Tailored Hydrogels as Delivery Platforms for Conditioned Medium from Mesenchymal Stem Cells in a Model of Acute Colitis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogel Development and Preparation
2.3. Hydrogel Characterization
2.3.1. Texturometric Analysis
2.3.2. Rheological Analysis
2.3.3. Stability Assessments
2.4. Cell Cultures and hUCESC-CM Preparation
2.5. Hydrogel Loading with hUCESC-CM
2.6. In Vivo Assay
2.6.1. Ethics Statement
2.6.2. Mice
2.6.3. Colitis Induction and Experimental Groups
2.6.4. Clinical Symptoms Evaluation and Colon Macroscopic Examination
2.6.5. Histological Evaluation
2.6.6. RNA Extraction and Quantitative Real Time PCR
2.7. Statistical Analysis
3. Results
3.1. Hydrogel Preparation and Characterization
3.2. Inducement of DSS-Associated Colitis in C57BL/6 Mice
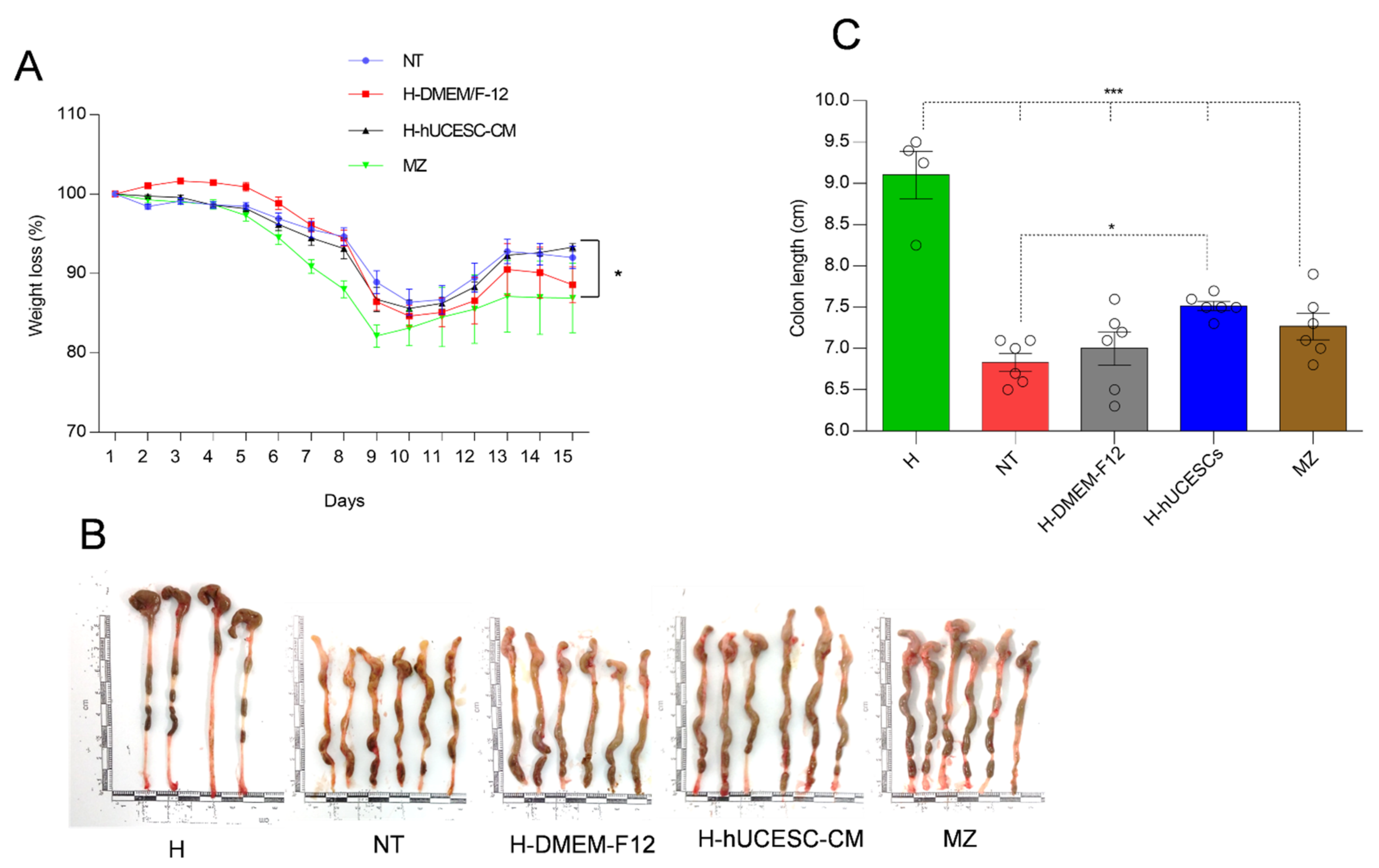
3.3. Effect of H-hUCESC-CM on Body Weight and Colon Length
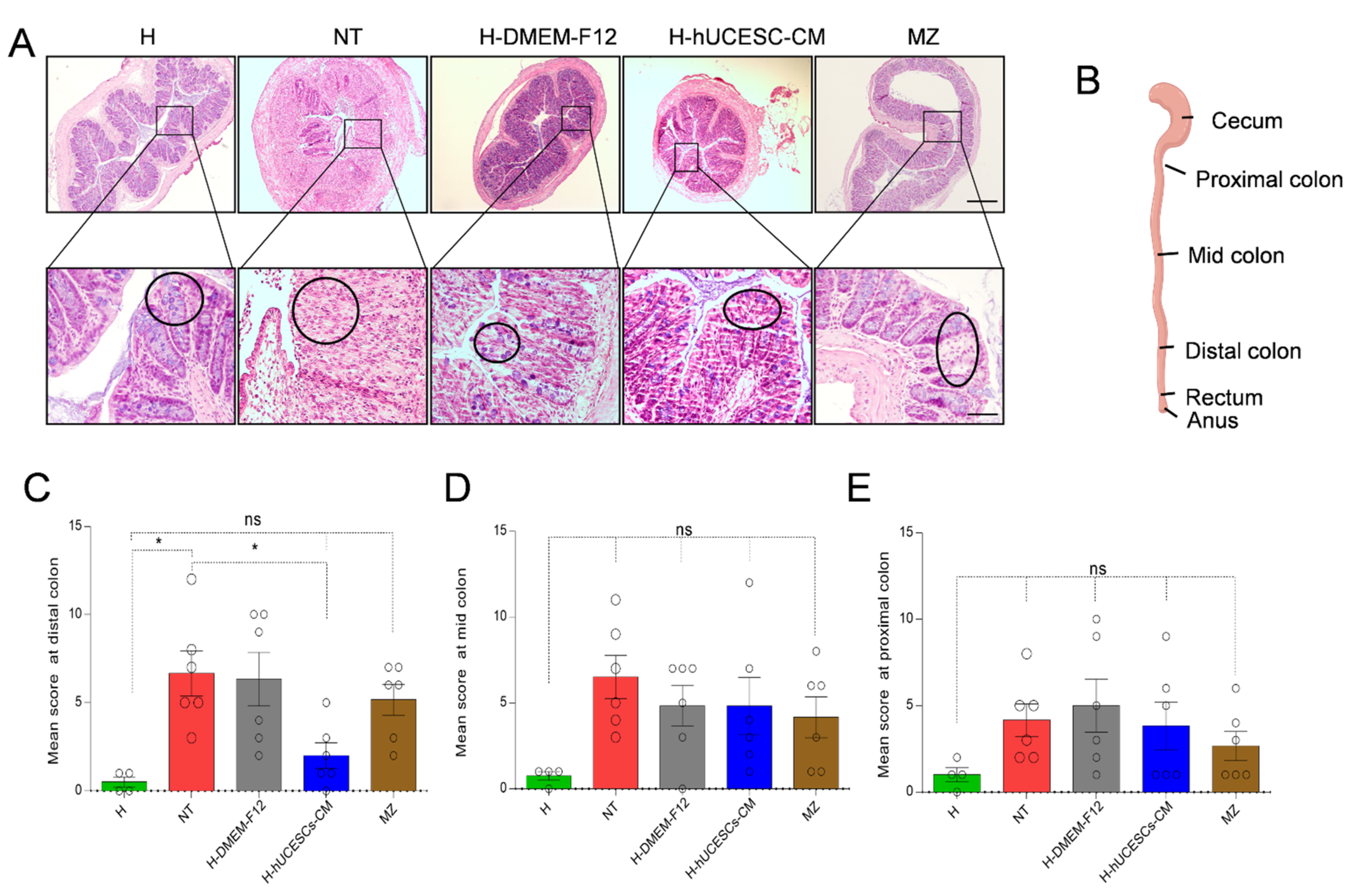
3.4. H-hUCESC-CM Hydrogel Reduced the Extension and Severity of Intestinal Lesions
3.5. H-hUCESC-CM Proinflammatory Cytokines in Colon
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef]
- Burisch, J.; Pedersen, N.; Cukovic-Cavka, S.; Brinar, M.; Kaimakliotis, I.; Duricova, D.; Shonova, O.; Vind, I.; Avnstrom, S.; Thorsgaard, N.; et al. East-West gradient in the incidence of inflammatory bowel disease in Europe: The ECCO-EpiCom inception cohort. Gut 2014, 63, 588–597. [Google Scholar] [CrossRef]
- Gomez-Gomez, G.J.; Masedo, A.; Yela, C.; Martinez-Montiel, M.P.; Casis, B. Current stage in inflammatory bowel disease: What is next? World J. Gastroenterol. 2015, 21, 11282–11303. [Google Scholar] [CrossRef]
- Chassaing, B.; Darfeuille-Michaud, A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, T.T.; Monteleone, I.; Fantini, M.C.; Monteleone, G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology 2011, 140, 1768–1775. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef] [Green Version]
