Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends
Abstract
:1. Introduction
2. Treatment
2.1. Iron Oxide Catalyzed Cancer Therapies
2.2. Drug and Gene Delivery
2.3. Magnetothermal Heating
3. Imaging
3.1. Magnetic Resonance Imaging (MRI) Contrast Agents
3.2. Magnetic Particle Imaging (MPI) Tracers
4. Movement
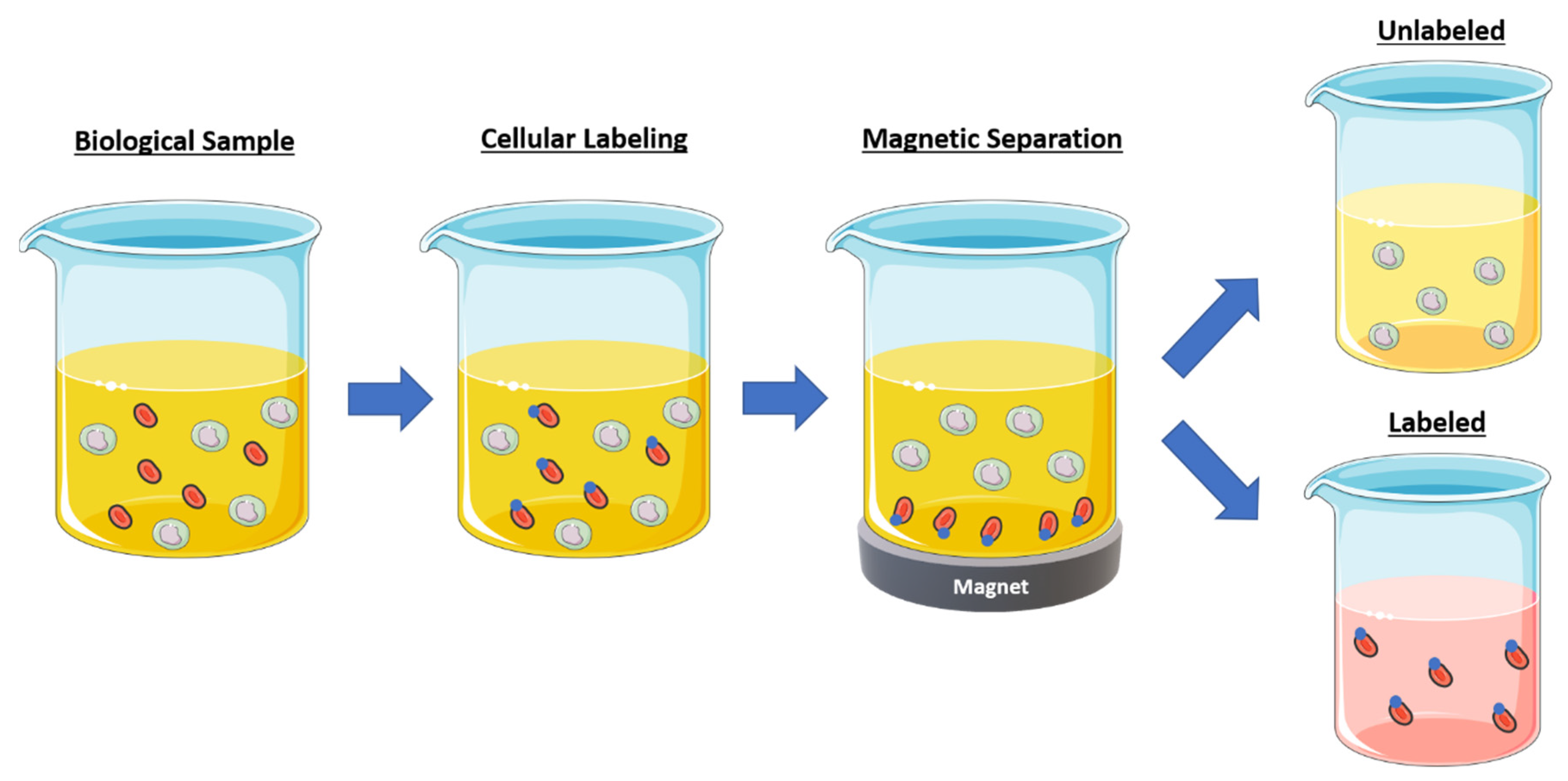
4.1. Cell Separation
4.2. Soft Robotics
5. Diagnostics
Immunoassays
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Häfeli, U. The History of Magnetism in Medicine. In Magnetism in Medicine; Wiley-VCH: Berlin, Germany, 2006; pp. 1–25. [Google Scholar]
- Peterson, F.; Kennelly, A.E. Some Physiological Experiments with Magnets at the Edison Laboratory; D. Appleton & Company: New York, NY, USA, 1892. [Google Scholar]
- Freeman, M.W.; Arrott, A.S.; Watson, J.H.L. Magnetism in Medicine. J. Appl. Phys. 1960, 31, S404–S405. [Google Scholar] [CrossRef]
- Damadian, R. Tumor Detection by Nuclear Magnetic Resonance. Science 1971, 171, 1151–1153. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Q.; Guo, X.; Villanova, J.; Hu, Y.; Külaots, I.; Garcia-Rojas, D.; Guo, W.; Colvin, V.L. Libraries of Uniform Magnetic Multicore Nanoparticles with Tunable Dimensions for Biomedical and Photonic Applications. ACS Appl. Mater. Interfaces 2020, 12, 41932–41941. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Gneveckow, U.; Taymoorian, K.; Thiesen, B.; Waldöfner, N.; Scholz, R.; Jung, K.; Jordan, A.; Wust, P.; Loening, S.A. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. Int. J. Hyperth. 2007, 23, 315–323. [Google Scholar] [CrossRef]
- Lubbe, A.S. Preclinical experiences with magnetic drug targeting: Tolerance and efficacy and clinical experiences with magnetic drug targeting: A phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors—Reply. Cancer Res. 1997, 57, 3064–3065. [Google Scholar]
- Merle, P.; Ahmed, S.S.; Habersetzer, F.; Abergel, A.; Taieb, J.; Bonyhay, L.; Costantini, D.; Dufour-Lamartinie, J.; Trepo, C.P. 384 Phase 1 study of intra-arterial hepatic (IAH) delivery of doxorubicin-transdrug® (DT) for patients with advanced hepatocellular carcinoma (HCC). J. Clin. Virol. 2006, 36, S179. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Delikanli, S.; Zeng, H.; Ferkey, D.M.; Pralle, A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol. 2010, 5, 602–606. [Google Scholar] [CrossRef]
- Henriksen, A.D.; Rozlosnik, N.; Hansen, M.F. Geometrical optimization of microstripe arrays for microbead magnetophoresis. Biomicrofluidics 2015, 9, 054123. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.F.; Lan, Z.; Ferrari, C.; Stein, J.M.; Higbee-Dempsey, E.; Yan, L.; Amirshaghaghi, A.; Cheng, Z.; Issadore, D.; Tsourkas, A. Use of Oppositely Polarized External Magnets to Improve the Accumulation and Penetration of Magnetic Nanocarriers into Solid Tumors. ACS Nano 2020, 14, 142–152. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Zhang, H.; Zhang, S.; Zhong, M.; Fan, H. Ferrite Nanoparticles-Based Reactive Oxygen Species-Mediated Cancer Therapy. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, Y.-X.; Gao, Y.-J.; Gao, F.-P.; Fan, Y.-S.; Li, X.-J.; Duan, Z.-Y.; Wang, H. Anti-bacterial and in vivo tumor treatment by reactive oxygen species generated by magnetic nanoparticles. J. Mater. Chem. B 2013, 1, 5100–5107. [Google Scholar] [CrossRef]
- Thoidingjam, S.; Tiku, A.B. Therapeutic efficacy of Phyllanthus emblica-coated iron oxide nanoparticles in A549 lung cancer cell line. Nanomedicine 2019, 14, 2355–2371. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Singh, K.; Subramanian, S.; Korde, A.; Singh, R.; Sawant, K. Heterogeneous surface architectured pH responsive Metal-Drug Nano-conjugates for mitochondria targeted therapy of Glioblastomas: A multimodal intranasal approach. Chem. Eng. J. 2020, 394, 124419. [Google Scholar] [CrossRef]
- Wu, H.; Liu, L.; Song, L.; Ma, M.; Gu, N.; Zhang, Y. Enhanced Tumor Synergistic Therapy by Injectable Magnetic Hydrogel Mediated Generation of Hyperthermia and Highly Toxic Reactive Oxygen Species. ACS Nano 2019, 13, 14013–14023. [Google Scholar] [CrossRef]
- Liu, X.; Yan, B.; Li, Y.; Ma, X.; Jiao, W.; Shi, K.; Zhang, T.; Chen, S.; He, Y.; Liang, X.-J.; et al. Graphene Oxide-Grafted Magnetic Nanorings Mediated Magnetothermodynamic Therapy Favoring Reactive Oxygen Species-Related Immune Response for Enhanced Antitumor Efficacy. ACS Nano 2020, 14, 1936–1950. [Google Scholar] [CrossRef]
