Photodynamic Therapy and Hyperthermia in Combination Treatment—Neglected Forces in the Fight against Cancer
Abstract
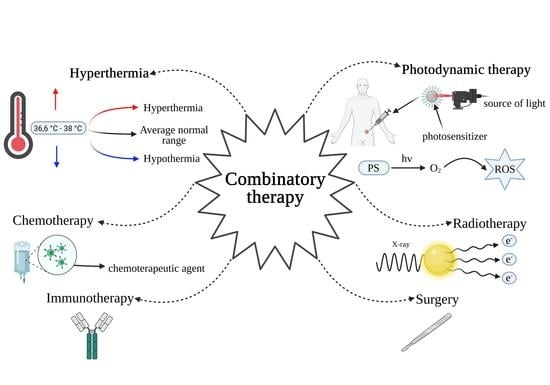
:1. Introduction: Aims of Anti-Cancer Combination Therapy
1.1. Combination Treatment–Definition, Pros, and Cons
1.2. Action Strategies and the Greatest Successes in the Fight against Cancer
2. General Information about Photodynamic Therapy and Hyperthermia
2.1. PDT
2.2. Hyperthermia
3. Why Are Hyperthermia and PDT Used in Treatment? Why Are They a Good Option for Combination Treatment?
3.1. Selectivity
3.2. Precision
3.3. Broad Effects on Tumor Cells
3.4. Increased Blood Flow in the Tumor
3.5. Vessels Pruning and Hypoxia
3.6. Inflammatory Reaction against Cancer
3.7. Induction of Long-Term Immune Response against Tumor
3.8. Targeting Possibility
3.9. Nanomedicine
4. What Other Methods Are PDT and Hyperthermia Combined with?
| Hyperthermia in Combinatory Anticancer Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Schema of Combinatory Treatment | Cancer | Stage of Trial | Country | Study Start Date–Study Completion Date | Enrollment | Results of Trials | ClinicalTrial. Gov ID | Ref. |
| H (FRWBH) + R + CH | Head and neck cancer | 1 phase | Germany | 2018–2020 | 10 | The median LRC and OS of all included patients * was 10 and 9 months, respectively. * Due to COVID-19 only 5 patients received all cycles of Fever-range whole body hyperthermia (FRWBH). | NCT03547388 [262] | [263,264] |
| H + CH + S (HIPEC + CRS) | Gastric cancer | 1 phase | United States | 2016–2018 | 4 | — | NCT02672865 [265] | — |
| H + CH | Bladder cancer | 1 phase (early) | United States | 2008–2011 | 15 | With a median follow-up of 3.18 years, 67% experienced another bladder cancer recurrence (none were muscle invasive) and 13% experienced an upper tract recurrence. | NCT00734994 [266] | [267] |
| H + CH + S (IPHC + CRS) | Appendix cancer colorectal cancer | 1 phase | United States | 2007–2007 | 16 | — | NCT00458809 [268] | [269] |
| H + S + CH (HIPEC) | Peritoneal cavity cancer | 1 phase | United States | 2007–2011 | 17 | — | NCT00625092 [270] | — |
| H + CH + LS | Lung cancer | 1 phase | United States | 1999–? | — | — | NCT00020007 [271] | — |
| H + TT | Liver tumor | 1 phase | United Kingdom | 2014–2017 | 10 | — | NCT02181075 [272] | [273,274,275] |
| S + HIPEC + IT | Ovarian adenocarcinoma fallopian tube adenocarcinoma, primary peritoneal carcinoma | 1 phase | France | 2011–2015 | 30 | With a median follow-up of 29.3 months since the diagnosis and 23 months after CCRS + HIPEC. Median DFS from CCRS + HIPEC was 16.7 months and after CCRS + HIPEC, 2-year DFS and OS were 27% and 71%, respectively. The median PFS was 16.7 months after surgery. | NCT02217956 [276] | [277] |
| H + DT | Non-small cell lung cancer | 1 phase 2 phase | China | 2015–2018 | 97 | The median OS for the active arm was 9.4 months and for the control arm was 5.6 months. The median PFS for the active arm was 3.0 months and for the control arm was 1.85 months. | NCT02655913 [278] | [279,280] |
| H + CH | Breast cancer | 1 phase 2 phase | United States | 2006–2016 | 29 (Trial A = 18 Trial B = 11) | In Trial A: TTLP, PFS and OS was 4.9, 4.8, 9.0 months, respectively.In trial B: 6 of 11 patients had a clinically significant quality of life (QoL) improvement. | NCT00346229 (trial A) [281] NCT00826085 (trial B) [282] | [283] |
