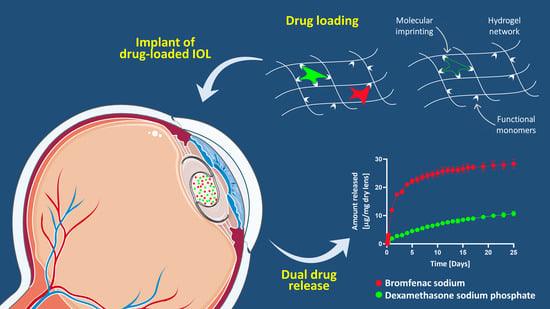
Drug-Loaded Hydrogels for Intraocular Lenses with Prophylactic Action against Pseudophakic Cystoid Macular Edema
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Molecular Interaction Analysis
2.3. Hydrogels Synthesis
2.4. Drug Loading and Release
2.5. Drug Quantification
2.6. Drug Amount Loaded
2.7. Physical and Mechanical Characterization
2.8. Prediction of In Vivo Efficacy
2.9. Cytotoxicity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Molecular Interaction Analysis
3.2. Physical and Mechanical Characterization of the Unloaded Hydrogels
3.3. Drug Release In Vitro
3.4. Drug Amount Loaded
3.5. Drug Permeability through the Cornea
3.6. Prediction of In Vivo Efficacy
3.7. Characterization of the Drug-Loaded Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCarty, C. Cataract in the 21st Century: Lessons from previous epidemiologic research. Clin. Exp. Optom. 2002, 85, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Asbell, P.; Dualan, I.; Mindel, J.; Brocks, D.; Ahmad, M.; Epstein, S. Age-related cataract. Lancet 2005, 365, 599–609. [Google Scholar] [CrossRef]
- González-Chomón, C.; Concheiro, A.; Alvarez-Lorenzo, C. Drug-Eluting Intraocular Lenses. Materials 2011, 4, 1927–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wielders, L.H.; Lambermont, V.A.; Schouten, J.S.; Biggelaar, F.J.V.D.; Worthy, G.; Simons, R.W.; Winkens, B.; Nuijts, R.M. Prevention of Cystoid Macular Edema After Cataract Surgery in Nondiabetic and Diabetic Patients: A Systematic Review and Meta-Analysis. Am. J. Ophthalmol. 2015, 160, 968–981.e33. [Google Scholar] [CrossRef]
- Allocco, A.; Ponce, J.; Quintana, N.; Magurno, M. Non steroidal anti-inflammatory drugs in the prevention of cystoid macular edema after uneventful cataract surgery. Clin. Ophthalmol. 2014, 8, 1209–1212. [Google Scholar] [CrossRef] [Green Version]
- McCafferty, S.; Harris, A.; Kew, C.; Kassm, T.; Lane, L.; Levine, J.; Raven, M. Pseudophakic cystoid macular edema prevention and risk factors; Prospective study with adjunctive once daily topical nepafenac 0.3% versus placebo. BMC Ophthalmol. 2017, 17, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Laursen, S.B.; Erichsen, J.H.; Holm, L.M.; Kessel, L. Prevention of macular edema in patients with diabetes after cataract surgery. J. Cataract. Refract. Surg. 2019, 45, 854–869. [Google Scholar] [CrossRef]
- Wilkins, C.S.; Sobol, E.K.; Ginsburg, R.N. Perioperative Management of Macular Edema in the Cataract Surgery Patient. Adv. Ophthalmol. Optom. 2019, 4, 193–210. [Google Scholar] [CrossRef]
- Kiernan, D.F.; Hariprasad, S.M. Controversies in the management of Irvine-Gass syndrome. Ophthalmic Surg. Lasers Imaging Retin. 2013, 44, 522–527. [Google Scholar] [CrossRef] [Green Version]
- Toffoletto, N.; Saramago, B.; Serro, A.P. Therapeutic Ophthalmic Lenses: A Review. Pharmaceutics 2020, 13, 36. [Google Scholar] [CrossRef]
- Grzybowski, A. The Role of Steroids and NSAIDs in Prevention and Treatment of Postsurgical Cystoid Macular Edema. Curr. Pharm. Des. 2019, 24, 4896–4902. [Google Scholar] [CrossRef]
- Filipe, H.P.; Bozukova, D.; Pimenta, A.; Vieira, A.P.; Oliveira, A.; Galante, R.; Topete, A.; Másson, M.; Alves, P.; Coimbra, P.; et al. Moxifloxacin-loaded acrylic intraocular lenses: In vitro and in vivo performance. J. Cataract. Refract. Surg. 2019, 45, 1808–1817. [Google Scholar] [CrossRef]