- Klein, S.; Kızaloğlu, M.; Portilla, L.; Park, H.; Rejek, T.; Hümmer, J.; Meyer, K.; Hock, R.; Distel, L.V.R.; Halik, M.; et al. Enhanced In Vitro Biocompatibility and Water Dispersibility of Magnetite and Cobalt Ferrite Nanoparticles Employed as ROS Formation Enhancer in Radiation Cancer Therapy. Small 2018, 14, 1704111. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Torrice, M. Does Nanomedicine Have a Delivery Problem? ACS Cent. Sci. 2016, 2, 434–437. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Jung, H.; Li, X. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, e1706759. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Li, G.; Su, M.; Chen, X.; Zhang, H.; Zhang, Y.; Jiao, W.; He, Y.; Yi, J.; et al. Engineering ferrite nanoparticles with enhanced magnetic response for advanced biomedical applications. Mater. Today Adv. 2020, 8, 100119. [Google Scholar] [CrossRef]
- Poon, W.; Kingston, B.R.; Ouyang, B.; Ngo, W.; Chan, W.C.W. A framework for designing delivery systems. Nat. Nanotechnol. 2020, 15, 819–829. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Srinivasan, S.Y.; Paknikar, K.M.; Gajbhiye, V.; Bodas, D. Magneto-Conducting Core/Shell Nanoparticles for Biomedical Applications. ChemNanoMat 2018, 4, 151–164. [Google Scholar] [CrossRef]
- Dai, Y.; Xu, C.; Sun, X.; Chen, X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem. Soc. Rev. 2017, 46, 3830–3852. [Google Scholar] [CrossRef]
- Nikazar, S.; Barani, M.; Rahdar, A.; Zoghi, M.; Kyzas, G.Z. Photo- and Magnetothermally Responsive Nanomaterials for Therapy, Controlled Drug Delivery and Imaging Applications. ChemistrySelect 2020, 5, 12590–12609. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Noh, S.-H.; Moon, S.H.; Shin, T.-H.; Lim, Y.; Cheon, J. Recent advances of magneto-thermal capabilities of nanoparticles: From design principles to biomedical applications. Nano Today 2017, 13, 61–76. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.A.E.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, K.T.; Bai, J.; Wang, T.-W.; Protti, A.; Southern, P.; Bogart, L.; Heidari, H.; Li, X.; Cakebread, A.; Asker, D.; et al. Magnetic Drug Targeting: Preclinical in Vivo Studies, Mathematical Modeling, and Extrapolation to Humans. Nano Lett. 2016, 16, 5652–5660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, Biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Thakor, A.S.; Jokerst, J.V.; Ghanouni, P.; Campbell, J.L.; Mittra, E.; Gambhir, S.S. Clinically Approved Nanoparticle Imaging Agents. J. Nucl. Med. 2016, 57, 1833–1837. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Ward, J.; Robinson, P.J. MR imaging of the liver and spleen: A comparison of the effects on signal intensity of two superparamagnetic iron oxide agents. Magn. Reson. Imaging 1999, 17, 549–556. [Google Scholar] [CrossRef]
- Anderson, S.; Gwenin, V.V.; Gwenin, C.D. Magnetic Functionalized Nanoparticles for Biomedical, Drug Delivery and Imaging Applications. Nanoscale Res. Lett. 2019, 14, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hervault, A.; Dunn, A.E.; Lim, M.; Boyer, C.; Mott, D.; Maenosono, S.; Thanh, N.T.K. Doxorubicin loaded dual pH- and thermo-responsive magnetic nanocarrier for combined magnetic hyperthermia and targeted controlled drug delivery applications. Nanoscale 2016, 8, 12152–12161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Ai, K.; Liu, J.; Sun, G.; Yin, Q.; Lu, L. Multifunctional envelope-type mesoporous silica nanoparticles for pH-responsive drug delivery and magnetic resonance imaging. Biomaterials 2015, 60, 111–120. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: A combinational approach for enhanced delivery of nanoparticles. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Luque-Michel, E.; Lemaire, L.; Blanco-Prieto, M.J. SPION and doxorubicin-loaded polymeric nanocarriers for glioblastoma theranostics. Drug Deliv. Transl. Res. 2021, 11, 515–523. [Google Scholar] [CrossRef]
- Shetty, A.; Chandra, S. Inorganic hybrid nanoparticles in cancer theranostics: Understanding their combinations for better clinical translation. Mater. Today Chem. 2020, 18, 100381. [Google Scholar] [CrossRef]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Gersting, S.W.; Schillinger, U.; Lausier, J.; Nicklaus, P.; Rudolph, C.; Plank, C.; Reinhardt, D.; Rosenecker, J. Gene delivery to respiratory epithelial cells by magnetofection. J. Gene Med. 2004, 6, 913–922. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Mantovani, D.; Candiani, G. Non-Viral in Vitro Gene Delivery: It is Now Time to Set the Bar! Pharmaceutics 2020, 12, 183. [Google Scholar] [CrossRef] [Green Version]