| H + CH + S (HIPEC + CRS) | Colorectal cancer | 1 phase 2 phase | Italia | 2006–2010 | 20 | Median follow-up was 65.2 months in the HIPEC group and 34.5 months in the control group. 5-year overall survival (OS) was 81.3 % in the HIPEC group versus 70.0 % in the control group. | NCT02575859 [284] | [285] |
| H + CH + S (HIPEC + CRS) | Peritoneal carcinomatosis | 1 phase 2 phase | France | 2007–2011 | 18 | — | NCT01226550 [286] | — |
| H + R + S + CH | Sarcoma | 1 phase 2 phase | United States | 1999–2007 | 15 | — | NCT00093509 [287] | [288] |
| H + CH + S + CH (HIPEC + S + CH) | Ovarian carcinoma, fallopian tube carcinoma, primary peritoneal carcinoma | 1 phase 2 phase | Belgium | 2010–2015 | 19 | The median follow-up was 30.9 and the median PFS was 33.2 months. The OS survival was not reached. | NCT01709487 [289] | [290] |
| H + CH + S + CH (HIPEC + S + CH) | Colorectal cancer | 2 phase | China | 2016–2020 | 100 | — | NCT02830139 [291] | — |
| H + CH + S + CH (HIPEC + S + CH) | Stomach cancer | 2 phase | China | 2015–2020 | 100 | — | NCT02528110 [292] | — |
| H + CH + R | Rectal cancer | 2 phase | Germany | 2012–2017 | 78 | 3-year evaluate for OS, DFS, LC and DC were 94%, 81%, 96%, and 87%, respectively. Higher cumulative temperatures associated with hyperthermia indicated stronger tumor regression in patients. | NCT02353858 [293] | [294]. |
| H + CH + S (HIPEC + LS) | Gastrointestinal cancer | 2 phase | United States | 2014–2020 | 21 | The median OS from the date of diagnosis of metastatic disease was 30.2 months. The median OS from the first laparoscopic HIPEC was 20.3 months. | NCT02092298 [295] | [296,297] |
| H + R | Prostate cancer | 2 phase | United States | 1997–2003 | 37 | With a median follow-up of 70 months (18–110 months) 7-year OS was 94% with 61% of patient failure free. | NCT00003045 [298] | [299] |
| H + CH +S (HIPEC + CRS) | Desmoplastic small round cell tumor (DSRCT) sarcoma | 2 phase | United States | 2011–2018 | 22 | The estimated median OS from the time of diagnosis was 58.44 months (for 20 patience). | NCT01277744 [300] | [301] |
| CRS + HIPEC + EPIC | Peritoneal carcinomatosis gastric cancer | 2 phase | Sweden | 2005–2009 | 18 | The OS was 14.3 months for 8 patients who received entire treatment. The median OS for the CRS + HIPEC + EPIC group of patience was 10.2 months. 6 patients had macroscopically radical surgery (CC0) and for this subgroup OS was 19.1 months. | NCT01379482 [302] | [303] |
| H + CH + S (HIPEC + CRS) | Peritoneal carcinomatosis, colorectal cancer, appendiceal cancer peritoneal mesothelioma, pseudomyxoma peritonei, gastric cancer | 2 phase | United States | 2011–2020 | 51 | — | NCT02040142 [304] | — |
| H + CH + S (HIPEC + CRS) | Adrenocortical carcinoma, peritoneal carcinomatosis | 2 phase | United States | 2013–2018 | 11 | The median follow-up was 23 months. The median IP-PFS was 19 months. The median OS had not yet been reached. | NCT01833832 [305] | [306] |
| H + CH + S + CH (IPHC + CRS + CH) | Colorectal cancer | 2 phase | United States | 2002–2012 | 27 | The median follow-up was 40.4 months. The median OS and PFS were 43.0 and 9.3 months, respectively. | NCT00310076 [307] | [308] |
| H + CH | Sarcoma | 2 phase | United States | 1996–2003 | 34 | — | NCT00002974 [309] | — |
| H + CH | Melanoma | 2 phase | United States | 1995–2000 | 34 | — | NCT00002973 [310] | — |