- Sehgal, P.; Colombel, J.F.; Aboubakr, A.; Narula, N. Systematic review: Safety of mesalazine in ulcerative colitis. Aliment. Pharmacol. Ther. 2018, 47, 1597–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Thomas, A.; Lodhia, N. Advanced therapy for inflammatory bowel disease: A guide for the primary care physician. J. Am. Board Fam. Med. 2014, 27, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffari, S.; Nikfar, S.; Abdolghaffari, A.H.; Abdollahi, M. New biologic therapeutics for ulcerative colitis and Crohn’s disease. Expert Opin. Biol. Ther. 2014, 14, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Young, H.E.; Mancini, M.L.; Wright, R.P.; Smith, J.C.; Black, A.C.; Reagan, C.R.; Lucas, P.A. Mesenchymal stem cells reside within the connective tissues of many organs. Dev. Dyn. 1995, 202, 137–144. [Google Scholar] [CrossRef]
- Meirelles, S.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef]
- Hosseini-Asl, S.-K.; Mehrabani, D.; Karimi-Busheri, F.K. Therapeutic effect of mesenchymal stem cells in ulcerative colitis: A review on achievements and challenges. J. Clin. Med. 2020, 9, 3922. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Q.; Wang, F. Mesenchymal stem cells for the treatment of ulcerative colitis: A systematic review and meta-analysis of experimental and clinical studies. Stem Cell Res. Ther. 2019, 10, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legaki, E.; Roubelakis, M.G.; Theodoropoulos, G.E.; Lazaris, A.; Kollia, A.; Karamanolis, G.; Marinos, E.; Gazouli, M. Therapeutic potential of secreted molecules derived from human amniotic fluid mesenchymal stem/stroma cells in a mice model of colitis. Stem Cell Rev. Rep. 2016, 12, 604–612. [Google Scholar] [CrossRef]
- Song, J.Y.; Kang, H.J.; Hong, J.S.; Kim, C.J.; Shim, J.Y.; Lee, C.W.; Choi, J. Umbilical cord-derived mesenchymal stem cell extracts reduce colitis in mice by re-polarizing intestinal macrophages. Sci. Rep. 2017, 7, 9412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Qiu, W.; Xu, X.; Kang, J.; Wang, J.; Wen, Y.; Tang, X.; Yan, Y.; Qian, H.; Zhang, X.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease in mice through ubiquitination. Am. J. Transl. Res. 2018, 10, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Panes, J.; Garcia-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Panes, J.; Garcia-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn’s disease. Gastroenterology 2018, 154, 1334–1342. [Google Scholar] [CrossRef] [Green Version]
