side effects during treatment [
4,
5], or present disease relapse [
6,
7].
IBD and intestinal cancers are associated with several gene dysregulations. Nucleic acid-based therapeutics present a promising approach for the treatment and/or prevention of the two aforementioned diseases [
8,
9]. TNF-a, IFN-γ, IL-4, IL-10 IL-21, activation of proto-oncogenes (KRAS) and inactivation of genes like APC, and p53, are some of the examples of gene dysregulations or genetic alterations during IBD and intestinal cancers [
10,
11,
12,
13,
14,
15]. Nucleic acid-based therapeutics, such as plasmids, small interfering RNAs (siRNAs), microRNAs (miRNAs), and messenger RNAs (mRNAs), are versatile molecules that can regulate gene expressions and disease progression [
16].
As patients with IBD have a 4.5-fold increased risk of CRC and both diseases may require protracted therapies [
17], the oral route of delivery for nucleic acids presents a potential benefit for long-term treatments against either disease. Unfortunately, the oral delivery of nucleic acids presents significant challenges. The stomach’s acidic environment and the enzyme-rich intestine present significant hurdles for the oral administration of nucleic acids [
1,
16,
18]. Therefore, a system capable of delivering nucleic acids to the intestinal cells following oral administration can provide significant advantages. Furthermore, the development of a delivery carrier that can not only transfect intestinal tissue, but also preferentially target cells of the immune system or cancer cells presents significant advantages over traditional approaches.
Nanotechnology-based approaches have propelled active targeting methodologies against cancer cells [
19]. For example, active targeting can be achieved by the direct conjugation of ligands, such as small molecules, proteins and peptides, onto the nanoparticles’ surface to hone towards receptors overexpressed in specific cell types [
19]. Representative examples include the Arg-Gly-Asp (RGD) peptide that binds to integrins [
20], folic acid that binds to the folate receptors [
21], and chlorotoxin that binds to MMP-2 receptors [
22]. Here, we report on the development of a novel delivery carrier for the oral delivery of nucleic acids, capable of targeting cells with overexpressed mannose receptors. This includes cells of the immune system, such as macrophages or cancer cells [
23,
24].
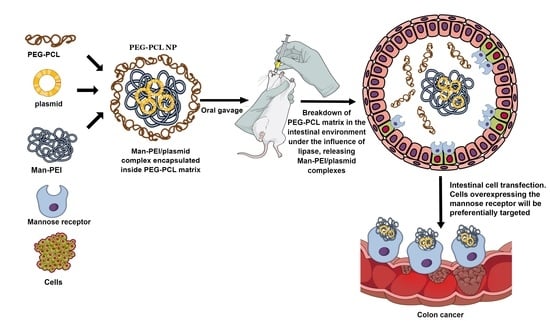
To evaluate the nanocarrier, we use two model nucleic acids, pGL3 luciferase-expressing plasmid and green fluorescence protein (GFP) expressing plasmid. Briefly, we prepared PEG-PCL nanoparticles (NPs) with encapsulated mannosylated PEI (Man-PEI)/plasmid complexes. The Man-PEI/plasmid complexes transfect cells with the nucleic acids while actively targeting cells with overexpressed mannose receptors, as presented here, for minimizing nonspecific targeting to healthy cells. For Man-PEI, we use PEI1800, due to its minimal toxicity and strong transfection efficiency [
1,
16]. The encapsulation of the Man-PEI/plasmid complexes inside PEG-PCL NPs protects them during their transition through the harsh acidic gastric environment. The PCL’s solid polymeric core remains stable in the stomach’s acidic environment but degrades in the neutral-to-basic intestinal area, aided by lipase enzymes present at the organ [
25], releasing the Man-PEI/nucleic acid complexes locally. As Man-PEI was previously studied for other routes of administration with advantageous and promising results [
26,
27,
28], here we present a new application for the successful oral delivery of nucleic acids with the polymer. Thus, such behavior potentiates passive targeting to the small and large intestine and active targeting through the mannose-receptor to mannose-overexpressing cells.