- Topete, A.; Serro, A.; Saramago, B. Dual drug delivery from intraocular lens material for prophylaxis of endophthalmitis in cataract surgery. Int. J. Pharm. 2019, 558, 43–52. [Google Scholar] [CrossRef]
- Pimenta, A.F.; Serro, A.P.; Colaço, R.; Chauhan, A. Optimization of intraocular lens hydrogels for dual drug release: Experimentation and modelling. Eur. J. Pharm. Biopharm. 2019, 141, 51–57. [Google Scholar] [CrossRef]
- Ongkasin, K.; Masmoudi, Y.; Tassaing, T.; Le-Bourdon, G.; Badens, E. Supercritical loading of gatifloxacin into hydrophobic foldable intraocular lenses–Process control and optimization by following in situ CO2 sorption and polymer swelling. Int. J. Pharm. 2020, 581, 119247. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Wang, K.; Wang, L.; Yang, X.; Zhu, S. Cyclodextrin-containing hydrogels as an intraocular lens for sustained drug release. PLoS ONE 2017, 12, e0189778. [Google Scholar] [CrossRef] [Green Version]
- Bouledjouidja, A.; Masmoudi, Y.; Li, Y.; He, W.; Badens, E. Supercritical impregnation and optical characterization of loaded foldable intraocular lenses using supercritical fluids. J. Cataract. Refract. Surg. 2017, 43, 1343–1349. [Google Scholar] [CrossRef]
- Eperon, S.; Rodriguez-Aller, M.; Balaskas, K.; Gurny, R.; Guex-Crosier, Y. A new drug delivery system inhibits uveitis in an animal model after cataract surgery. Int. J. Pharm. 2013, 443, 254–261. [Google Scholar] [CrossRef]
- Kassumeh, S.; Kueres, A.; Hillenmayer, A.; von Studnitz, A.; Elhardt, C.; Ohlmann, A.; Priglinger, S.G.; Wertheimer, C.M. Development of a drug-eluting intraocular lens to deliver epidermal growth factor receptor inhibitor gefitinib for posterior capsule opacification prophylaxis. Eur. J. Ophthalmol. 2021, 31, 436–444. [Google Scholar] [CrossRef]
- Wertheimer, C.; Kassumeh, S.; Piravej, N.P.; Nilmayer, O.; Braun, C.; Priglinger, C.; Luft, N.; Wolf, A.; Mayer, W.J.; Eibl-Lindner, K.H. The Intraocular Lens as a Drug Delivery Device: In Vitro Screening of Pharmacologic Substances for the Prophylaxis of Posterior Capsule Opacification. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6408. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Lin, Q.; Li, J.; Han, Y.; Chang, P.; Lu, F.; Zhao, Y.-E. ROCK inhibitor modified intraocular lens as an approach for inhibiting the proliferation and migration of lens epithelial cells and posterior capsule opacification. Biomater. Sci. 2019, 7, 4208–4217. [Google Scholar] [CrossRef]
- Ongkasin, K.; Masmoudi, Y.; Wertheimer, C.M.; Hillenmayer, A.; Eibl-Lindner, K.H.; Badens, E. Supercritical fluid technology for the development of innovative ophthalmic medical devices: Drug loaded intraocular lenses to mitigate posterior capsule opacification. Eur. J. Pharm. Biopharm. 2020, 149, 248–256. [Google Scholar] [CrossRef]
- Han, Y.; Tang, J.; Xia, J.; Wang, R.; Qin, C.; Liu, S.; Zhao, X.; Chen, H.; Lin, Q. Anti-Adhesive and Antiproliferative Synergistic Surface Modification of Intraocular Lens for Reduced Posterior Capsular Opacification. Int. J. Nanomed. 2019, 14, 9047–9061. [Google Scholar] [CrossRef] [Green Version]
- Campa, C.; Salsini, G.; Perri, P. Comparison of the Efficacy of Dexamethasone, Nepafenac, and Bromfenac for Preventing Pseudophakic Cystoid Macular Edema: An Open-label, Prospective, Randomized Controlled Trial. Curr. Eye Res. 2017, 43, 362–367. [Google Scholar] [CrossRef]