- Eiro, N.; Sendon-Lago, J.; Seoane, S.; Bermudez, M.A.; Lamelas, M.L.; Garcia-Caballero, T.; Schneider, J.; Perez-Fernandez, R.; Vizoso, F.J. Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget 2014, 5, 10692–10708. [Google Scholar] [CrossRef] [Green Version]
- Bermudez, M.A.; Sendon-Lago, J.; Eiro, N.; Trevino, M.; Gonzalez, F.; Yebra-Pimentel, E.; Giraldez, M.J.; Macia, M.; Lamelas, M.L.; Saa, J.; et al. Corneal epithelial wound healing and bactericidal effect of conditioned medium from human uterine cervical stem cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 983–992. [Google Scholar] [CrossRef]
- Bermudez, M.A.; Sendon-Lago, J.; Seoane, S.; Eiro, N.; Gonzalez, F.; Saa, J.; Vizoso, F.J.; Perez-Fernandez, R. Anti-inflammatory effect of conditioned medium from human uterine cervical stem cells in uveitis. Exp. Eye Res. 2016, 149, 84–92. [Google Scholar] [CrossRef]
- Sendon-Lago, J.; Seoane, S.; Martinez-Ordonez, A.; Eiro, N.; Saa, J.; Vizoso, F.; Gonzalez, F.; Perez-Fernandez, R.; Bermudez, M.A. Corneal regeneration by conditioned medium of human uterine cervical stem cells is mediated by TIMP-1 and TIMP-2. Exp. Eye Res. 2019, 180, 110–121. [Google Scholar] [CrossRef]
- Schneider, J.; Mateo, E.; Marcos-Arias, C.; Eiro, N.; Vizoso, F.; Perez-Fernandez, R.; Eraso, E.; Quindos, G. Antifungal activity of the human uterine cervical stem cells conditioned medium (hUCESC-CM) against candida albicans and other medically relevant species of candida. Front. Microbiol. 2018, 9, 2818. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Seremeta, K.P. Polymeric Hydrogels as Technology Platform for Drug Delivery Applications. Gels 2017, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Hui, P.C.; Kan, C.W. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-del Rio, L.; Diaz-Rodriguez, P.; Landin, M. New tools to design smart thermosensitive hydrogels for protein rectal delivery in IBD. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110252. [Google Scholar] [CrossRef]