The nanocarrier is designed to overcome the limitations of oral nucleic acid delivery and can be potentially utilized for the treatment of both intestinal diseases, IBD or cancer. In this study, we focus on the potential targeting to CRC cells due to the disease’s severity on survival. We conclusively demonstrate the expression of a non-endogenous gene in the intestinal tissue following the oral administration of the respective plasmid. Our analysis does not only present a visual and quantitative successful transfection following oral delivery using an exogenous gene but also the potential for intestinal and, more importantly, colonic delivery of nucleic acids with active targeting, based on our nanocarrier system.
2. Methods
2.1. Materials
Cell culture reagents and trypsin were purchased from VWR (Radnor, PA, USA) or Corning Cellgro (Manassas, VA, USA). Fetal Bovine Serum (FBS) was obtained from Atlanta Biologicals. Opti-MEM was purchased from GibcoTM (Life technologies, Carlsbad, CA, USA). mPEG-PCL (5000:20,000) and PEG-PCL conjugated with aminofluorescein were purchased from PolySciTech Inc. (West Lafayette, IN, USA). α-D-mannopyranosylphenyl isothiocyanate (MPITC) was purchased from Carbosynth Ltd. (Berkshire, UK). Simulated gastric fluid, simulated intestinal fluid, lipase were obtained from Fisher (Hampton, NH, USA). Anti-mannose receptor (CD206) antibody (ab64693) was acquired from Abcam (Cambridge, MA, USA), Alexa Fluor 488 goat anti-rabbit IgG (A11008) was purchased from Invitrogen (Carlsbad, CA, USA). ONE-GLO + Tox Luciferase Reporter and cell viability assay kit were from Promega (Madison, WI, USA). GFP quantification kit (ab235672) was purchased from Abcam (Cambridge, MA, USA).
2.2. Cell Cultures and Plasmid DNA
Colon cancer cell lines SW480, HCT-15, HT-29, and normal cells HEK-293, HFL1 were obtained from ATCC. We cultured the SW480 cells in L-15 media, HCT-15 and HT-29 cells in RPMI1640 media, HEK-293 in EMEM media, and HFL1 in F-12K media. All media were supplemented with 10% FBS and 1% antibiotics. Cell cultures were maintained at 37 °C, under a 5% CO2 environment, with the exception for SW480 that no CO2 was supplemented. We used the pGL-3 luciferase-expressing plasmid DNA (Promega, Madison, WI, USA) in vitro and the green-fluorescent-protein (GFP) expressing pLenti-III-mir-GFP plasmid for the in vivo transfection as model nucleic acids (ABM, Richmond, BC, Canada). We extracted and purified the plasmids using the QIAGEN Plasmid Giga Kit (Chatsworth, CA, USA).
2.3. Mannose Receptor Expression
To analyze mannose receptors’ relative expression level in colon cancer vs. normal cell lines, we used the SW480, HCT-15, HT-29 colon cancer cell lines, and the HFL1, HEK-293 normal epithelial cell lines. We harvested approximately 106 cells from each cell line and treated them with anti-mannose receptor antibodies at 1:50 dilution. Following incubation and washing, we treated the cells with the secondary antibody, Alexa Flour 488 goat anti-rabbit IgG, at 1:50 dilution. The expression of mannose receptors was detected in 10,000 events of the gated populations using a BD FACSCalibur Flow Cytometer with CellQuest Pro software (BD Biosciences, Franklin Lakes, NJ, USA).
2.4. Synthesis of Mannosylated Polyethyleneimine 1800 (Man-PEI)
We utilized a previously published protocol for the synthesis of Man-PEI, with minor modifications [
26]. Briefly, we linked mannose to PEI via a phenyl isothiocyanate bridge using mannopyranosylphenyl isothiocyanate as the coupling reagent. We dissolved 25 mg (80 μmol) of α-D-mannopyranosylphenyl isothiocyanate (MPITC) in methanol (5 mL) and added the resulting mixture to a solution of equal methanol volume containing 23.4 mg PEI 1800 (13 μmol) in a drop-wise manner. We allowed the reaction to progress under stirring at room temperature until no MPITC could be detected by thin-layer chromatography (TLC, methanol: chloroform 1:1
v/v). We purified the final product through dialysis (MWCO: 1 kDa) in water and freeze-dried it until further use.