| H + CH + S (HIPEC + CRS) | Ovarian cancer | 2 phase 3 phase | Republic of Korea | 2010–2020 | 184 (HIPEC, 92; control, 92) | Two-year PFS was 43.2% and 43.5% and 5-year PFS was 20.9% and 16.0% in HIPEC and control group, respectively. Five-year OS was 51.0% and 49.4% in HIPEC and control group, respectively. In women who received NAC, the median PFS for HIPEC and control group were 20 and 19 months and the median OS for HIPEC and control group were 54 and 51 months, respectively. In the subgroup with NAC, 2-year PFS was 37.2% in HIPEC group and 29.5% in control group and 5-year OS was 47.9% in HIPEC group and 27.7% in control group. After 20 months in PFS and 30 months in OS. | NCT01091636 [311] | [312] |
| H + B | Cervical cancer | 3 phase | Poland | 2006–2009 | 224 | Statistical differences were not observed for the distribution of early and late complications between the HT and non HT groups. | NCT01474356 [313] | [314] |
| H + CH + S + CH (HIPEC + CRS + CH) | Colorectal cancer primary peritoneal cavity cancer | 3 phase | France | 2008–2015 | 265 | The median follow-up of was 63.8 months, the median OS was 41.7 months in the cytoreductive surgery plus HIPEC group and 41.2 months in the cytoreductive surgery group. | NCT00769405 [315] | [316] |
| CRS + HIPEC | Ovarian cancer | 3 phase | Netherlands | 2007–2017 | 242 | The median OS was 45.7 months in the surgery-plus-HIPEC group and for surgery group of patience the median OS was 33.9 months. | NCT00426257 [317] | [318,319,320] |
| H + CH | Sarcoma | 3 phase | Germany | 1997–2012 | 340 | Median follow-up was 11.3 years. Patients randomized to chemotherapy plus hyperthermia had prolonged survival rates compared with those randomized to neoadjuvant chemotherapy alone with 5-year survival of 62.7% vs 51.3%, respectively, and 10-year survival of 52.6% vs 42.7%. | NCT00003052 [321] | [322,323] |
| H + B | Cervical cancer, prostate cancer | N/A | United States | 2009–2020 | 13 | — | NCT00911079 [324] | [325] |
| H + CH | Bladder cancer | N/A | Turkey | 2012–2017 | 44 | In the intermediate- and high-risk groups, the recurrence free survival rates at the 24th month were 78.6% and 80% and the progression free survival rates were 92.6% and 76.7%, respectively. | NCT03694535 [326] | [327] |
| PDT + TT | Basal cell carcinoma | 1 phase | United States | 2015–2017 | 4 | ORR showed 90% CR and 10% PR for the study. | NCT02639117 [328] | [329] |
| PDT + CH | Pancreatic cancer | 1 phase | United States | 2013–2018 | 12 | The median follow-up of 10.5 months, PFS and OS were 2.6 months and 11.5 months, respectively. | NCT01770132 [330] | [331] |
| PDT + S | Non-small cell lung cancer | 1 phase | United States | 2014–2018 | 8 | — | NCT01854684 [332] | — |
| PDT + S | Head and neck cancer | 1 phase | United States | 2006–2018 | 15 | The clinical follow-up visits at 48 months showed OS of 10 patients and PFS of 7 patients. The primary objective was to determine the safety of HPPH-mediated intraoperative adjuvant PDT immediately after tumor resection and to determine the highest dose of laser light that can be safely used in treatment. | NCT00470496 [333] | [334] |
| PDT + B | Lung cancer | 1 phase | United States | 1993–2004 | — | — | NCT00014066 [335] | — |