- White, C.J.; McBride, M.K.; Pate, K.M.; Tieppo, A.; Byrne, M.E. Extended release of high molecular weight hydroxypropyl methylcellulose from molecularly imprinted, extended wear silicone hydrogel contact lenses. Biomaterials 2011, 32, 5698–5705. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Anguiano-Igea, S.; Varela-García, A.; Vivero-Lopez, M.; Concheiro, A. Bioinspired hydrogels for drug-eluting contact lenses. Acta Biomater. 2019, 84, 49–62. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Yañez, F.; Concheiro, A. Ocular drug delivery from molecularly-imprinted contact lenses. J. Drug Deliv. Sci. Technol. 2010, 20, 237–248. [Google Scholar] [CrossRef]
- Topete, A.; Barahona, I.; Santos, L.F.; Pinto, C.A.; Saraiva, J.A.; Serro, A.P.; Saramago, B. The effects of addition of functional monomers and molecular imprinting on dual drug release from intraocular lens material. Int. J. Pharm. 2021, 600, 120513. [Google Scholar] [CrossRef]
- Zaidi, S.A. Latest trends in molecular imprinted polymer based drug delivery systems. RSC Adv. 2016, 6, 88807–88819. [Google Scholar] [CrossRef]
- Hiratani, H.; Mizutani, Y.; Alvarez-Lorenzo, C. Controlling Drug Release from Imprinted Hydrogels by Modifying the Characteristics of the Imprinted Cavities. Macromol. Biosci. 2005, 5, 728–733. [Google Scholar] [CrossRef]
- Sheppard, J.D. Topical bromfenac for prevention and treatment of cystoid macular edema following cataract surgery: A review. Clin. Ophthalmol. 2016, 10, 2099–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, D.T. Intracameral dexamethasone reduces inflammation on the first postoperative day after cataract surgery in eyes with and without glaucoma. Clin. Ophthalmol. 2009, 3, 345–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Chomón, C.; Braga, M.E.; de Sousa, H.C.; Concheiro, A.; Alvarez-Lorenzo, C. Antifouling foldable acrylic IOLs loaded with norfloxacin by aqueous soaking and by supercritical carbon dioxide technology. Eur. J. Pharm. Biopharm. 2012, 82, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Varela-Garcia, A.; Gomez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C. Imprinted Contact Lenses for Ocular Administration of Antiviral Drugs. Polymers 2020, 12, 2026. [Google Scholar] [CrossRef]
- Kim, J.; Chauhan, A. Dexamethasone transport and ocular delivery from poly(hydroxyethyl methacrylate) gels. Int. J. Pharm. 2008, 353, 205–222. [Google Scholar] [CrossRef]
- Kotsmar, C.; Sells, T.; Taylor, N.; Liu, D.E.; Prausnitz, J.M.; Radke, C.J. Aqueous Solute Partitioning and Mesh Size in HEMA/MAA Hydrogels. Macromolecules 2012, 45, 9177–9187. [Google Scholar] [CrossRef]
- Pimenta, A.; Serro, A.P.; Colaco, R.; Chauhan, A. Drug delivery to the eye anterior chamber by intraocular lenses: An in vivo concentration estimation model. Eur. J. Pharm. Biopharm. 2018, 133, 63–69. [Google Scholar] [CrossRef]
- Dixon, P.; Chauhan, A. Effect of the surface layer on drug release from delefilcon-A (Dailies Total1®) contact lenses. Int. J. Pharm. 2017, 529, 89–101. [Google Scholar] [CrossRef]
- Toffoletto, N.; Chauhan, A.; Alvarez-Lorenzo, C.; Saramago, B.; Serro, A. Asymmetry in Drug Permeability through the Cornea. Pharmaceutics 2021, 13, 694. [Google Scholar] [CrossRef]
- OECD2020: Guidelines for the Testing of Chemicals, Section 4, Test N.437. n.d. Available online: https://www.oecd-ilibrary.org/environment/test-no-437-bovine-corneal-opacity-and-permeability-test-method-for-identifying-i-chemicals-inducing-serious-eye-damage-and-ii-chemicals-not-requiring-classification-for-eye-irritation-or-serious-eye-damage_978926 (accessed on 28 October 2020).
