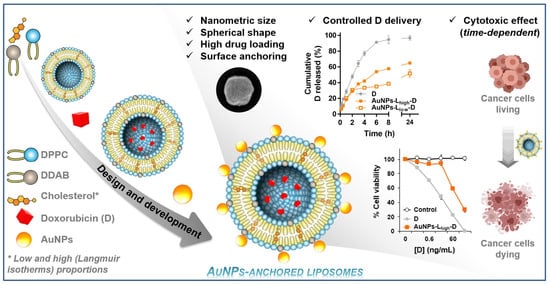
Cholesterol Levels Affect the Performance of AuNPs-Decorated Thermo-Sensitive Liposomes as Nanocarriers for Controlled Doxorubicin Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Cultures
2.3. Solubility Studies of Doxorubicin
2.3.1. Doxorubicin Quantification by HPLC-Fluorescence Detection
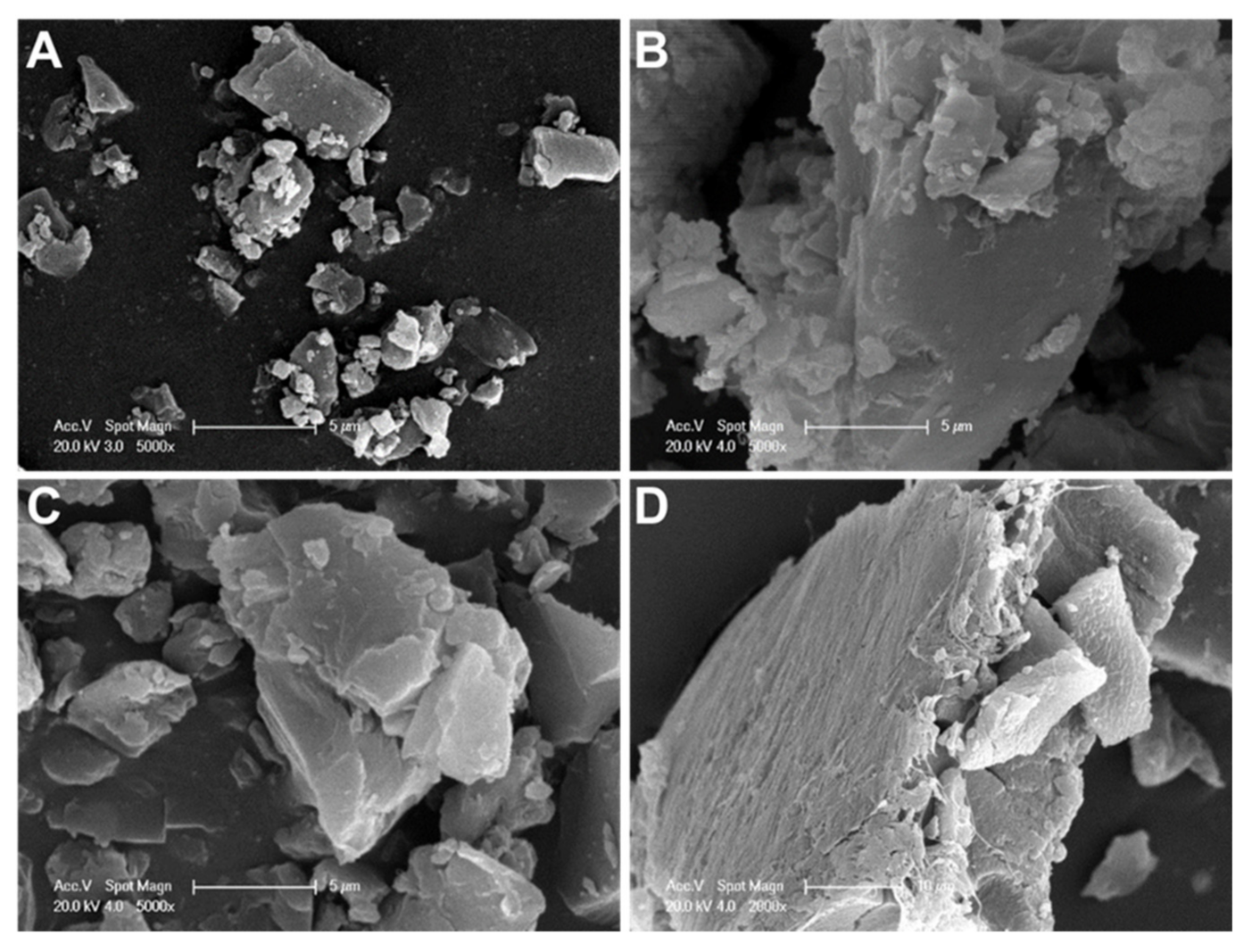
2.3.2. Morphological Characterization of Doxorubicin Crystals by SEM
2.4. Preparation of Monolayers at the Air/Water Interphase and Measurement of the Langmuir Isotherms
2.5. Liposome Preparation
2.6. Doxorubicin Encapsulation
2.7. Functionalization with AuNPs
2.8. Characterization of Liposomal Formulations
2.8.1. Size and Surface Charge
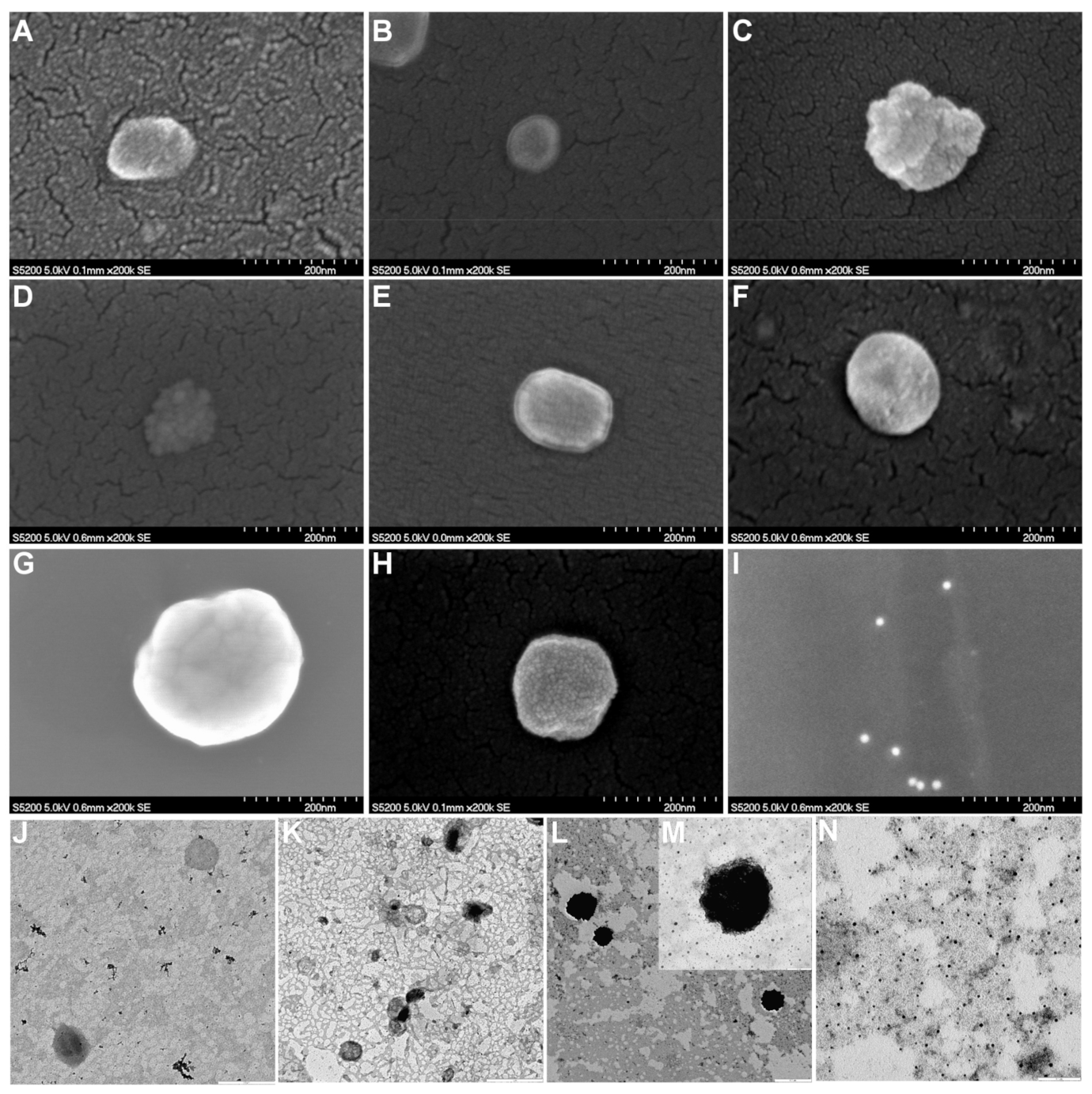
2.8.2. Morphological Analysis
2.8.3. UV-Visible Spectroscopy Studies
2.8.4. Encapsulation Efficiency
2.9. In Vitro Release Studies
2.10. Cell Viability Assay
3. Results
3.1. Solubility Studies
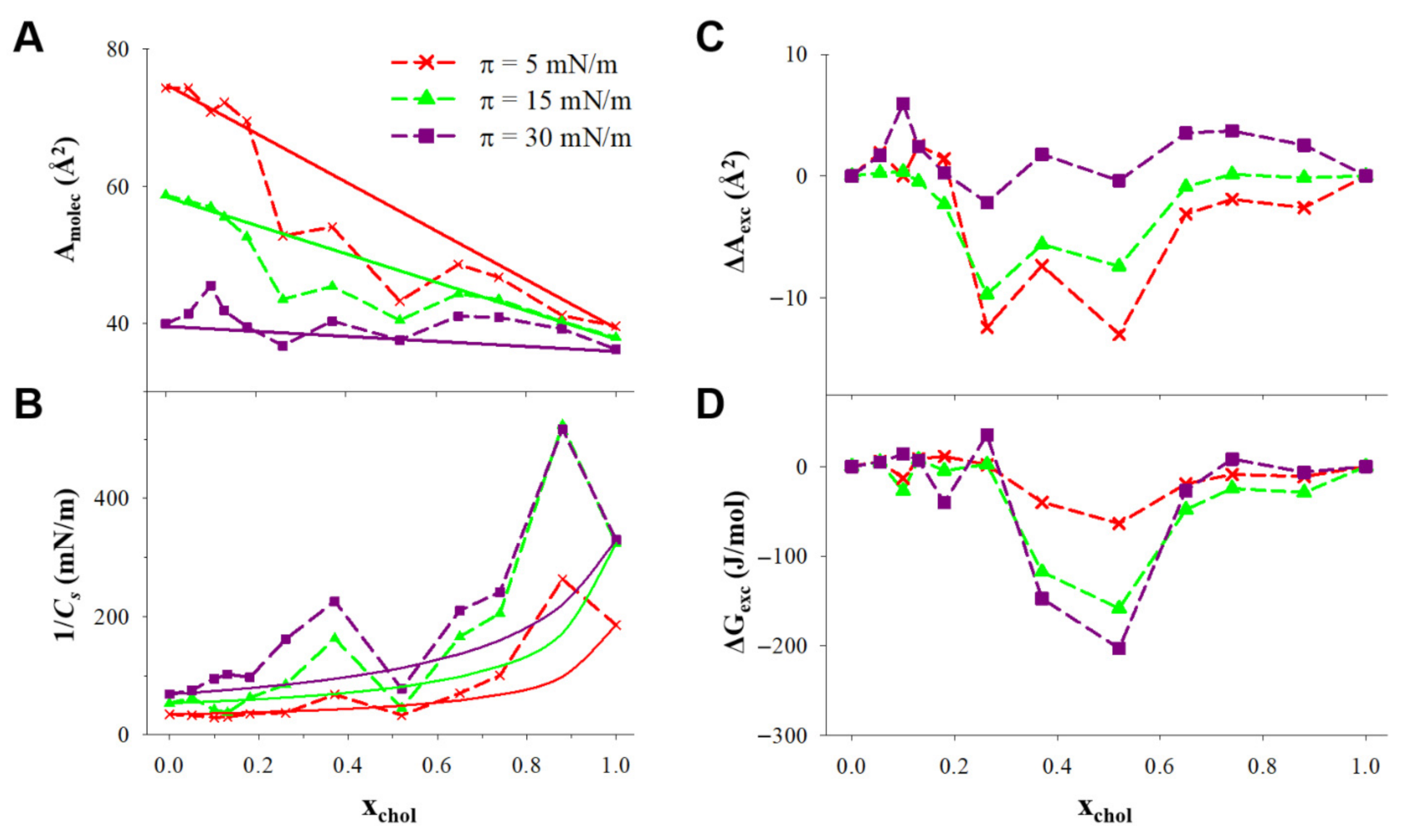
3.2. Langmuir Isotherms of DPPC:DDAB:Cholesterol Ternary Monolayers
3.3. Characterization of Liposomal Dispersions
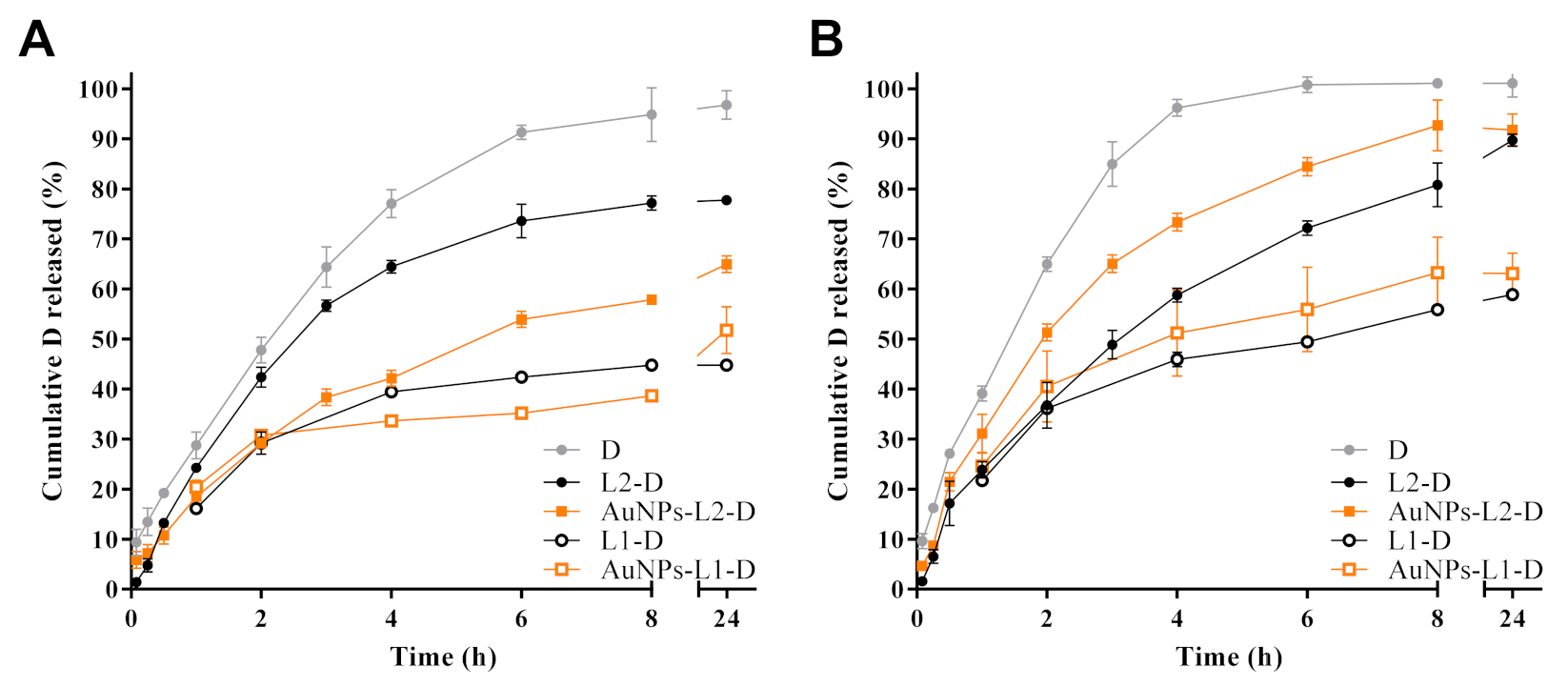
3.4. In Vitro Doxorubicin Release
3.5. In Vitro Cytotoxic Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naitlho, N.; Prieto-Dapena, F.; Rabasco, A.; Rueda, M.; González-Rodríguez, M.L. Didodecyldimethylammonium bromide role in anchoring gold nanoparticles onto liposome surface for triggering the drug release. AAPS PharmSciTech 2019, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- García, M.C.; Aloisio, C.; Onnainty, R.; Ullio-Gamboa, G. Self Assembled Nanomaterials in Nanobiomaterials: Nanostructured Materials for Biomedical Applications, 1st ed.; Narayan, R., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 41–94. [Google Scholar]
- García, M.C. Nano-And Microparticles as Drug Carriers. In Engineering Drug Delivery Systems; Elsevier: Cambridge, UK, 2019; pp. 71–110. [Google Scholar]
- Wissing, S.; Kayser, O.; Müller, R. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 1–13. [Google Scholar]