The final product, Man-PEI, was analyzed using 1H-NMR (CD3OD; JEOL Eclipse ECS-400). We perform Fourier Transformed Infrared Spectroscopy (FTIR) spectroscopic analysis from 400 to 4000 cm−1 on Man-PEI and its reaction components using a Spectrum Two FTIR spectrometer (PerkinElmer, Waltham, MA, USA). Data were analyzed with PerkinElmer Spectrum Quant software.
2.5. Gel Retardation and DNAse Stability Assay
We evaluated the ability of Man-PEI to complex with a plasmid-based on a gel retardation assay, using agarose gel electrophoresis. Briefly, we complexed the Man-PEI at different nitrogen to phosphate (N/P; indicates the ratio between the number of nitrogen atoms present in the Man-PEI to the phosphate atoms in the nucleic acid) ratios and run the samples in a 1% agarose gel with 0.1 μg/mL ethidium bromide, as previously described [
29]. The nucleic acid’s signal was visualized under a Chemidoc Touch Imaging (Biorad, Hercules, CA, USA).
To evaluate the ability of Man-PEI to protect nucleic acid degradation in the presence of DNAses, we complexed the pGL-3 plasmid with Man-PEI at different N/P ratios and incubated the samples in the presence of DNase I (2U of DNase/600 ng of plasmid for 30 min at 37 ºC; naked plasmid with DNAses was used as positive control). Following the deactivation of the DNAses using EDTA, the nucleic acids were released from the complexes using 8% polyacrylic acid (PAA) and run in the agarose gel, as described above.
2.6. Transfection Assay for Man-PEI/Plasmid Complexes
We incubated SW480 and HCT-15 cells seeded in 96-well Optical-Bottom Plates (Fisher, Hampton, NH, USA) in the presence of 10 μg of pGL-3 plasmid complexed with either Man-PEI or PEI at various N/P ratios (six replicates per sample). After 6 h of incubation at 37 °C, the transfection media in each well was replaced with fresh complete media. Following 24 or 48 h, we determined the luciferase activity and cell survival of the transfected cells using the ONE-GLO + Tox Luciferase Reporter and Cell Viability Assay Kit, according to the manufacturer’s instructions (Promega, Madison, WI, USA). Transfection was calculated by obtaining the ratio of luminescence of cells over cell survival for each well and sample.
2.7. Cellular Uptake Study Using Fluorescent Microscopy and Flow cytometer
We conjugated fluorescein-NHS (excitation/emission: 498/517 nm; Lumiprobe, Cockeysville, MD) to Man-PEI and complexed it with pGL-3 plasmid. We investigated the cellular uptake using (a) fluorescence microscopy with an Olympus BX63 microscope, and (b) flow cytometry using a BD FACS Calibur Flow Cytometer along with CellQuest Pro software. Briefly, for fluorescent microscopy, we incubated SW480 and HCT-15 cells with Man-PEI-fluorescein complexed with pGL-3 plasmid at N/P ratio 20:1 in chambered cell culture slides (Falcon, Corning, NY, USA). Following predetermined incubation periods, cells were washed with 1× PBS, fixed with 4% formaldehyde, and covered with 4′,6-diamidino-2-phenylindole (DAPI)-containing mounting media before visualizing under the microscope.
For the flow cytometric analysis, we incubated the SW480 and HCT-15 cells in the presence of Man-PEI-fluorescein/pGL-3 complexes, as described above, for predetermined periods. Subsequently, the cells were washed with 1× PBS, harvested, fixed with 4% formaldehyde and analyzed under a flow cytometer.
2.8. Competition Transfection Assay
We seeded SW480 and HCT-15 cells in 96-well optical-bottom plates (Fisher, Hampton, NH, USA) for transfection with Man-PEI or PEI complexed with pGL-3 at N/P ratio 20:1, as above. Some cells were pretreated with free mannose (1.5 mmoles/liter) for an hour prior to transfection. After 48 h, we determined luciferase activity and cell survival of the transfected cells using ONE-Glo + Tox Luciferase Reporter and Cell Viability Assay Kit, according to the manufacturer’s protocol. We calculated transfection by obtaining the ratio of luminescence over the cells’ survival in each well [
29].