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Soluplus micelles for acyclovir ocular delivery: Formulation and cornea and sclera permeability. Int. J. Pharm. 2018, 552, 39–47. [Google Scholar] [CrossRef]
- Dudley, R.; McDowell, B.; Mahl, C.; Sarkar, A.B. Influence of temperature on the thirty-day chemical stability of extemporaneously prepared dexa-methasone paste. Int. J. Pharm. Compd. 2012, 16, 258–261. [Google Scholar]
- Zhang, Z.; Huang, W.; Lei, M.; He, Y.; Yan, M.; Zhang, X.; Zhao, C. Laser-triggered intraocular implant to induce photodynamic therapy for posterior capsule opacification prevention. Int. J. Pharm. 2016, 498, 1–11. [Google Scholar] [CrossRef]
- Song, W.; Quan, P.; Li, S.; Liu, C.; Lv, S.; Zhao, Y.; Fang, L. Probing the role of chemical enhancers in facilitating drug release from patches: Mechanistic insights based on FT-IR spectroscopy, molecular modeling and thermal analysis. J. Control. Release 2016, 227, 13–22. [Google Scholar] [CrossRef]
- Palazi, E.; Karavas, E.; Barmpalexis, P.; Kostoglou, M.; Nanaki, S.; Christodoulou, E.; Bikiaris, D.N. Melt extrusion process for adjusting drug release of poorly water soluble drug felodipine using different polymer matrices. Eur. J. Pharm. Sci. 2018, 114, 332–345. [Google Scholar] [CrossRef]
- Lu, J.; Dong, Y.; Ng, E.C.; Siehl, D.L. Novel form of the Michaelis–Menten equation that enables accurate estimation of (kcat/KM)*KI with just two rate measurements; utility in directed evolution. Protein Eng. Des. Sel. 2017, 30, 395–399. [Google Scholar] [CrossRef]
- Tanito, M.; Okuno, T.; Ishiba, Y.; Ohira, A. Measurements of transmission spectrums and estimation of retinal blue-light irradiance values of currently available clear and yellow-tinted intraocular lenses. Jpn. J. Ophthalmol. 2011, 56, 82–90. [Google Scholar] [CrossRef]
- Topete, A.; Oliveira, A.; Fernandes, A.; Nunes, T.G.; Serro, A.; Saramago, B. Improving sustained drug delivery from ophthalmic lens materials through the control of temperature and time of loading. Eur. J. Pharm. Sci. 2018, 117, 107–117. [Google Scholar] [CrossRef]
- Trusted Quality IOL Materials—Website Brochure by Contamac Ltd. Available online: https://www.contamac.com/sites/default/files/documents/documents/IOL%20Brochure_Website_2019.pdf (accessed on 1 March 2021).
- Canal, T.; Peppas, N.A. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J. Biomed. Mater. Res. 1989, 23, 1183–1193. [Google Scholar] [CrossRef]
- Pimenta, A.; Ascenso, J.; Fernandes, J.C.S.; Colaco, R.; Serro, A.; Saramago, B. Controlled drug release from hydrogels for contact lenses: Drug partitioning and diffusion. Int. J. Pharm. 2016, 515, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Buga, I.; Uzoma, J.I.; Reindel, K.; Rashid, K.; Diep, T.; McCartan, P.; Zhao, F. Physical and Chemical Stability of Dexamethasone Sodium Phosphate in Intravenous Admixtures Used to Prevent Chemotherapy-Induced Nausea and Vomiting. Hosp. Pharm. 2019. [Google Scholar] [CrossRef] [Green Version]
- Milne, G.W.A. Drugs: Synonyms and Properties, 2nd ed.; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Vulic, K.; Shoichet, M.S. Affinity-Based Drug Delivery Systems for Tissue Repair and Regeneration. Biomacromolecules 2014, 15, 3867–3880. [Google Scholar] [CrossRef]