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Aghdam, M.A.; Bagheri, R.; Mosafer, J.; Baradaran, B.; Hashemzaei, M.; Baghbanzadeh, A.; de la Guardia, M.; Mokhtarzadeh, A. Recent advances on thermosensitive and pH-sensitive liposomes employed in controlled release. J. Control. Release 2019, 315, 1–22. [Google Scholar] [CrossRef]
- Lyon, P.C.; Gray, M.D.; Mannaris, C.; Folkes, L.K.; Stratford, M.; Campo, L.; Chung, D.Y.F.; Scott, S.; Anderson, M.; Goldin, R. Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): A single-centre, open-label, phase 1 trial. Lancet Oncol. 2018, 19, 1027–1039. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Kataoka, K. Chemo-physical Strategies to Advance the in ivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, F.; Ju, R.; Lu, W. Advances in liposomal drug delivery system in the field of chemotherapy. Clin. Oncol. 2016, 1, 1–10. [Google Scholar]
- Pippa, N.; Meristoudi, A.; Pispas, S.; Demetzos, C. Temperature-dependent drug release from DPPC: C12H25-PNIPAM-COOH liposomes: Control of the drug loading/release by modulation of the nanocarriers’ components. Int. J. Pharm. 2015, 485, 374–382. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, M.S.; Park, S.J.; Park, E.S.; Choi, K.S.; Kim, Y.S.; Kim, H.R. Novel temperature-triggered liposome with high stability: Formulation, in vitro evaluation, and in vivo study combined with high-intensity focused ultrasound (HIFU). J. Control. Release 2013, 170, 373–379. [Google Scholar] [CrossRef]
- Milczewska, K.; Voelkel, A.; Zwolińska, J.; Jędro, D. Preparation of hybrid materials for controlled drug release. Drug Dev. Ind. Pharm. 2016, 42, 1058–1065. [Google Scholar] [CrossRef]
- García, M.C.; Uberman, P. Nanohybrid fillers-based drug delivery systems. In Nanocarriers for Drug Delivery. Nanoscience and Nanotechnology in Drug Delivery, 1st ed.; Shyam, M., Shivendu, R., Nandita, D., Raghvendra, M., Sabu, T., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 43–79. [Google Scholar]
- Paasonen, L.; Laaksonen, T.; Johans, C.; Yliperttula, M.; Kontturi, K.; Urtti, A. Gold nanoparticles enable selective light-induced contents release from liposomes. J. Control. Release 2007, 122, 86–93. [Google Scholar] [CrossRef]
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer coated gold nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef] [Green Version]