| PDT + ER | Early cancer in Barrett’s esophagus | 2 phase | United States | 2005–2012 | 73 | — | NCT00217087 [336] | — |
| PDT + S | Malignant mesothelioma | 2 phase | United States | 1999–2010 | 12 | — | NCT00054002 [337] | — |
| PDT + S | Non-melanoma Skin cancer | 2 phase | United States | 1993–2007 | — | — | NCT00002963 [338] | — |
| PDT + CH | Perihilar cholangiocarcinoma | 3 phase | Republic of Korea | 2009–2013 | 43 | Patients treated with combinatory therapy showed higher 1-year SR compared with the patients treated with PDT alone: 76.2% vs. 32% and median prolonged OS was 17 months vs. 8 months. Median PFS for combinatory treatment was 10 months and for patients with PDT alone was 2 months. | NCT00869635 [339] | [340] |
4.1. The Synergistic Effect
4.2. Attempts to Treat Tumors with PDT and Hyperthermia
5. Combination Therapy’s Effect on Drug Uptake and Delivery
6. Photodynamic Therapy and Hyperthermia in Combination Treatment
7. Proposed Combinations That Are Currently Used in Multimodal Cancer Treatment
7.1. Radiotherapy
7.2. Chemotherapy
7.3. Surgical Intervention
8. Hypoxia as a Treatment Imitation Factor
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Me, J.F.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Nicholas, C.; Lesinski, G.B. Immunomodulatory cytokines as therapeutic agents for melanoma. Immunomodulatory 2013, 3, 673–690. [Google Scholar] [CrossRef] [Green Version]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Narayanaswamy, R.; Ren, H.; Torchilin, V.P. Combination therapy targeting both cancer stem-like cells and bulk tumor cells for improved efficacy of breast cancer treatment. Cancer Biol. Ther. 2016, 17, 698–707. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Wang, F.; Chen, G.; Zhang, H.; Feng, L.; Wang, L.; Colman, H.; Keating, M.J.; Li, X.; Xu, R.-H.; et al. Effective Elimination of Cancer Stem Cells By a Novel Drug Combination Strategy. STEM CELLS 2012, 31, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delbaldo, C.; Michiels, S.; Syz, N.; Soria, J.-C.; Le Chevalier, T.; Pignon, J.-P. Benefits of Adding a Drug to a Single-Agent or a 2-Agent Chemotherapy Regimen in Advanced Non–Small-Cell Lung Cancer. JAMA 2004, 292, 470–484. [Google Scholar] [CrossRef] [PubMed]
- García-Román, J.; Zentella-Dehesa, A. Vascular permeability changes involved in tumor metastasis. Cancer Lett. 2013, 335, 259–269. [Google Scholar] [CrossRef]
- Chen, J.; Weihs, D.; Vermolen, F.J. Computational modeling of therapy on pancreatic cancer in its early stages. Biomech. Model. Mechanobiol. 2019, 19, 427–444. [Google Scholar] [CrossRef] [Green Version]
- Srivani, G.; Behera, S.K.; Dariya, B.; Chalikonda, G.; Alam, A.; Nagaraju, G.P. HIF-1α and RKIP: A computational approach for pancreatic cancer therapy. Mol. Cell. Biochem. 2020, 472, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Lala, M.; Li, T.R.; de Alwis, D.P.; Sinha, V.; Mayawala, K.; Yamamoto, N.; Siu, L.L.; Chartash, E.; Aboshady, H.; Jain, L. A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur. J. Cancer 2020, 131, 68–75. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Chiang, C.-S. Challenges of Using High-Dose Fractionation Radiotherapy in Combination Therapy. Front. Oncol. 2016, 6, 165. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Chen, Y.; Zheng, D.; Xiao, Y.; Chen, J.; Pan, J.; Chen, W. Diffusion kurtosis imaging and tumour microstructure for monitoring response to radiotherapy in human nasopharyngeal carcinoma xenografts. Jpn. J. Clin. Oncol. 2020, 50, 548–555. [Google Scholar] [CrossRef]