- Xu, X.; Yu, N.; Bai, Z.; Xun, Y.; Jin, D.; Li, Z.; Cui, H. Effect of menthol on ocular drug delivery. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1503–1510. [Google Scholar] [CrossRef]
- Kida, T.; Kozai, S.; Takahashi, H.; Isaka, M.; Tokushige, H.; Sakamoto, T. Pharmacokinetics and Efficacy of Topically Applied Nonsteroidal Anti-Inflammatory Drugs in Retinochoroidal Tissues in Rabbits. PLoS ONE 2014, 9, e96481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gökçe, B.; Gençer, N.; Arslan, O.; Turkoğlu, S.A.; Alper, M.; Köçkar, F. Evaluation of in vitro effects of some analgesic drugs on erythrocyte and recombinant carbonic anhydrase I and II. J. Enzym. Inhib. Med. Chem. 2011, 27, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinna, A.; Blasetti, F.; Ricci, G.D.; Boscia, F. Bromfenac Eyedrops in the Treatment of Diabetic Macular Edema: A Pilot Study. Eur. J. Ophthalmol. 2017, 27, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Bandello, F.; Coassin, M.; Di Zazzo, A.; Rizzo, S.; Biagini, I.; Pozdeyeva, N.; Sinitsyn, M.; Verzin, A.; De Rosa, P.; Calabrò, F.; et al. One week of levofloxacin plus dexamethasone eye drops for cataract surgery: An innovative and rational therapeutic strategy. Eye 2020, 34, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.J.; Conway, M.D.; Peyman, G.A. Intracameral dexamethasone injection in the treatment of cataract surgery induced inflammation: Design, development, and place in therapy. Clin. Ophthalmol. 2018, 12, 2223–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, R.C.; Yang, H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release 2019, 306, 29–39. [Google Scholar] [CrossRef]
- Jünemann, A.G.; Chorągiewicz, T.; Ozimek, M.; Grieb, P.; Rejdak, R. Drug bioavailability from topically applied ocular drops. Does drop size matter? Ophthalmol. J. 2016, 1, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, E.; del Amo, E.M.; Toropainen, E.; Tengvall-Unadike, U.; Ranta, V.-P.; Urtti, A.; Ruponen, M. Corneal and conjunctival drug permeability: Systematic comparison and pharmacokinetic impact in the eye. Eur. J. Pharm. Sci. 2018, 119, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Topete, A.; Pinto, C.A.; Barroso, H.; Saraiva, J.A.; Barahona, I.; Saramago, B.; Serro, A.P. High Hydrostatic Pressure as Sterilization Method for Drug-Loaded Intraocular Lenses. ACS Biomater. Sci. Eng. 2020, 6, 4051–4061. [Google Scholar] [CrossRef]
- Mylona, I.; Tsinopoulos, I. A Critical Appraisal of New Developments in Intraocular Lens Modifications and Drug Delivery Systems for the Prevention of Cataract Surgery Complications. Pharmaceutics 2020, 13, 448. [Google Scholar] [CrossRef]





| Code | HEMA | BEM | APMA | AAm | Bromfenac Sodium | Dexamethasone Sodium | AIBN | EGDMA |
|---|---|---|---|---|---|---|---|---|
| A1 | 2.4 mL | 600 µL | 10 mM | 80 mM | ||||
| A2 | 2.4 mL | 600 µL | 100 mM | 10 mM | 80 mM | |||
| A3 | 2.4 mL | 600 µL | 100 mM | 10 mM | 80 mM | |||
| B1 | 2.4 mL | 600 µL | 25 mM | 10 mM | 80 mM | |||
| B2 | 2.4 mL | 600 µL | 100 mM | 25 mM | 10 mM | 80 mM | ||