- Yuan, Y.; Cui, Y.; Zhang, L.; Zhu, H.P.; Guo, Y.S.; Zhong, B.; Hu, X.; Zhang, L.; Wang, X.H.; Chen, L. Thermosensitive and mucoadhesive in situ gel based on poloxamer as new carrier for rectal administration of nimesulide. Int. J. Pharm. 2012, 430, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Santos Akkari, A.C.; Ramos Campos, E.V.; Keppler, A.F.; Fraceto, L.F.; de Paula, E.; Tofoli, G.R.; de Araujo, D.R. Budesonide-hydroxypropyl-beta-cyclodextrin inclusion complex in binary poloxamer 407/403 system for ulcerative colitis treatment: A physico-chemical study from micelles to hydrogels. Colloids Surf. B Biointerfaces 2016, 138, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Zhang, M.; Djabourov, M.; Bourgaux, C.; Bouchemal, K. Nanostructured fluids from pluronic(R) mixtures. Int. J. Pharm. 2013, 454, 599–610. [Google Scholar] [CrossRef]
- Bouchemal, K.; Aka-Any-Grah, A.; Dereuddre-Bosquet, N.; Martin, L.; Lievin-Le-Moal, V.; Le Grand, R.; Nicolas, V.; Gibellini, D.; Lembo, D.; Pous, C.; et al. Thermosensitive and mucoadhesive pluronic-hydroxypropylmethylcellulose hydrogel containing the mini-CD4 M48U1 is a promising efficient barrier against HIV diffusion through macaque cervicovaginal mucus. Antimicrob. Agents Chemother. 2015, 59, 2215–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Laroui, H.; Ingersoll, S.A.; Liu, H.C.; Baker, M.T.; Ayyadurai, S.; Charania, M.A.; Laroui, F.; Yan, Y.; Sitaraman, S.V.; Merlin, D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS ONE 2012, 7, e32084. [Google Scholar] [CrossRef] [PubMed]
- Koffi, A.A.; Agnely, F.; Ponchel, G.; Grossiord, J.L. Modulation of the rheological and mucoadhesive properties of thermosensitive poloxamer-based hydrogels intended for the rectal administration of quinine. Eur. J. Pharm. Sci. 2006, 27, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Mustapha, O.; Kim, D.W.; Rashid, R.; Park, J.H.; Choi, J.Y.; Ku, S.K.; Yong, C.S.; Kim, J.O.; Choi, H.G. Novel dual-reverse thermosensitive solid lipid nanoparticle-loaded hydrogel for rectal administration of flurbiprofen with improved bioavailability and reduced initial burst effect. Eur. J. Pharm. Biopharm. 2015, 94, 64–72. [Google Scholar] [CrossRef]