- Lajunen, T.; Viitala, L.; Kontturi, L.-S.; Laaksonen, T.; Liang, H.; Vuorimaa-Laukkanen, E.; Viitala, T.; Le Guével, X.; Yliperttula, M.; Murtomäki, L. Light induced cytosolic drug delivery from liposomes with gold nanoparticles. J. Control. Release 2015, 203, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.K.; Urban, A.S.; Dondapati, S.K.; Fedoruk, M.; Horton, M.R.; Rogach, A.L.; Stefani, F.D.; Rädler, J.O.; Feldmann, J. Controlling loading and optical properties of gold nanoparticles on liposome membranes. Colloids Surf. A Physicochem. Eng. Asp. 2009, 342, 92–96. [Google Scholar] [CrossRef]
- Hou, K.; Bao, M.; Xin, C.; Wang, L.; Zhang, H.; Zhao, H.; Wang, Z. Green synthesis of gold nanoparticles coated doxorubicin liposomes using procyanidins for light–controlled drug release. Adv. Powder Technol. 2020, 31, 3640–3649. [Google Scholar] [CrossRef]
- Kanwar, R.; Kaur, G.; Mehta, S. Revealing the potential of Didodecyldimethylammonium bromide as efficient scaffold for fabrication of nano liquid crystalline structures. Chem. Phys. Lipids 2016, 196, 61–68. [Google Scholar] [CrossRef]
- Łudzik, K.; Kacperska, A.; Kustrzepa, K.; Dychto, R. Interactions between sodium dodecylsulphate and didodecyldimethylammonium bromides vesicles in aqueous solutions. J. Mol. Liq. 2017, 240, 273–279. [Google Scholar] [CrossRef]
- Manojlovic, V.; Winkler, K.; Bunjes, V.; Neub, A.; Schubert, R.; Bugarski, B.; Leneweit, G. Membrane interactions of ternary phospholipid/cholesterol bilayers and encapsulation efficiencies of a RIP II protein. Colloids Surfaces B Biointerfaces 2008, 64, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Najafinobar, N.; Mellander, L.J.; Kurczy, M.E.; Dunevall, J.; Angerer, T.B.; Fletcher, J.S.; Cans, A.S. Cholesterol alters the dynamics of release in protein independent cell models for exocytosis. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Takechi-Haraya, Y.; Sakai-Kato, K.; Abe, Y.; Kawanishi, T.; Okuda, H.; Goda, Y. Atomic force microscopic analysis of the effect of lipid composition on liposome membrane rigidity. Langmuir 2016, 32, 6074–6082. [Google Scholar] [CrossRef]