- Krötz, F.; Sohn, H.-Y.; Gloe, T.; Plank, C.; Pohl, U. Magnetofection potentiates gene delivery to cultured endothelial cells. J. Vasc. Res. 2003, 40, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Nacev, A.; Weinberg, I.N.; Stepanov, P.Y.; Kupfer, S.; Mair, L.O.; Urdaneta, M.G.; Shimoji, M.; Fricke, S.T.; Shapiro, B. Dynamic Inversion Enables External Magnets to Concentrate Ferromagnetic Rods to a Central Target. Nano Lett. 2014, 15, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-L.; Chen, D.; Shang, P.; Yin, D.-C. A review of magnet systems for targeted drug delivery. J. Control. Release 2019, 302, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective Inductive Heating of Lymph Nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, M.G.; Senko, A.W.; Chen, R.; Romero, G.; Anikeeva, P. Magnetically multiplexed heating of single domain nanoparticles. Appl. Phys. Lett. 2014, 104, 213103. [Google Scholar] [CrossRef] [Green Version]
- Curley, S. Thermal cancer ablation therapies using nanoparticles. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 2697–2704. [Google Scholar]
- Silva, P.L.; Savchuk, O.A.; Gallo, J.; García-Hevia, L.; Bañobre-López, M.; Nieder, J.B. Mapping intracellular thermal response of cancer cells to magnetic hyperthermia treatment. Nanoscale 2020, 12, 21647–21656. [Google Scholar] [CrossRef]
- Liu, J.; Guo, X.; Zhao, Z.; Li, B.; Qin, J.; Peng, Z.; He, G.; Brett, D.J.; Wang, R.; Lu, X. Fe3S4 nanoparticles for arterial inflammation therapy: Integration of magnetic hyperthermia and photothermal treatment. Appl. Mater. Today 2020, 18, 100457. [Google Scholar] [CrossRef]
- Yang, F.; Skripka, A.; Tabatabaei, M.S.; Hong, S.H.; Ren, F.; Benayas, A.; Oh, J.K.; Martel, S.; Liu, X.; Vetrone, F.; et al. Multifunctional Self-Assembled Supernanoparticles for Deep-Tissue Bimodal Imaging and Amplified Dual-Mode Heating Treatment. ACS Nano 2019, 13, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Dai, X.; Zhang, P.; Tan, X.; Zhong, Y.; Yao, C.; Song, M.; Song, G.; Zhang, Z.; Peng, G.; et al. Fe3O4@Au composite magnetic nanoparticles modified with cetuximab for targeted magneto-photothermal therapy of glioma cells. Int. J. Nanomed. 2018, 13, 2491–2505. [Google Scholar] [CrossRef] [Green Version]
- Alhasan, A.H.; Fardous, R.S.; Alsudir, S.A.; Majrashi, M.A.; Alghamdi, W.M.; Alsharaeh, E.H.; Almalik, A.M. Polymeric Reactor for the Synthesis of Superparamagnetic-Thermal Treatment of Breast Cancer. Mol. Pharm. 2019, 16, 3577–3587. [Google Scholar] [CrossRef]
- Pardo, A.; Pelaz, B.; Gallo, J.; Bañobre-López, M.; Parak, W.J.; Barbosa, S.; Del Pino, P.; Taboada, P. Synthesis, Characterization, and Evaluation of Superparamagnetic Doped Ferrites as Potential Therapeutic Nanotools. Chem. Mater. 2020, 32, 2220–2231. [Google Scholar] [CrossRef]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for In Vivo Imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef]
- Shen, Z.; Wu, A.; Chen, X. Iron Oxide Nanoparticle Based Contrast Agents for Magnetic Resonance Imaging. Mol. Pharm. 2017, 14, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E. Ten Things You Might Not Know about Iron Oxide Nanoparticles. Radiology 2017, 284, 616–629. [Google Scholar] [CrossRef]
- Shen, S.; Ding, W.; Ahmed, S.; Hu, R.; Opalacz, A.; Roth, S.; You, Z.; Wotjkiewicz, G.R.; Lim, G.; Chen, L.; et al. Ultrasmall Superparamagnetic Iron Oxide Imaging Identifies Tissue and Nerve Inflammation in Pain Conditions. Pain Med. 2018, 19, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Moon, W.-J. Gadolinium Deposition in the Brain: Current Updates. Korean J. Radiol. 2019, 20, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.; Napolitano, A.; Visconti, E.; Longo, D.; Romano, A.; Tomà, P.; Espagnet, M.C.R. Gadolinium-Based Contrast Agent-Related Toxicities. CNS Drugs 2018, 32, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.-A. Magnetic Nanoparticles Used as Contrast Agents in MRI: Relaxometric Characterisation. In Magnetic Characterization Techniques for Nanomaterials; Kumar, C.S.S.R., Ed.; Springer: Berlin, Germany, 2017; pp. 511–555. [Google Scholar]
- Laurent, S.; Elst, L.V.; Muller, R.N. Superparamagnetic Iron Oxide Nanoparticles for MRI. In The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging; John Wiley & Sons: New York, NY, USA, 2013; pp. 427–447. [Google Scholar]
- Laurent, S.; Céline, H.; Stanicki, D.; Boutry, S.; Lipani, E.; Belaid, S.; Muller, R.N.; Elst, L.V. MRI Contrast Agents: From Molecules to Particles, 1st ed.; Springer: Singapore, 2017; p. 125. [Google Scholar]
- Ni, D.; Bu, W.; Ehlerding, E.B.; Cai, W.; Shi, J. Engineering of inorganic nanoparticles as magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2017, 46, 7438–7468. [Google Scholar] [CrossRef]
- Stanicki, D.; Elst, L.V.; Muller, R.N.; Laurent, S.; Felder-Flesch, D.; Mertz, D.; Parat, A.; Begin-Colin, S.; Cotin, G.; Greneche, J.-M.; et al. Chapter 4. Iron-oxide Nanoparticle-based Contrast Agents. In Contrast Agents for MRI: Experimental Methods; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 318–447. [Google Scholar]