- Salamat-Miller, N.; Johnston, T.P. Current strategies used to enhance the paracellular transport of therapeutic polypeptides across the intestinal epithelium. Int. J. Pharm. 2005, 294, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int. J. Nanomed. 2020, 15, 5911–5926. [Google Scholar] [CrossRef]
- Ahangar, P.; Mills, S.J.; Cowin, A.J. Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. Int. J. Mol. Sci. 2020, 21, 7038. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Yan, L.; Wang, C.Z.; Wang, W.H.; Shi, H.; Su, B.B.; Zeng, Q.H.; Du, H.T.; Wan, J. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J. Gastroenterol. 2013, 19, 4702–4717. [Google Scholar] [CrossRef]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Peña, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef]
- Danese, S.; Fiocchi, C. Ulcerative colitis. N. Engl. J. Med. 2011, 365, 1713–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, G.M.; Fetih, G.; Abbas, A.M. Thermosensitive bioadhesive gels for the vaginal delivery of sildenafil citrate: In vitro characterization and clinical evaluation in women using clomiphene citrate for induction of ovulation. Drug Dev. Ind. Pharm. 2017, 43, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, W.-L.; Wang, J.-C.; Zhang, X.; Zhang, H.; Wang, X.-Q.; Zhou, T.-Y.; Zhang, Q. Controlled delivery of recombinant hirudin based on thermo-sensitive Pluronic F127 hydrogel for subcutaneous administration: In vitro and in vivo characterization. J. Control. Release 2007, 117, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Maguire, G. Stem cell therapy without the cells. Commun. Integr. Biol. 2013, 6, e26631. [Google Scholar] [CrossRef]
- De Aguiar, C.F.; Castoldi, A.; Andrade-Oliveira, V.; Ignacio, A.; da Cunha, F.F.; Felizardo, R.J.F.; Bassi, E.J.; Camara, N.O.S.; de Almeida, D.C. Mesenchymal stromal cells modulate gut inflammation in experimental colitis. Inflammopharmacology 2018, 26, 251–260. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Costa, L.; Esparza, P.; Landin, M.; Diaz-Rodriguez, P.; Schneider, J.; Perez-Fernandez, R. Mesenchymal stem cells in homeostasis and systemic diseases: Hypothesis, evidence, and therapeutic opportunities. Int. J. Mol. Sci. 2019, 20, 3738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [Green Version]





| Gene | Forward | Reverse |