2.9. Formulation and Characterization of PEG-PCL NPs
To prepare the PEG-PCL NPs with encapsulated Man-PEI/plasmid complexes, we used the double-emulsion solvent evaporation technique with minor modifications, as previously described [
30]. Based on the transfection data, we complexed the plasmid with Man-PEI at the optimal N/P ratio of 20:1 in 200 μL of Tris-EDTA, which formed the inner aqueous phase. The oil phase consisted of 100 mg PEG-PCL dissolved in 2 mL dichloromethane (DCM) solution. The outer aqueous phase consisted 6 mL of a sodium cholate solution at a concentration of 6 mg/mL. The inner aqueous phase was transferred into the oil phase and emulsified the system using a probe sonicator for 30 s (Misonix Ultrasonic liquid processors, 15 output wattage), to form the primary-water-in-oil (
w/o) emulsion. We subsequently placed this emulsion in the outer aqueous phase and probe-sonicated for 60 s to form the final water-in-oil-in-water (
w/o/w) emulsion. We allowed the DCM to evaporate under continuous stirring for approximately 3 h. We collected the NPs via centrifugation at 14,000×
g for 1 h and washed them using nuclease-free water. The supernatant obtained from the centrifugation was collected to calculate the plasmid’s encapsulation efficiency, using the PicoGreen assay (Invitrogen, Waltham, MA, USA) at wavelengths of 485/520 nm. We determined the plasmid loading in NPs by subtracting the total amount of plasmid recovered in the supernatant from the initial amount of plasmid added into the formulation. We calculated the encapsulation efficiency, loading capacity, and production yield of the NPs according to the following equations [
31]:
We determined the particle size, size distribution and zeta potential of the PEG-PCL NPs using NanoBrook 90Plus PALS (Brookhaven, Holtsville, NY, USA) at room temperature. SEM images of the PEG-PCL NPs were obtained under a FEI Quanta 3D FEG FIB/SEM dual beam system interfaced with the EDAX Apollo XL EDS detector. The powder samples of air-dried NPs were fixed on SEM stub with double-sided carbon tape and then coated with a thin layer of Pt to avoid charging effect during imaging and analysis.
We evaluated the plasmid retention from PEG-PCL NPs with encapsulated Man-PEI/pGL-3 complexes at two N/P ratios, 7:1 or 20:1. Briefly, 10 mg of lyophilized NPs were suspended in 1 mL PBS and incubated at 37 °C for 40 min under constant shaking. Then, the suspension was centrifuged for 15 min at 14,000× g and any plasmid was detected using the agarose gel electrophoresis described above.
2.10. Cell Viability Assay
We used a standard 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay to determine the PEG-PCL NPs’ cytotoxicity in SW80 and HCT-15 cells, as previously described [
29]. Briefly, we treated SW480 and HCT-15 cells with different concentrations of PEG-PCL NPs (15–1000 μg/mL) containing Man-PEI/plasmid complexes in complete media. Following 24, 48, and 72 h incubation and addition of MTT, the absorption of solubilized formazan crystals in acidified SDS at 570/630 nm was acquired using a plate reader (BioTek H1 Synergy Plate Reader, Winooski, VT). Cell viability was determined as a percentage of the negative control (untreated cells).
2.11. Plasmid Release and Stability Assay from PEG-PCL NPs
A PEG-PCL NPs suspension containing 50 μg plasmid complexed with Man-PEI in 10 mL of simulated gastric fluid (SGF), simulated intestinal fluid (SIF), and simulated intestinal fluid with lipases (SIF + lipase; 1 mg/mL). At predetermined time points, 0.5, 1, 2, 4, 8, 24, 48, and 72 h, we withdrew 0.5 mL samples from each release media and collected the supernatant after centrifuging the samples for 20 min at 14,000× g. To maintain the volume of the release media unchanged, 0.5 mL of fresh release media was supplemented to each tube, respectively. We measured plasmid concentration with the PicoGreen assay kit, as described above. As SGF’s acidity interfered with plasmid quantification, the measurements took place after dilution with NaOH to neutralize SGF’s acidity. The plasmid detection under the different incubation conditions was confirmed with unentrapped, non-complexed, naked plasmids.