- Simons, K.; Sampaio, J.L. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.L.; Deng, Y.J.; Du, H.Y.; Suo, X.B.; Wang, X.Y.; Wang, X.; Wang, L.; Cui, L.J.; Duan, N. Preparation of a liposomal delivery system and its in vitro release of rapamycin. Exp. Ther. Med. 2015, 9, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Haeri, A.; Alinaghian, B.; Daeihamed, M.; Dadashzadeh, S. Preparation and characterization of stable nanoliposomal formulation of fluoxetine as a potential adjuvant therapy for drug-resistant tumors. Iran. J. Pharm. Res. 2014, 13, 3–14. [Google Scholar]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Villasmil-Sánchez, S.; Rabasco, A.M.; González-Rodríguez, M.L. Thermal and 31P-NMR studies to elucidate sumatriptan succinate entrapment behavior in phosphatidylcholine/cholesterol liposomes. Comparative 31P-NMR analysis on negatively and positively-charged liposomes. Colloids Surf. B Biointerfaces 2013, 105, 14–23. [Google Scholar] [CrossRef]
- Briuglia, M.-L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Magarkar, A.; Dhawan, V.; Kallinteri, P.; Viitala, T.; Elmowafy, M.; Róg, T.; Bunker, A. Cholesterol level affects surface charge of lipid membranes in saline solution. Sci. Rep. 2014, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Pathak, S.K.; Mishra, R.; Kumar, S.; Prakash, G.; Parthasarthy, R. Effect of cholesterol concentration on size of liposome. J. Pharm. Biol. Sci. 2013, 1, 50–53. [Google Scholar] [CrossRef]
- Bober, P.; Alexovič, M.; Tomková, Z.; Kilík, R.; Sabo, J. RHOA and mDia1 promotes apoptosis of breast cancer cells via a high dose of doxorubicin treatment. Open Life Sci. 2019, 14, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Douedi, S.; Carson, M.P. Anthracycline medications (Doxorubicin). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551633/ (accessed on 25 April 2021).
- National Cancer Institute. Doxorubicin Hydrochloride. In NIH Turning Discovery into Health [Internet]; Department of Health and Human Services: Bethesda, MD, USA. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/doxorubicinhydrochloride (accessed on 4 December 2020).