- Zeng, L.; Wu, D.; Zou, R.; Chen, T.; Zhang, J.; Wu, A. Paramagnetic and Superparamagnetic Inorganic Nanoparticles for T1-Weighted Magnetic Resonance Imaging. Curr. Med. Chem. 2018, 25, 2970–2986. [Google Scholar] [CrossRef]
- Cho, M.; Sethi, R.; Narayanan, J.S.A.; Lee, S.S.; Benoit, D.N.; Taheri, N.; Decuzzi, P.; Colvin, V.L. Gadolinium oxide nanoplates with high longitudinal relaxivity for magnetic resonance imaging. Nanoscale 2014, 6, 13637–13645. [Google Scholar] [CrossRef] [Green Version]
- Stinnett, G.; Taheri, N.; Villanova, J.; Bohloul, A.; Guo, X.; Esposito, E.P.; Xiao, Z.; Stueber, D.; Avendano, C.; Decuzzi, P.; et al. 2D Gadolinium Oxide Nanoplates as T 1 Magnetic Resonance Imaging Contrast Agents. Adv. Heal. Mater. 2021, 10, 2001780. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Chen, X.; Du, X.-S.; Zhang, J.-L.; Liu, G.; Zhang, W.-G. Application of iron oxide nanoparticles in glioma imaging and therapy: From bench to bedside. Nanoscale 2016, 8, 7808–7826. [Google Scholar] [CrossRef]
- Bernsen, M.R.; Guenoun, J.; Van Tiel, S.; Krestin, G. Nanoparticles and clinically applicable cell tracking. Br. J. Radiol. 2015, 88, 20150375. [Google Scholar] [CrossRef] [Green Version]
- Weissig, V.; Pettinger, T.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar] [CrossRef]
- Langsjoen, J.; Neuwelt, A.; Eberhardt, S.; Mlady, G.; Shukla, U.; Murali, S.; Pizanis, C.; Sillerud, L.O. A comparison of ferumoxytol with gadolinium as contrast agents for the diagnostic magnetic resonance imaging of osteomyelitis. Magn. Reson. Imaging 2020, 71, 45–54. [Google Scholar] [CrossRef]
- Siedek, F.; Muehe, A.M.; Theruvath, A.J.; Avedian, R.; Pribnow, A.; Spunt, S.L.; Liang, T.; Farrell, C.; Daldrup-Link, H.E. Comparison of ferumoxytol- and gadolinium chelate-enhanced MRI for assessment of sarcomas in children and adolescents. Eur. Radiol. 2020, 30, 1790–1803. [Google Scholar] [CrossRef]
- Wáng, Y.X.J.; Idée, J.-M. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant. Imaging Med. Surg. 2017, 7, 88–122. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron Oxide Nanoparticles as T 1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2021, 33, e1906539. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Bruns, O.T.; Kaul, M.G.; Hansen, E.C.; Barch, M.; Wisniowska, A.E.; Chen, O.; Chen, Y.; Li, N.; Okada, S.; et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl. Acad. Sci. USA 2017, 114, 2325–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Y.; Sherwood, J.A.; Sun, Z. Magnetic iron oxide nanoparticles asT1contrast agents for magnetic resonance imaging. J. Mater. Chem. C 2018, 6, 1280–1290. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Y.-J.; Zhang, G.-B.; Ling, D.; Wang, M.-Q.; Zhou, Y.; Wu, Y.-D.; Wu, T.; Hackett, M.J.; Kim, B.H.; et al. Iron oxide nanoclusters for T 1 magnetic resonance imaging of non-human primates. Nat. Biomed. Eng. 2017, 1, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, E.M. Biodegradable, polymer encapsulated, metal oxide particles for MRI-based cell tracking. Magn. Reson. Med. 2015, 73, 376–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.; Jin, S.; Lee, D.; Cho, H. MRI Visualization of Whole Brain Macro- and Microvascular Remodeling in a Rat Model of Ischemic Stroke: A Pilot Study. Sci. Rep. 2020, 10, 4989. [Google Scholar] [CrossRef] [Green Version]
- Guldris, N.; Argibay, B.; Gallo, J.; Iglesias-Rey, R.; Carbó-Argibay, E.; Kolen’Ko, Y.V.; Campos, F.; Sobrino, T.; Salonen, L.M.; Bañobre-López, M.; et al. Magnetite Nanoparticles for Stem Cell Labeling with High Efficiency and Long-Term In Vivo Tracking. Bioconjugate Chem. 2017, 28, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Ohki, A.; Saito, S.; Fukuchi, K. Magnetic resonance imaging of umbilical cord stem cells labeled with superparamagnetic iron oxide nanoparticles: Effects of labelling and transplantation parameters. Sci. Rep. 2020, 10, 13684. [Google Scholar] [CrossRef]
- Wierzbiński, K.R.; Szymanski, T.; Rozwadowska, N.; Rybka, J.D.; Zimna, A.; Zalewski, T.; Nowicka-Bauer, K.; Malcher, A.; Nowaczyk, M.; Krupinski, M.; et al. Potential use of superparamagnetic iron oxide nanoparticles for in vitro and in vivo bioimaging of human myoblasts. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherwood, J.; Rich, M.; Lovas, K.; Warram, J.; Bolding, M.S.; Bao, Y. T1-Enhanced MRI-visible nanoclusters for imaging-guided drug delivery. Nanoscale 2017, 9, 11785–11792. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Zu, G.; Kuang, Y.; Dong, J.; Cao, Y.; Zhang, T.; Liu, M.; Luo, L.; Pei, R. Gadolinium(III)-based Polymeric Magnetic Resonance Imaging Agents for Tumor Imaging. Curr. Med. Chem. 2018, 25, 2910–2937. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liang, Z.; Liu, J.; Sun, J.; Hu, X.; Zhao, M.; Liu, J.; Bai, R.; Kim, D.; Sun, X.; et al. Dynamically Reversible Iron Oxide Nanoparticle Assemblies for Targeted Amplification of T1-Weighted Magnetic Resonance Imaging of Tumors. Nano Lett. 2019, 19, 4213–4220. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.F.; Lam, R.W.M.; Hu, T.; Ng, M.W.F.; Liau, Z.Q.G.; Nagata, K.; Khanna, S.; Lam, Y.; Bhakoo, K.; Ho, R.C.M.; et al. Locating the Site of Neuropathic Pain In Vivo Using MMP-12-Targeted Magnetic Nanoparticles. Pain Res. Manag. 2019, 2019, 9394715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Xiong, S.; Zhang, K.; Feng, L.; Chen, X.; Wu, Y.; Huang, X.; Xiong, Y. Quantum bead-based fluorescence-linked immunosorbent assay for ultrasensitive detection of aflatoxin M1 in pasteurized milk, yogurt, and milk powder. J. Dairy Sci. 2019, 102, 3985–3993. [Google Scholar] [CrossRef]
- Barajas, R.F.; Hamilton, B.E.; Schwartz, D.; McConnell, H.; Pettersson, D.R.; Horvath, A.; Szidonya, L.; Varallyay, C.G.; Firkins, J.; Jaboin, J.J.; et al. Combined iron oxide nanoparticle ferumoxytol and gadolinium contrast enhanced MRI define glioblastoma pseudoprogression. Neuro-Oncology 2019, 21, 517–526. [Google Scholar] [CrossRef]
- Richard, S.; Boucher, M.; Lalatonne, Y.; Mériaux, S.; Motte, L. Iron oxide nanoparticle surface decorated with cRGD peptides for magnetic resonance imaging of brain tumors. Biochim. Biophys. Acta BBA Gen. Subj. 2017, 1861, 1515–1520. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Y.; Shi, Y.; Li, Y.; Xiao, Z.; Zhang, X. Traceable Nanoparticles with Spatiotemporally Controlled Release Ability for Synergistic Glioblastoma Multiforme Treatment. Adv. Funct. Mater. 2017, 27, 1703967. [Google Scholar] [CrossRef]
- Bulte, J.W.M. Superparamagnetic iron oxides as MPI tracers: A primer and review of early applications. Adv. Drug Deliv. Rev. 2019, 138, 293–301. [Google Scholar] [CrossRef]
- Du, Y.; Lai, P.T.; Leung, C.H.; Pong, P.W.T. Design of Superparamagnetic Nanoparticles for Magnetic Particle Imaging (MPI). Int. J. Mol. Sci. 2013, 14, 18682–18710. [Google Scholar] [CrossRef] [Green Version]
- Gleich, B.; Weizenecker, J. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2005, 435, 1214–1217. [Google Scholar] [CrossRef]
- Weizenecker, J.; Gleich, B.; Rahmer, J.; Dahnke, H.; Borgert, J. Three-dimensional real-time in vivo magnetic particle imaging. Phys. Med. Biol. 2009, 54, L1–L10. [Google Scholar] [CrossRef]
- Talebloo, N.; Gudi, M.; Robertson, N.; Wang, P. Magnetic Particle Imaging: Current Applications in Biomedical Research. J. Magn. Reson. Imaging 2020, 51, 1659–1668. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Jeffris, K.E.; Yu, E.Y.; Zheng, B.; Goodwill, P.W.; Nahid, P.; Conolly, S.M. First in vivo magnetic particle imaging of lung perfusion in rats. Phys. Med. Biol. 2017, 62, 3510–3522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, E.Y.; Bishop, M.; Zheng, B.; Ferguson, R.M.; Khandhar, A.P.; Kemp, S.J.; Krishnan, K.M.; Goodwill, P.W.; Conolly, S.M. Magnetic Particle Imaging: A Novel in Vivo Imaging Platform for Cancer Detection. Nano Lett. 2017, 17, 1648–1654. [Google Scholar] [CrossRef]
- Zhu, X.; Li, J.; Peng, P.; Hosseini-Nassab, N.; Smith, B.R. Quantitative Drug Release Monitoring in Tumors of Living Subjects by Magnetic Particle Imaging Nanocomposite. Nano Lett. 2019, 19, 6725–6733. [Google Scholar] [CrossRef]
- Zheng, B.; Von See, M.P.; Yu, E.; Gunel, B.; Lu, K.; Vazin, T.; Schaffer, D.V.; Goodwill, P.W.; Conolly, S.M. Quantitative Magnetic Particle Imaging Monitors the Transplantation, Biodistribution, and Clearance of Stem Cells In Vivo. Theranostics 2016, 6, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Yu, E.Y.; Chandrasekharan, P.; Berzon, R.; Tay, Z.W.; Zhou, X.Y.; Khandhar, A.P.; Ferguson, R.M.; Kemp, S.J.; Zheng, B.; Goodwill, P.W.; et al. Magnetic Particle Imaging for Highly Sensitive, Quantitative, and Safe in Vivo Gut Bleed Detection in a Murine Model. ACS Nano 2017, 11, 12067–12076. [Google Scholar] [CrossRef] [PubMed]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.; Zarek, M.; Elyashiv, A.; Abu Sbitan, M.; Sharma, V.; Ramanujan, R.V. Remotely triggered morphing behavior of additively manufactured thermoset polymer-magnetic nanoparticle composite structures. Smart Mater. Struct. 2021, 30, 045022. [Google Scholar] [CrossRef]
- Wyatt Shields, C., IV; Reyes, C.D.; López, G.P. Microfluidic cell sorting: A review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef] [Green Version]
- Vermesh, O.; Aalipour, A.; Ge, T.J.; Saenz, Y.; Guo, Y.; Alam, I.S.; Park, S.-M.; Adelson, C.N.; Mitsutake, Y.; Vilches-Moure, J.; et al. An intravascular magnetic wire for the high-throughput retrieval of circulating tumour cells in vivo. Nat. Biomed. Eng. 2018, 2, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, A.H.; Faghih, Z.; Khorasani, M.T.; Farjadian, F. Antibody conjugated onto surface modified magnetic nanoparticles for separation of HER2+ breast cancer cells. J. Magn. Magn. Mater. 2019, 490, 165479. [Google Scholar] [CrossRef]