- Bhuyan, B.K. Kineticsof Cell Kill by Hyperthermia. Cancer Res. 1979, 39, 2277–2284. [Google Scholar] [PubMed]
- Overgaard, J.; Overgaard, J. The current and potential role of hyperthermia in radiotherapy. Int. J. Radiat. Oncol. 1989, 16, 535–549. [Google Scholar] [CrossRef]
- Cheng, Y.; Weng, S.; Yu, L.; Zhu, N.; Yang, M.; Yuan, Y. The Role of Hyperthermia in the Multidisciplinary Treatment of Malignant Tumors. Integr. Cancer Ther. 2019, 18. [Google Scholar] [CrossRef]
- Pritchard, J.R.; Lauffenburger, D.A.; Hemann, M.T. Understanding resistance to combination chemotherapy. Drug Resist. Updat. 2012, 15, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, E.L.; Prosnitz, L.R.; Dewhirst, M.W.; Marcom, P.K.; Hardenbergh, P.H.; Marks, L.B.; Brizel, D.; Vujaskovic, Z. Thermochemoradiotherapy Improves Oxygenation in Locally Advanced Breast Cancer. Clin. Cancer Res. 2004, 10, 4287–4293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrouzkia, Z.; Joveini, Z.; Keshavarzi, B.; Eyvazzadeh, N.; Aghdam, R.Z. Hyperthermia: How Can It Be Used? Oman Med. J. 2016, 31, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Krzykawska-Serda, M.; Dąbrowski, J.M.; Arnaut, L.G.; Szczygieł, M.; Urbanska, K.; Stochel, G.; Elas, M. The role of strong hypoxia in tumors after treatment in the outcome of bacteriochlorin-based photodynamic therapy. Free. Radic. Biol. Med. 2014, 73, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Arnaut, L.G.; Pereira, M.M.; Dabrowski, J.; Silva, E.F.F.; Schaberle, F.; Abreu, A.R.; Rocha, L.B.; Barsan, M.M.; Urbanska, K.; Stochel, G.; et al. Photodynamic Therapy Efficacy Enhanced by Dynamics: The Role of Charge Transfer and Photostability in the Selection of Photosensitizers. Chem. A Eur. J. 2014, 20, 5346–5357. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; De Ruysscher, D. NI volumab CO mbination with Standard First-line Chemotherapy and Radiotherapy in Locally Advanced Stage IIIA/B Non-Small Cell Lung Carcinoma (NICOLAS). European Thoracic Oncology Platform 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02434081 (accessed on 26 April 2021).
- Peters, S.; Felip, E.; Dafni, U.; Belka, C.; Guckenberger, M.; Irigoyen, A.; Nadal, E.; Becker, A.; Vees, H.; Pless, M.; et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer—The ETOP NICOLAS trial. Lung Cancer 2019, 133, 83–87. [Google Scholar] [CrossRef]
- Peters, S.; Felip, E.; Dafni, U.; Tufman, A.; Guckenberger, M.; Álvarez, R.; Nadal, E.; Becker, A.; Vees, H.; Pless, M.; et al. Progression-Free and Overall Survival for Concurrent Nivolumab with Standard Concurrent Chemoradiotherapy in Locally Advanced Stage IIIA-B NSCLC: Results From the European Thoracic Oncology Platform NICOLAS Phase II Trial (European Thoracic Oncology Platform 6-14). J. Thorac. Oncol. 2021, 16, 278–288. [Google Scholar] [CrossRef]
- Provencio, M. No TitleLocally Advanced Trial of Tri-weekly Metronomic Oral Vinorelbine and Cisplatin as Induc-tion Therapy and Subsequent Concomitance with Radiation Therapy in Patients with Unresectable Non Small Cell Lung Cancer (NSCLC) (NORA). Spanish Lung Cancer Group; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02709720 (accessed on 26 April 2021).