- Cai, F.; Luis, M.A.F.; Lin, X.; Wang, M.; Cai, L.; Cen, C.; Biskup, E. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: Preventive strategies and treatment. Mol. Clin. Oncol. 2019, 11, 15–23. [Google Scholar] [CrossRef]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Schrand, A.M.; Miller, J.M.; Hutchison, J.; Schlager, J.J.; Hussain, S.M. Surface charge of gold nanoparticles mediates mechanism of toxicity. Nanoscale 2011, 3, 410–420. [Google Scholar] [CrossRef]
- Balazs, D.A.; Godbey, W. Liposomes for use in gene delivery. J. Drug Deliv. 2011, 2011, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Pharmacopoeial Convention. United States Pharmacopoeia (38). The National Formulary and Dispensing Information (33); USP 38–NF 33; United States Pharmacopeia Convention Inc.: Rockville, MD, USA, 2015. [Google Scholar]
- Olson, F.; Hunt, C.; Szoka, F.; Vail, W.; Papahadjopoulos, D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim. Biophys. Acta Biomembr. 1979, 557, 9–23. [Google Scholar] [CrossRef]
- Bolotin, E.M.; Cohen, R.; Bar, L.K.; Emanuel, N.; Ninio, S.; Barenholz, Y.; Lasic, D.D. Ammonium sulfate gradients for efficient and stable remote loading of amphipathic weak bases into liposomes and ligandoliposomes. J. Liposome Res. 1994, 4, 455–479. [Google Scholar] [CrossRef]
- Abraham, S.A.; Edwards, K.; Karlsson, G.; Hudon, N.; Mayer, L.D.; Bally, M.B. An evaluation of transmembrane ion gradient-mediated encapsulation of topotecan within liposomes. J. Control. Release 2004, 96, 449–461. [Google Scholar] [CrossRef]
- Fritze, A.; Hens, F.; Kimpfler, A.; Schubert, R.; Peschka-Süss, R. Remote loading of doxorubicin into liposomes driven by a transmembrane phosphate gradient. Biochim. Biophys. Acta (BBA) Biomembranes 2006, 1758, 1633–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 1–40. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto, F.; Rueda, M.; Naitlho, N.; Vázquez-González, M.; González-Rodríguez, M.L.; Rabasco, A.M. Electrochemical characterization of a mixed lipid monolayer supported on Au (111) electrodes with implications for doxorubicin delivery. J. Electroanal. Chem. 2018, 815, 246–254. [Google Scholar] [CrossRef]
- Burdach, K.; Tymecka, D.; Urban, A.; Lasek, R.; Bartosik, D.; Sek, S. Interactions of Linear Analogues of Battacin with Negatively Charged Lipid Membranes. Membranes 2021, 11, 192. [Google Scholar] [CrossRef]