- Liang, W.; Liu, J.; Yang, X.; Zhang, Q.; Yang, W.; Zhang, H.; Liu, L. Microfluidic-based cancer cell separation using active and passive mechanisms. Microfluid. Nanofluidics 2020, 24, 1–19. [Google Scholar] [CrossRef]
- Saei, A.; Asfia, S.; Kouchakzadeh, H.; Rahmandoust, M. Antibody-modified magnetic nanoparticles as specific high-efficient cell-separation agents. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2633–2642. [Google Scholar] [CrossRef]
- Wilson, R.E.; O’Connor, R.T.; Gallops, C.E.; Kwizera, E.A.; Noroozi, B.; Morshed, B.I.; Wang, Y.; Huang, X. Immunomagnetic Capture and Multiplexed Surface Marker Detection of Circulating Tumor Cells with Magnetic Multicolor Surface-Enhanced Raman Scattering Nanotags. ACS Appl. Mater. Interfaces 2020, 12, 47220–47232. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, W.; Li, S.; Ya, S.; Li, F.; Qiu, B. On-chip analysis of magnetically labeled cells with integrated cell sorting and counting techniques. Talanta 2020, 220, 121351. [Google Scholar] [CrossRef]
- Korangath, P.; Ivkov, R. Magnet-assisted Flow Cytometry of in vivo Tumors to Quantitate Cell-specific Responses to Magnetic Iron Oxide Nanoparticles. Bio-Protocol 2020, 10, e3822. [Google Scholar] [CrossRef]
- Robert, D.; Pamme, N.; Conjeaud, H.; Gazeau, F.; Iles, A.; Wilhelm, C. Cell sorting by endocytotic capacity in a microfluidic magnetophoresis device. Lab Chip 2011, 11, 1902–1910. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, T.; Xu, R.; Gao, W.; Zhao, H.; Shapter, J.G.; Wang, K.; Shen, Y.; Huang, P.; Gao, G.; et al. Large-scale immuno-magnetic cell sorting of T cells based on a self-designed high-throughput system for potential clinical application. Nanoscale 2017, 9, 13592–13599. [Google Scholar] [CrossRef] [Green Version]
- Tran, M.V.; Susumu, K.; Medintz, I.L.; Algar, W.R. Supraparticle Assemblies of Magnetic Nanoparticles and Quantum Dots for Selective Cell Isolation and Counting on a Smartphone-Based Imaging Platform. Anal. Chem. 2019, 91, 11963–11971. [Google Scholar] [CrossRef]
- Shcmitt, F.; Piccin, O.; Barbé, L.; Bayle, B. Soft Robots Manufacturing: A Review. Front. Robot AI 2018, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Cianchetti, M.; Laschi, C.; Menciassi, A.; Dario, P. Biomedical applications of soft robotics. Nat. Rev. Mater. 2018, 3, 143–153. [Google Scholar] [CrossRef]
- Cao, Q.; Fan, Q.; Chen, Q.; Liu, C.; Han, X.; Li, L. Recent advances in manipulation of micro- and nano-objects with magnetic fields at small scales. Mater. Horizons 2020, 7, 638–666. [Google Scholar] [CrossRef]
- Hu, W.; Lum, G.Z.; Mastrangeli, M.; Sitti, M. Small-scale soft-bodied robot with multimodal locomotion. Nature 2018, 554, 81–85. [Google Scholar] [CrossRef]
- Gu, H.; Boehler, Q.; Cui, H.; Secchi, E.; Savorana, G.; De Marco, C.; Gervasoni, S.; Peyron, Q.; Huang, T.-Y.; Pane, S.; et al. Magnetic cilia carpets with programmable metachronal waves. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Bayaniahangar, R.; Ahangar, S.B.; Zhang, Z.; Lee, B.P.; Pearce, J.M. 3-D printed soft magnetic helical coil actuators of iron oxide embedded polydimethylsiloxane. Sensors Actuators B Chem. 2021, 326, 128781. [Google Scholar] [CrossRef]
- Goudu, S.R.; Yasa, I.C.; Hu, X.; Ceylan, H.; Hu, W.; Sitti, M. Biodegradable Untethered Magnetic Hydrogel Milli-Grippers. Adv. Funct. Mater. 2020, 30, 9. [Google Scholar] [CrossRef]
- Breger, J.C.; Yoon, C.; Xiao, R.; Kwag, H.R.; Wang, M.O.; Fisher, J.P.; Nguyen, T.D.; Gracias, D.H. Self-Folding Thermo-Magnetically Responsive Soft Microgrippers. ACS Appl. Mater. Interfaces 2015, 7, 3398–3405. [Google Scholar] [CrossRef]
- Hwang, G.; Paula, A.J.; Hunter, E.E.; Liu, Y.; Babeer, A.; Karabucak, B.; Stebe, K.; Kumar, V.; Steager, E.; Koo, H. Catalytic antimicrobial robots for biofilm eradication. Sci. Robot. 2019, 4, eaaw2388. [Google Scholar] [CrossRef] [PubMed]
- Brisbois, C.A.; Tasinkevych, M.; Vázquez-Montejo, P.; de la Cruz, M.O. Actuation of magnetoelastic membranes in precessing magnetic fields. Proc. Natl. Acad. Sci. USA 2019, 116, 2500–2505. [Google Scholar] [CrossRef] [Green Version]
- Chin, S.Y.; Poh, Y.C.; Kohler, A.-C.; Sia, S.K. An Additive Manufacturing Technique for the Facile and Rapid Fabrication of Hydrogel-based Micromachines with Magnetically Responsive Components. J. Vis. Exp. 2018, 12, e56727. [Google Scholar] [CrossRef] [Green Version]
- Ugelstad, J.; Berge, A.; Ellingsen, T.; Schmid, R.; Nilsen, T.-N.; Mørk, P.; Stenstad, P.; Hornes, E.; Olsvik, Ø. Preparation and application of new monosized polymer particles. Prog. Polym. Sci. 1992, 17, 87–161. [Google Scholar] [CrossRef]