Finally, we evaluated the stability of the plasmid complexed with Man-PEI with or without encapsulation in PEG-PCL NPs and incubated in SIF in the presence or absence of lipases. Briefly, for the NPs, we incubated 10 mg of lyophilized NPs suspended in 1 mL of SIF with lipases (1 mg/mL) for 72 h at 37 °C. Subsequently, we centrifuged the sample for 20 min at 14,000× g, and supernatants were collected. The collected supernatant was then run directly into the gel under the presence or absence of polyacrylic acid (PAA). Similarly, the non-encapsulated Man-PEI/plasmid complexes were treated under similar conditions.
2.12. In Vivo Biodistribution Assay
We performed a biodistribution analysis of the PEG-PCL NPs in C57BL/6J wild-type female mice (4–6 weeks old, Envigo, Indianapolis, IN, USA). The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC; Protocol number: 20OCT-GM-01; October 2020) of the University of Louisiana Monroe, based on Office of Laboratory Animal Welfare (OLAW), National Institute of Health (NIH) guidelines, and the Guide for the Care and Use of Laboratory Animals, 8th ed. For our analysis, we used PEG-PCL polymer tagged with fluorescein (Ex: 492 nm, Em: 516 nm). We formulated the NPs with Man-PEI/plasmid complexes, as described above. To minimize the food’s interference, the mice were under an Alfalfa-free diet for at least five days before our analysis. Furthermore, the mice were fasted for 24 h before oral administration. Subsequently, 12.5 mg of the PEG-PCL NPs in 300 μL suspension were given via oral gavage per mouse. Animals were sacrificed at each predetermined time point (0 h- no dose, 1, 2, 4, 8, and 24 h; 2 animals per time point). We collected the stomach, small intestine, cecum, and colon of the mice and obtained fluorescent images using IVIS (Perkin-Elmer, Waltham, MA, USA).
2.13. In Vivo Plasmid Transfection
The transfection potential of our system following oral administration was assessed in C57BL/6J wild-type mice. To detect the in vivo transfection of plasmids in the intestine, we used the GFP-expressing plasmid complexed with Man-PEI at N/P ratio 20:1. The complexes were encapsulated in PEG-PCL NPs, as described above, and given orally to mice (n = 3 for treatment, n = 2 for control) at 100 μg of plasmid/mouse, daily for five days (1×/day). Subsequently, the mice were sacrificed, and the intestinal tissues were harvested. The tissues were opened, cleaned, rolled, and fixed using cold-isopentane. The samples were sectioned at 20 µm thickness using a cryostat (Leica CM3050 S), such that each section included the complete length of the intestine. Sectioned intestinal tissues were viewed under a fluorescence microscope for GFP signal. For the negative control, mice that were not fed with PEG-PCL NPs were used. As a positive control, we transfected SW480 cells with GFP-expressing plasmid and lipofectamine 2000 for 48 h.
GFP expression in the tissues was verified using GFP quantification kit (Abcam, Cambridge, MA, USA). Briefly, 50 mg of intestinal tissue samples were harvested from untreated and treated mice (two tissue samples per animal, two animals for control, three animals for treatment). The harvested tissues were lysed in 500 μL of GFP assay buffer, using a homogenizer for 60 s. The samples were then incubated in ice for 10 min to ensure complete lysis and centrifuged for 10 min at 10,000 RCF to separate the supernatant. An amount of 100 μL of the supernatant were used to quantify the amount of GF-protein present, following the manufacturer’s protocol.
4. Discussion
Nanoparticles incorporating nucleic acids have primarily focused on the intravenous route of administration, but the process is invasive and unsuitable for prolonged therapy [
32]. In contrast, oral administration has multiple advantages, such as sustained and controllable delivery, ease of administration, patient compliance, and efficiency in targeting diseases of the gastrointestinal tract, such as IBD or colon cancer [
1,
33]. Oral administration of nucleic acids presents challenges, such as the harsh acidic condition in the stomach with pH 1–2.5, gastric enzymes like pepsin, strong nuclease activity, and the presence of a mucus layer that is difficult to surpass [
32,
33]. The development of a carrier system that could overcome these barriers and provide an efficient nucleic acid delivery will propel significant advances in nucleic acid-based therapies against diseases related to the gastrointestinal tract through oral delivery.