- Miyoshi, T.; Kato, S. Detailed analysis of the surface area and elasticity in the saturated 1, 2-diacylphosphatidylcholine/cholesterol binary monolayer system. Langmuir 2015, 31, 9086–9096. [Google Scholar] [CrossRef] [PubMed]
- Cern, A.; Golbraikh, A.; Sedykh, A.; Tropsha, A.; Barenholz, Y.; Goldblum, A. Quantitative structure-property relationship modeling of remote liposome loading of drugs. J. Control. Release 2012, 160, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haran, G.; Cohen, R.; Bar, L.K.; Barenholz, Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim. Biophys. Acta (BBA) Biomembranes 1993, 1151, 201–215. [Google Scholar] [CrossRef]
- Li, X.; Cabral-Lilly, D.; Janoff, A.; Perkins, W. Complexation of internalized doxorubicin into fiber bundles affects its release rate from liposomes. J. Liposome Res. 2000, 10, 15–27. [Google Scholar] [CrossRef]
- Abraham, S.A.; Waterhouse, D.N.; Mayer, L.D.; Cullis, P.R.; Madden, T.D.; Bally, M.B. The liposomal formulation of doxorubicin. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 391, pp. 71–97. [Google Scholar]
- Coderch, L.; Fonollosa, J.; De Pera, M.; Estelrich, J.; De La Maza, A.; Parra, J.L. Influence of cholesterol on liposome fluidity by EPR: Relationship with percutaneous absorption. J. Control. Release 2000, 68, 85–95. [Google Scholar] [CrossRef]
- Farzaneh, H.; Nik, M.E.; Mashreghi, M.; Saberi, Z.; Jaafari, M.R.; Teymouri, M. A study on the role of cholesterol and phosphatidylcholine in various features of liposomal doxorubicin: From liposomal preparation to therapy. Int. J. Pharm. 2018, 551, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Mady, M.M.; Fathy, M.M.; Youssef, T.; Khalil, W.M. Biophysical characterization of gold nanoparticles-loaded liposomes. Phys. Med. 2012, 28, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Hindley, J.W.; Macdonald, T.J.; Barritt, J.D.; Ces, O.; Quirke, N. Size dependency of gold nanoparticles interacting with model membranes. Commun. Chem. 2020, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sułkowski, W.W.; Pentak, D.; Nowak, K.; Sułkowska, A. The influence of temperature, cholesterol content and pH on liposome stability. J. Mol. Struct. 2005, 744, 737–747. [Google Scholar] [CrossRef]