- Fonnum, G.; Johansson, C.; Molteberg, A.; Mørup, S.; Aksnes, E. Characterisation of Dynabeads® by magnetization measurements and Mössbauer spectroscopy. J. Magn. Magn. Mater. 2005, 293, 41–47. [Google Scholar] [CrossRef]
- Treleaven, J.G.; Ugelstad, J.; Philip, T.; Gibson, F.M.; Rembaum, A.; Caine, G.D.; Kemshead, J.T. Removal of Neuro-Blastoma Cells from Bone-Marrow with Monoclonal-Antibodies Conjugated to Magnetic Microspheres. Lancet 1984, 323, 70–73. [Google Scholar] [CrossRef]
- Kemshead, J.T.; Heath, L.; Gibson, F.M.; Katz, F.; Richmond, F.; Treleaven, J.; Ugelstad, J. Magnetic microspheres and monoclonal antibodies for the depletion of neuroblastoma cells from bone marrow: Experiences, improvements and observations. Br. J. Cancer 1986, 54, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Uhlén, M.; Hultman, T.; Wahlberg, J.; Lundeberg, J.; Bergh, S.; Petterson, B.; Holmberg, A.; Ståhl, S.; Moks, T. Semi-automated solid-phase DNA sequencing. Trends Biotechnol. 1992, 10, 52–55. [Google Scholar] [CrossRef]
- Rimstad, E.; Hornes, E.; Olsvik, O.; Hyllseth, B. Identification of a double-stranded RNA virus by using polymerase chain reaction and magnetic separation of the synthesized DNA segments. J. Clin. Microbiol. 1990, 28, 2275–2278. [Google Scholar] [CrossRef] [Green Version]
- Wright, D.J.; Chapman, P.A.; Siddons, C.A. Immunomagnetic separation as a sensitive method for isolating Escherichia coli O157 from food samples. Epidemiol. Infect. 1994, 113, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woywodt, A.; Schroeder, M.; Gwinner, W.; Mengel, M.; Jaeger, M.; Schwarz, A.; Haller, H.; Haubitz, M. Elevated Numbers of Circulating Endothelial Cells in Renal Transplant Recipients. Transplantation 2003, 76, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Bäckman, A.; Lantz, P.-G.; Rådström, P.; Olcén, P. Evaluation of an extended diagnostic PCR assay for detection and verification of the common causes of bacterial meningitis in CSF and other biological samples. Mol. Cell. Probes 1999, 13, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Satterly, N.G.; Voorhees, M.A.; Ames, A.D.; Schoepp, R.J. Comparison of MagPix Assays and Enzyme-Linked Immunosorbent Assay for Detection of Hemorrhagic Fever Viruses. J. Clin. Microbiol. 2017, 55, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Baker, H.N.; Murphy, R.; Lopez, E.; Garcia, C. Conversion of a Capture ELISA to a Luminex xMAP Assay using a Multiplex Antibody Screening Method. J. Vis. Exp. 2012, e4084. [Google Scholar] [CrossRef]
- Kyaw, S.P.; Hanthamrongwit, J.; Jangpatarapongsa, K.; Khaenam, P.; Leepiyasakulchai, C. Sensitive detection of the IS6110 sequence of Mycobacterium tuberculosis complex based on PCR-magnetic bead ELISA. RSC Adv. 2018, 8, 33674–33680. [Google Scholar] [CrossRef] [Green Version]
- Su, R.; Tang, X.; Feng, L.; Yao, G.-L.; Chen, J. Development of quantitative magnetic beads-based flow cytometry fluorescence immunoassay for aflatoxin B1. Microchem. J. 2020, 155, 104715. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Lien, K.-Y.; Huang, K.-J.; Lei, H.-Y.; Lee, G.-B. Micro flow cytometry utilizing a magnetic bead-based immunoassay for rapid virus detection. Biosens. Bioelectron. 2008, 24, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Furuya, H.; Pagano, I.; Chee, K.; Kobayashi, T.; Wong, R.S.; Lee, R.; Rosser, C.J. Comparison of Commercial ELISA Kits, a Prototype Multiplex Electrochemoluminescent Assay, and a Multiplex Bead-Based Immunoassay for Detecting a Urine-Based Bladder-Cancer-Associated Diagnostic Signature. Diagnostics 2019, 9, 166. [Google Scholar] [CrossRef] [Green Version]
- Srisa-Art, M.; Boehle, K.E.; Geiss, B.J.; Henry, C.S. Highly Sensitive Detection ofSalmonella typhimuriumUsing a Colorimetric Paper-Based Analytical Device Coupled with Immunomagnetic Separation. Anal. Chem. 2018, 90, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Perraut, R.; Richard, V.; Varela, M.-L.; Trape, J.-F.; Guillotte, M.; Tall, A.; Toure, A.; Sokhna, C.; Vigan-Womas, I.; Mercereau-Puijalon, O. Comparative analysis of IgG responses to Plasmodium falciparum MSP1p19 and PF13-DBL1α1 using ELISA and a magnetic bead-based duplex assay (MAGPIX®-Luminex) in a Senegalese meso-endemic community. Malar. J. 2014, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koffi, D.; Touré, A.O.; Varela, M.-L.; Vigan-Womas, I.; Beourou, S.; Brou, S.; Ehouman, M.-F.; Gnamien, L.; Richard, V.; Djaman, J.A.; et al. Analysis of antibody profiles in symptomatic malaria in three sentinel sites of Ivory Coast by using multiplex, fluorescent, magnetic, bead-based serological assay (MAGPIX™). Malar. J. 2015, 14, 1–11. [Google Scholar] [CrossRef]