|---|---|---|
| TNF-α | aggctgccccgactacgt | gactttctcctggtatgagatagcaaa |
| IFN-γ | cagcaacagcaaggcgaaa | ctggacctgtgggttgttgac |
| IL-6 | acaagtcggaggcttaattacacat | ttgccattgcacaactctttt |
| 18S | cccctcgatgactttagctgagtgt | cgccggtccaagaatttcacctct |
| Parameter | Predicted by ANN | H-PBS |
|---|---|---|
| Syringeability work (mJ) | 141.92 | 170 ± 0.95 |
| Bioadhesion work (mJ) | 0.33 | 0.31 ± 0.02 |
| Tgel (°C) | 27.2 | 26.9 ± 0.20 |
| Parameter | Day 0 | 2 Weeks | 4 Weeks |
|---|---|---|---|
| Syringeability work (mJ) | 176.17 ± 1.30 | 182.15 ± 1.64 | 175.79 ± 1.19 |
| Bioadhesion work (mJ) | 0.270 ± 0.01 | 0.327 ± 0.03 | 0.520 ± 0.03 |
| Tgel (°C) | 26.89 ± 0.07 | 26.77 ± 0.18 | 26.17 ± 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sendon-Lago, J.; Rio, L.G.-d.; Eiro, N.; Diaz-Rodriguez, P.; Avila, L.; Gonzalez, L.O.; Vizoso, F.J.; Perez-Fernandez, R.; Landin, M. Tailored Hydrogels as Delivery Platforms for Conditioned Medium from Mesenchymal Stem Cells in a Model of Acute Colitis in Mice. Pharmaceutics 2021, 13, 1127. https://doi.org/10.3390/pharmaceutics13081127
Sendon-Lago J, Rio LG-d, Eiro N, Diaz-Rodriguez P, Avila L, Gonzalez LO, Vizoso FJ, Perez-Fernandez R, Landin M. Tailored Hydrogels as Delivery Platforms for Conditioned Medium from Mesenchymal Stem Cells in a Model of Acute Colitis in Mice. Pharmaceutics. 2021; 13(8):1127. https://doi.org/10.3390/pharmaceutics13081127
Chicago/Turabian StyleSendon-Lago, Juan, Lorena Garcia-del Rio, Noemi Eiro, Patricia Diaz-Rodriguez, Leandro Avila, Luis O. Gonzalez, Francisco J. Vizoso, Roman Perez-Fernandez, and Mariana Landin. 2021. "Tailored Hydrogels as Delivery Platforms for Conditioned Medium from Mesenchymal Stem Cells in a Model of Acute Colitis in Mice" Pharmaceutics 13, no. 8: 1127. https://doi.org/10.3390/pharmaceutics13081127
APA StyleSendon-Lago, J., Rio, L. G.-d., Eiro, N., Diaz-Rodriguez, P., Avila, L., Gonzalez, L. O., Vizoso, F. J., Perez-Fernandez, R., & Landin, M. (2021). Tailored Hydrogels as Delivery Platforms for Conditioned Medium from Mesenchymal Stem Cells in a Model of Acute Colitis in Mice. Pharmaceutics, 13(8), 1127. https://doi.org/10.3390/pharmaceutics13081127