- Jiang, W.; Lionberger, R.; Yu, L.X. In vitro and in vivo characterizations of PEGylated liposomal doxorubicin. Bioanalysis 2011, 3, 333–344. [Google Scholar] [CrossRef]
- Al-Ahmady, Z.; Lozano, N.; Mei, K.-C.; Kostarelos, K. Engineering thermosensitive liposome-nanoparticle hybrids loaded with doxorubicin for heat-triggered drug release. Int. J. Pharm. 2016, 514, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.J.; Byeon, Y.; Jeon, H.N.; Cho, S.H.; Han, H.D.; Shin, B.C. Gold cluster-labeled thermosensitive liposmes enhance triggered drug release in the tumor microenvironment by a photothermal effect. J. Control. Release 2015, 216, 132–139. [Google Scholar] [CrossRef]
- Russell, L.M.; Hultz, M.; Searson, P.C. Leakage kinetics of the liposomal chemotherapeutic agent Doxil: The role of dissolution, protonation, and passive transport, and implications for mechanism of action. J. Control. Release 2018, 269, 171–176. [Google Scholar] [CrossRef]
- Haghiralsadat, F.; Amoabediny, G.; Helder, M.N.; Naderinezhad, S.; Sheikhha, M.H.; Forouzanfar, T.; Zandieh-Doulabi, B. A comprehensive mathematical model of drug release kinetics from nano-liposomes, derived from optimization studies of cationic PEGylated liposomal doxorubicin formulations for drug-gene delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 169–177. [Google Scholar] [CrossRef] [Green Version]



| Sample | Chol (3.35) | Chol (40) | AuNPs | D |
|---|---|---|---|---|
| L1 | ||||
| L2 | ||||
| AuNPs-L1 | ||||
| AuNPs-L2 | ||||
| L1-D | ||||
| L2-D | ||||
| AuNPs-L1-D | ||||
| AuNPs-L2-D |
| Sample | EE % | dH (nm) | PDI | ζ (mV) |
|---|---|---|---|---|
| L1 | – | 245 ± 2 | 0.27 ± 0.01 | 18.8 ± 0.5 |
| AuNPs-L1 | – | 320 ± 4 | 0.22 ± 0.02 | 15.7 ± 0.9 |
| L1-D | 84 ± 1 | 293 ± 4 | 0.24 ± 0.04 | 20.8 ± 0.7 |
| AuNPs-L1-D | 78 ± 2 | 347 ± 6 | 0.22 ± 0.03 | 1.6 ± 0.6 |
| L2 | – | 149 ± 2 | 0.16 ± 0.02 | 14.7 ± 0.9 |
| AuNPs-L2 | – | 293 ± 8 | 0.30 ± 0.02 | 7.4 ± 0.8 |
| L2-D | 94 ± 2 | 240 ± 4 | 0.25 ± 0.02 | 14 ± 1 |
| AuNPs-L2-D | 78 ± 3 | 304 ± 5 | 0.22 ± 0.03 | –1.9 ± 0.9 |
| Temperature (°C) | Kinetic Models | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Zero Order | Higuchi | Korsmeyer-Peppas | |||||
| kZ | R2 | kH | R2 | kP | n | R2 | ||
| 37 | D | 18.8 | 0.998 | 38.5 | 0.978 | 30.8 | 0.65 | 0.996 |