- Provencio, M.; Majem, M.; Guirado, M.; Massuti, B.; Peñas, R.D.L.; Ortega, A.L.; Dómine, M.; Marsé, R.; Sala, M.; Paredes, A.; et al. Phase II clinical trial with metronomic oral vinorelbine and tri-weekly cisplatin as induction therapy, subsequently concomitant with radiotherapy (RT) in patients with locally advanced, unresectable, non-small cell lung cancer (NSCLC). Analysis of survival and value of ctDNA for patient selection. Lung Cancer 2021, 153, 25–34. [Google Scholar] [CrossRef]
- Keam, B. Pembrolizumab and Paclitaxel in Refractory Small Cell Lung Cancer (MISP-MK3475). Seoul National University Hospital; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02551432 (accessed on 26 April 2021).
- Kim, Y.; Keam, B.; Ock, C.-Y.; Song, S.; Kim, M.; Kim, S.H.; Kim, K.H.; Kim, J.-S.; Kim, T.M.; Kim, D.-W.; et al. A phase II study of pembrolizumab and paclitaxel in refractory extensive disease small cell lung cancer. J. Clin. Oncol. 2018, 36, 8575. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Keam, B.; Ock, C.-Y.; Song, S.; Kim, M.; Kim, S.H.; Kim, K.H.; Kim, J.-S.; Kim, T.M.; Kim, D.-W.; et al. A phase II study of pembrolizumab and paclitaxel in patients with relapsed or refractory small-cell lung cancer. Lung Cancer 2019, 136, 122–128. [Google Scholar] [CrossRef]
- Reck, M. A Study Comparing SB8 and Avastin® in Patients with Advanced Non-squamous Non-small Cell Lung Cancer. Samsung Bioepis Co., Ltd.; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02754882 (accessed on 26 April 2021).
- Reck, M.; Luft, A.; Bondarenko, I.; Shevnia, S.; Trukhin, D.; Kovalenko, N.V.; Vacharadze, K.; Andrea, F.; Hontsa, A.; Choi, J.; et al. A phase III, randomized, double-blind, multicenter study to compare the efficacy, safety, pharmacokinetics, and immunogenicity between SB8 (proposed bevacizumab biosimilar) and reference bevacizumab in patients with metastatic or recurrent nonsquamous non-small cell lung cancer. Lung Cancer 2020, 146, 12–18. [Google Scholar] [CrossRef]
- Shaw, A. Nivolumab in Combination with Chemotherapy, or Nivolumab in Combination with Ipilimumab, in Advanced EGFR-Mutant or ALK-Rearranged NSCLC. Massachusetts General Hospital; 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03256136 (accessed on 26 April 2021).
- Owonikoko, T.K. Tremelimumab and Durvalumab with or without Radiation Therapy in Patients with Relapsed Small Cell Lung Cancer. Emory University; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02701400 (accessed on 26 April 2021).
- Owonikoko, T.K.; Higgins, K.A.; Chen, Z.; Zhang, C.; Pillai, R.N.; Steuer, C.E.; Saba, N.F.; Pakkala, S.; Shin, D.M.; Zhang, G.; et al. A randomized phase II study of tremelimumab and durvalumab with or without radiation for patients with relapsed small cell lung cancer (SCLC). J. Clin. Oncol. 2019, 37, 8515. [Google Scholar] [CrossRef]
- Gandhi, L. Phase 1/2 Study of Ensartinib and Durvalumab, in ALK-Rearranged Non-Small Cell Lung Cancer. Ludwig Institute for Cancer Research; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02898116 (accessed on 26 April 2021).