- Varela, M.L.; Mbengue, B.; Basse, A.; Loucoubar, C.; Vigan-Womas, I.; Dièye, A.; Toure, A.; Perraut, R. Optimization of a magnetic bead-based assay (MAGPIX®-Luminex) for immune surveillance of exposure to malaria using multiple Plasmodium antigens and sera from different endemic settings. Malar. J. 2018, 17, 324. [Google Scholar] [CrossRef]
- Hung, L.-Y.; Chang, J.-C.; Tsai, Y.-C.; Huang, C.-C.; Chang, C.-P.; Yeh, C.-S.; Lee, G.-B. Magnetic nanoparticle-based immunoassay for rapid detection of influenza infections by using an integrated microfluidic system. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.Y.; Paknikar, K.M.; Bodas, D.; Gajbhiye, V. Applications of cobalt ferrite nanoparticles in biomedical nanotechnology. Nanomedicine 2018, 13, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Kohama, N.; Suwabe, C.; Ishii, H.; Hayashi, K.; Nagao, D. Characterization on magnetophoretic velocity of the cluster of submicron-sized composite particles applicable to magnetic separation and purification. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 141–146. [Google Scholar] [CrossRef]
- Fan, A.; Lau, C.; Lu, J. Magnetic Bead-Based Chemiluminescent Metal Immunoassay with a Colloidal Gold Label. Anal. Chem. 2005, 77, 3238–3242. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niessner, R.; Knopp, D. Magnetic Bead-Based Colorimetric Immunoassay for Aflatoxin B1 Using Gold Nanoparticles. Sensors 2014, 14, 21535–21548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, L.-H.; He, X.; Xia, J.; Ma, H.; Yang, F.; Zhang, Q.; Huang, D.; Chen, L.; Wu, C.; Zhang, X.; et al. Highly Sensitive Naked-Eye Assay for Enterovirus 71 Detection Based on Catalytic Nanoparticle Aggregation and Immunomagnetic Amplification. ACS Appl. Mater. Interfaces 2017, 9, 14691–14699. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Spiller, D.G.; Prior, I.; Veltkamp, K.J.; Hutchinson, A. A Simple Method for Preparing Spectrally Encoded Magnetic Beads for Multiplexed Detection. ACS Nano 2007, 1, 487–493. [Google Scholar] [CrossRef]
- Kim, C.; Hoffmann, G.; Searson, P.C. Integrated Magnetic Bead–Quantum Dot Immunoassay for Malaria Detection. ACS Sens. 2017, 2, 766–772. [Google Scholar] [CrossRef]








| IOP Name | IOP Type | Core Size/DH (nm) | r1/r2 (mM−1s−1) | B0 (T) | t1/2 (h) |
MRI Applications | Commercial Status | Clinical Approval | References |
|---|---|---|---|---|---|---|---|---|---|
| Ferristene (Abdoscan) | MIOP | -/~3500 | - | - | oral | GI | discontinued (2000) | - | [7,71,79] |
|
Ferumoxsil (AMI-121, GastroMARK, Lumirem) | MIOP | -/300 | 3.4, 2/3.8, 47 | 1, 1.5 | oral | GI | discontinued (2012) | 1996 US/EU (GI MRI) | [7,71,72,79] |
| Ferumoxides (AMI-25, Feridex, Endorem) | SPION | 4.5–5.6/50–100 | 40, ~10/~120–160 | 0.47, 1.5 | 2 | L, S, BM, CTL, BT | discontinued (2008) | 1996 US (L and S MRI) | [7,66,70,71,72,79,80,81,82] |
| Ferrixan (SHU 555A, Resovist, Cliavist) | SPION | ~10/60–80 | 25.4, 9.7/~150–190 | 1.5 | 2.4–3.6 | L, S, MRA, CTL | available in limited countries | 2001 EU/JP/AU (L MRI) | [7,66,70,71,72,79,80,82] |
| Ferumoxtran-10 (AMI-227, Combidex, Sinerem) | USPION | 4–6/20–50 | 23, ~10–20/53, ~65–88 | 0.47, 1.5 | 24–36 | L, LN, S, MRA, M, CTL, BT | discontinued (2007) | - | [7,66,70,71,72,79,82] |
| Ferumoxytol (AMI-7228, Feraheme, Rienso) | USPION | 6.7/20–30 | 38, 15 | 0.47, 1.5 | 10–14 | L, LN, MRA, M, I, CTL, BT, BL, S | available | 2009 US, 2013 EU (iron deficiency treatment) | [7,66,70,71,72,79,81,83,84] |
| Ferucarbotran C (SHU 555C, Supravist) | USPION | 3–5/20–25 | 24, 10.7/60, 38 | 0.47, 1.5 | 6–8 | MRA, CTL, M | discontinued | - | [66,70,71,72,79] |
| Feruglose (NC100150, PEG-feron, Clariscan) | USPION | 5–7/11–15 | 20 | 0.5 | 2–6 | L, LN, P, MRA | discontinued (early 2000s) | - | [7,66,70,71,72,79,82] |
| VSOP-C184 | USPION | 4–5 | 20.1, 14 | 0.94, 1.5 | 0.6–1.3 | L, MRA, CTL, M | stopped development | - | [7,70,71,72,79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z.; Colvin, V.L. Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics 2021, 13, 943. https://doi.org/10.3390/pharmaceutics13070943
Stueber DD, Villanova J, Aponte I, Xiao Z, Colvin VL. Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics. 2021; 13(7):943. https://doi.org/10.3390/pharmaceutics13070943
Chicago/Turabian StyleStueber, Deanna D., Jake Villanova, Itzel Aponte, Zhen Xiao, and Vicki L. Colvin. 2021. "Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends" Pharmaceutics 13, no. 7: 943. https://doi.org/10.3390/pharmaceutics13070943
APA StyleStueber, D. D., Villanova, J., Aponte, I., Xiao, Z., & Colvin, V. L. (2021). Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics, 13(7), 943. https://doi.org/10.3390/pharmaceutics13070943