| L1-D | 3.7 | 0.823 | 15.1 | 0.910 | 20.4 | 0.40 | 0.922 | |
| AuNPs-L1-D | 2.2 | 0.787 | 8.7 | 0.863 | 23.1 | 0.25 | 0.960 | |
| L2-D | 16.5 | 0.979 | 39.1 | 0.994 | 23.6 | 0.76 | 0.983 | |
| AuNPs-L2-D | 6.9 | 0.930 | 22.3 | 0.991 | 19.4 | 0.55 | 0.998 | |
| 42 | D | 28.3 | 0.990 | 39.3 | 0.988 | 41.0 | 0.65 | 0.996 |
| L1-D | 4.4 | 0.881 | 17.4 | 0.944 | 25.4 | 0.39 | 0.956 | |
| AuNPs-L1-D | 5.0 | 0.894 | 19.8 | 0.954 | 28.4 | 0.39 | 0.963 | |
| L2-D | 13.2 | 0.976 | 33.2 | 0.996 | 23.1 | 0.68 | 0.992 | |
| AuNPs-L2-D | 20.6 | 0.971 | 43.1 | 0.994 | 31.0 | 0.69 | 0.993 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, M.C.; Naitlho, N.; Calderón-Montaño, J.M.; Drago, E.; Rueda, M.; Longhi, M.; Rabasco, A.M.; López-Lázaro, M.; Prieto-Dapena, F.; González-Rodríguez, M.L. Cholesterol Levels Affect the Performance of AuNPs-Decorated Thermo-Sensitive Liposomes as Nanocarriers for Controlled Doxorubicin Delivery. Pharmaceutics 2021, 13, 973. https://doi.org/10.3390/pharmaceutics13070973
García MC, Naitlho N, Calderón-Montaño JM, Drago E, Rueda M, Longhi M, Rabasco AM, López-Lázaro M, Prieto-Dapena F, González-Rodríguez ML. Cholesterol Levels Affect the Performance of AuNPs-Decorated Thermo-Sensitive Liposomes as Nanocarriers for Controlled Doxorubicin Delivery. Pharmaceutics. 2021; 13(7):973. https://doi.org/10.3390/pharmaceutics13070973
Chicago/Turabian StyleGarcía, Mónica C., Nabila Naitlho, José Manuel Calderón-Montaño, Estrella Drago, Manuela Rueda, Marcela Longhi, Antonio M. Rabasco, Miguel López-Lázaro, Francisco Prieto-Dapena, and María Luisa González-Rodríguez. 2021. "Cholesterol Levels Affect the Performance of AuNPs-Decorated Thermo-Sensitive Liposomes as Nanocarriers for Controlled Doxorubicin Delivery" Pharmaceutics 13, no. 7: 973. https://doi.org/10.3390/pharmaceutics13070973
APA StyleGarcía, M. C., Naitlho, N., Calderón-Montaño, J. M., Drago, E., Rueda, M., Longhi, M., Rabasco, A. M., López-Lázaro, M., Prieto-Dapena, F., & González-Rodríguez, M. L. (2021). Cholesterol Levels Affect the Performance of AuNPs-Decorated Thermo-Sensitive Liposomes as Nanocarriers for Controlled Doxorubicin Delivery. Pharmaceutics, 13(7), 973. https://doi.org/10.3390/pharmaceutics13070973