In this study, we formulated a polymer-based nanocarrier system encapsulating nucleic acids, to overcome the above-presented barriers, when given orally and transfect the nucleic acids to the intestinal tissue. We selected for our study polyethylene glycol (PEG) and poly-caprolactone (PCL) polymers that are FDA-approved, biocompatible, biodegradable, and non-immunogenic [
34,
35]. PEG, a neutral, hydrophilic polymer, has been widely used in drug delivery applications to endow nanocarriers with long-circulating properties, protect them from trypsinization and increase their hydrophilicity [
36]. Furthermore, pegylated nanoparticles transverse through the mucus layer more efficiently than non-pegylated or larger (micro-sized) particles [
1]. PCL permits slow drug release and does not get degraded in the acidic gastric pH. However, the PCL matrix gets degraded in the intestinal tissue under the increasingly basic environment, accelerated by the presence of lipases, which are abundantly present in the intestinal tract [
37]. Polyethyleneimine (PEI) is a widely used cationic polymer for cell transfection with nucleic acids. PEI demonstrates a buffering capacity over a wide pH range, and it prevents hydrolysis in endosomes. This helps the PEI–nucleic acid complex escape from the endosomal compartment through a proton sponge mechanism [
16,
29,
38].
This study uses PEI conjugated with mannose (Man-PEI) as a transfecting agent. Mannose receptors, 175-kDa transmembrane proteins of the C-type lectin family, are overexpressed in the different cells, including cells of the immune system [
39] or colon cancer cells [
40]. In the interest of focusing our research, and the higher impact of CRC on patient survival, we focus on CRC in this study. Here, we confirmed mannose receptor overexpression in colon cancer cells vs. normal epithelial cells using flow cytometry, which indicated an up to a 3.1-fold increase of mannose receptor expression in cancer cells compared to the normal epithelial cells.
Following the Man-PEI’s synthesis and characterization via FTIR and NMR, we confirmed its ability to complex with the negatively charged plasmid DNA and protect it from degradation by DNAse I, using agarose gel electrophoresis. Although at lower N/P values, i.e., 2:1, 4:1, 7:1, Man-PEI did not efficiently complex with the plasmids, at N/P ratios of 10:1 and above, Man-PEI strongly complexed with the plasmid, and protected it from DNAse-mediated degradation. Our study aligns with previous findings [
29,
41].
We evaluated Man-PEI’s transfection capacity in SW480 and HCT-15 cells at different N/P ratios and compared it to PEI. As the N/P ratio increased, the luciferase expression, due to the transfection of the pGL-3 luciferase-expressing plasmid, becomes stronger for both polymers, as detected by luminescence, though the Man-PEI induced an overall stronger luciferase expression when compared to PEI for the same N/P ratios. Representatively, in SW480, at the N/P ratio of 20:1, Man-PEI had approximately a two-fold and 1.76-fold higher transfection compared to PEI, at 24 and 48 h, respectively. In contrast, PEI induced stronger transfection at the N/P ratio of 30:1, though only significant at 48 h. For HCT-15, Man-PEI induced stronger transfection compared to PEI at all N/P ratios and time points, though the differences reached statistical significance at N/P ratios of 30:1 at 24 h, and 20:1 and 30:1 at 48 h. These results indicate that mannosylation of PEI is overall increasing the transfection potential of the polymer.
The critical role of a drug delivery system is the successful uptake of its load by the cells. Our analysis confirmed a time-dependent uptake of the Man-PEI/plasmid complexes by the cells using a fluorescence microscope and flow cytometer. As mannose receptors undergo high-affinity binding to agents containing mannose residues, triggering their transport into endocytic pathways [
42], we evaluated whether Man-PEI can promote a receptor-ligand binding and endocytosis. Through a competition transfection assay using SW480 and HCT-15 cells that were preincubated with or without free mannose, we determined that pretreatment of cells with free mannose significantly decreased the luminescence compared to cells without mannose pretreatment, i.e., luciferase activity produced from successful transfection with the pGL-3 luciferase-expressing plasmid, after 48 h. In comparison, we detected no significant difference in the control groups, i.e., transfection with PEI (non-mannosylated) with or without pretreatment with free mannose. The purpose of pretreating the cells with free mannose is to saturate the mannose receptors beforehand. Doing this creates a competitive environment between free mannose and mannose present in Man-PEI to bind to mannose receptors. Thus, the mannose receptors’ availability plays a significant role in the enhanced uptake of the Man-PEI/plasmid complexes and they promote the internalization of the mannosylated complexes via a mannose receptor-dependent mechanism. The observed difference in the transfection ability between the mannosylated and parent PEI described above is also supported by the fact that mannose receptor-mediated endocytosis has previously been studied and the receptor has been characterized as a rapid and highly effective endocytic receptor [
24,
43,
44].