| B3 | 2.4 mL | 600 µL | 100 mM | 25mM | 10 mM | 80 mM | ||
| C1 | 2.4 mL | 600 µL | 12.5 mM | 10 mM | 80 mM | |||
| C2 | 2.4 mL | 600 µL | 100 mM | 12.5 mM | 10 mM | 80 mM | ||
| C3 | 2.4 mL | 600 µL | 100 mM | 12.5 MM | 10 mM | 80 mM |
| DRUG | HEMA | BEM | APMA | AAm | ||||
|---|---|---|---|---|---|---|---|---|
| E [Kcal/mol] | Ki [mM] | E [Kcal/mol] | Ki [mM] | E [Kcal/mol] | Ki [mM] | E [Kcal/mol] | Ki [mM] | |
| Dexamethasone sodium | −1.47 | 83.32 | −1.10 | 156.55 | −2.77 | 9.30 | −2.06 | 30.74 |
| Bromfenac sodium | −1.54 | 74.48 | −1.27 | 117.06 | −3.45 | 2.95 | −2.20 | 24.43 |
| Code | Bromfenac Sodium | Dexamethasone Sodium | ||||
|---|---|---|---|---|---|---|
| Drug Amount Loaded (µg/mg) | Drug Released after 25 Days (µg/mg) | Unreleased Drug (%) | Drug Amount Loaded (µg/mg) | Drug Released after 25 Days (µg/mg) | Unreleased Drug (%) | |
| A1 | 23 ± 1 | 20.0 ± 0.6 | 13 | 3.4 ± 0.1 | 3.7 ± 0.5 | 0 |
| A2 | 37 ± 1 | 32.9 ± 0.6 | 11 | 11.2 ± 0.8 | 8 ± 1 | 28 |
| B2 | 31 ± 2 | 26.1 ± 0.9 | 16 | 8.8 ± 0.7 | 6.2 ± 0.4 | 30 |
| C2 | 32 ± 1 | 28 ± 2 | 11 | 13.0 ± 0.8 | 10.7 ± 0.8 | 17 |
| Code | Cumulative Mass Permeated (µg/cm2) | J (µg/cm2/h) | Pcornea × 107 (cm/s) | R2 |
|---|---|---|---|---|
| A2 | 2 ± 1 | 0.30 ± 0.04 | 18 ± 2 | 0.96 ± 0.03 |
| C2 | 0.9 ± 0.4 | 0.27 ± 0.08 | 17 ± 5 | 0.93 ± 0.08 |
| Bromfenac Sodium | Dexamethasone Sodium | |||||||
|---|---|---|---|---|---|---|---|---|
| Code | K | D × 1014 [m2/s] | Peak Concentration [µg/mL] | Time with [Drug] > IC50 | K | D × 1014 [m2/s] | Peak Concentration [µg/mL] | Time with [Drug] > IC50 |
| A1 | 13.0 ± 0.8 | 26 ± 2 | 24.8 | 8 days | 1.2 ± 0.2 | 5.7 ± 0.1 | 5.1 | 4 weeks |
| A2 | 51 ± 4 | 17.8 ± 0.6 | 71.0 | 14 days | 2.6 ± 0.8 | 2.6 ± 0.6 | 7.2 | 7 weeks |
| B2 | 33.5 ± 0.6 | 21.6 ± 1.3 | 53.8 | 11 days | 2 ± 1 | 3.2 ± 0.4 | 7.1 | 6 weeks |
| C2 | 37 ± 3 | 21.6 ± 1.3 | 59.0 | 11 days | 5 ± 2 | 2.5 ± 0.6 | 12.6 | 8 weeks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toffoletto, N.; Salema-Oom, M.; Anguiano Igea, S.; Alvarez-Lorenzo, C.; Saramago, B.; Serro, A.P. Drug-Loaded Hydrogels for Intraocular Lenses with Prophylactic Action against Pseudophakic Cystoid Macular Edema. Pharmaceutics 2021, 13, 976. https://doi.org/10.3390/pharmaceutics13070976
Toffoletto N, Salema-Oom M, Anguiano Igea S, Alvarez-Lorenzo C, Saramago B, Serro AP. Drug-Loaded Hydrogels for Intraocular Lenses with Prophylactic Action against Pseudophakic Cystoid Macular Edema. Pharmaceutics. 2021; 13(7):976. https://doi.org/10.3390/pharmaceutics13070976
Chicago/Turabian StyleToffoletto, Nadia, Madalena Salema-Oom, Soledad Anguiano Igea, Carmen Alvarez-Lorenzo, Benilde Saramago, and Ana Paula Serro. 2021. "Drug-Loaded Hydrogels for Intraocular Lenses with Prophylactic Action against Pseudophakic Cystoid Macular Edema" Pharmaceutics 13, no. 7: 976. https://doi.org/10.3390/pharmaceutics13070976
APA StyleToffoletto, N., Salema-Oom, M., Anguiano Igea, S., Alvarez-Lorenzo, C., Saramago, B., & Serro, A. P. (2021). Drug-Loaded Hydrogels for Intraocular Lenses with Prophylactic Action against Pseudophakic Cystoid Macular Edema. Pharmaceutics, 13(7), 976. https://doi.org/10.3390/pharmaceutics13070976