The major concern for oral delivery of nucleic acids is to protect them from the harsh gastric environment. To protect the Man-PEI/plasmid complexes, we encapsulated them inside PEG-PCL NPs. Although oral administration does not inherently present limitations in particle sizes, it has been reported that particles ranging between 200 and 500 nm can efficiently transport through the mucus layer [
45]. As detected by DLS and SEM analysis, the PEG-PCL NPs possessed an ~140 nm diameter and were spherical.
We evaluated the release of plasmid from PEG-PCL NPs using different simulated gastrointestinal conditions. The incubation of the NPs in a simulated gastric fluid (SGF) induced a minimal release of the plasmids up to 72 h. In contrast, incubation of the NPs in SIF presented an increased plasmid release, further accelerated by the presence of lipases. More importantly, through agarose gel electrophoresis, we determined that the plasmid release from the PEG-PCL NPs takes place with the plasmid still complexed with Man-PEI. This is important, as the purpose of the PEG-PCL is to protect the Man-PEI/plasmid complexes from the gastric environment, but release them in the intestinal environment. At that point, the Man-PEI will protect the plasmids from any nuclease activity, preferentially targeting cells with overexpressed mannose receptor and ensuring successful cell transfection. It is important to note that although a small amount of plasmid was released from the PEG-PCL NPs without being complexed with Man-PEI, the majority of the plasmid maintained its complexation with Man-PEI.
We evaluated in vivo the PEG-PCL NPs biodistribution and their ability to transfect the intestinal area. Following oral administration of fluorescein-labeled PEG-PCL NPs with encapsulated Man-PEI/plasmid complexes and in vivo imaging of the excised stomach, small intestine, and colon tissue, we detected a gradual progression of the fluorescence signal from the stomach area to the colon as a function of time. The fluorescence signal reached the cecum within 2 h of administration and progressed to the colon within 8 h. 24 h post-administration, no fluorescence signal was detected. Although the fluorescein was attached to the PEG-PCL through an ether-triazine type bond, which should minimize leaching of the tag out the NPs, it is not possible to confirm if the observed signal originates from intact, partially broken, or entirely degraded NPs and freed fluorescein. Thus, the signal observed during the later time points may originate from a mixture of the above. A more reliable evaluation of the system is its capacity to transfect the intestinal tissue, as a successful expression of exogenous genes can only be achieved if the system successfully transfects cells.
We used a model plasmid, which induced the expression of the GFP protein and evaluated the intestinal tissue under fluorescence microscopy, following oral delivery. The GFP-expressing plasmid was complexed with the Man-PEI and the complexes were entrapped in PEG-PCL NPs. After five days of daily administration of the NPs, we harvested the intestinal tissue and observed them under the microscope. The green fluorescence seen in the mice’s intestinal tissue sections confirmed the successful transfection of the intestinal tissue. The GFP expression was also confirmed through GFP quantification, indicating a strong expression of the protein along the intestinal tissue. More importantly, the GFP expression in the colon remained high and comparable to the prior intestinal sections. Although the colon presented a relatively higher GFP expression than the proximal or distal portions of the small intestines, the GFP was overall evenly expressed along the intestine. Nonetheless, this indicates that the transfection was feasible throughout the intestinal tissues and up to the colon tissue; thus, the proposed system can efficiently reach the lower section of the intestinal tissue, which is also necessary for colon cancer treatments.
Overall, this behavior indicates that the proposed PEG-PCL NPs can effectively orally deliver nucleic acids to the intestines and transfect the intestinal tissue for the potential treatment of IBD or CRC. This study provides compelling evidence that the formulation can protect nucleic acids from stomach acids, release the Man-PEI/nucleic acid complexes in the intestinal tract through PEG-PCL’s degradation in the tissue, and actively target a mannose receptor-overexpressing cells through the mannose’s decoration of the PEI.