- Combination of Cryosurgery and NK Immunotherapy for Advanced Non-Small Cell Lung Cancer; Fuda Cancer Hospital: Guangzhou, China, 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02843815 (accessed on 26 April 2021).
- Lin, M.; Liang, S.-Z.; Wang, X.-H.; Liang, Y.-Q.; Zhang, M.-J.; Niu, L.-Z.; Chen, J.-B.; Li, H.-B.; Xu, K.-C. Clinical efficacy of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced non-small cell lung cancer. Immunol. Res. 2017, 65, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Liu, L. Cryotherapy Combine Icotinib for Advanced NSCLC Treatment; Fuda Cancer Hospital: Guangzhou, China, 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02744664 (accessed on 26 April 2021).
- A Study of Atezolizumab in Combination with Carboplatin Plus (+) Paclitaxel with or without Bevacizumab Compared with Carboplatin + Paclitaxel + Bevacizumab in Participants with Stage IV Non-Squamous Non-Small Cell Lung Cancer (NSCLC) (IMpower150). Hoffmann-La Roche; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02366143 (accessed on 26 April 2021).
- Reck, M.; Mok, T.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir. Med. 2019, 7, 387–401. [Google Scholar] [CrossRef]
- Reck, M.; Wehler, T.; Orlandi, F.; Nogami, N.; Barone, C.; Moro-Sibilot, D.; Shtivelband, M.; Larriba, J.L.G.; Rothenstein, J.; Früh, M.; et al. Safety and Patient-Reported Outcomes of Atezolizumab Plus Chemotherapy with or without Bevacizumab Versus Bevacizumab Plus Chemotherapy in Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 2530–2542. [Google Scholar] [CrossRef]
- Reck, M.; Shankar, G.; Lee, A.; Coleman, S.; McCleland, M.; Papadimitrakopoulou, V.A.; Socinski, M.A.; Sandler, A. Atezolizumab in combination with bevacizumab, paclitaxel and carboplatin for the first-line treatment of patients with metastatic non-squamous non-small cell lung cancer, including patients with EGFR mutations. Expert Rev. Respir. Med. 2019, 14, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Dinutuximab and Irinotecan Versus Irinotecan to Treat Subjects with Relapsed or Refractory Small Cell Lung Cancer. United Therapeutics; 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03098030 (accessed on 26 April 2021).
- Edelman, M.J.; Juan, O.; Navarro, A.; Golden, G.; Borg, E.; Saunders, A.V. A two-part, open-label, randomized, phase 2/3 study of dinutuximab and irinotecan versus irinotecan for second-line treatment of subjects with relapsed or refractory small cell lung cancer. J. Clin. Oncol. 2018, 36, TPS8588. [Google Scholar] [CrossRef]
- Ghebeh, H.; Al-Tweigeri, T. Study of Safety and Efficacy of Durvalumab in Combination with Paclitaxel in Metastatic Triple Negative Breast Cancer Patients. King Faisal Specialist Hospital & Research Center; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02628132 (accessed on 26 April 2021).
- Loibl, S. Addition of PD-L1 Antibody MEDI4736 to a Taxane-anthracycline Chemotherapy in Triple Negative Breast Cancer (GeparNuevo). German Breast Group; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02685059 (accessed on 26 April 2021).
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.; Blohmer, J.-U.; Grischke, E.-M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortes, J. PQR309 and Eribulin in Metastatic HER2 Negative and Triple-negative Breast Cancer (PIQHASSO). PIQUR Therapeutics AG; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02723877 (accessed on 26 April 2021).
- López-Miranda, E.; Gávila, J.; Pernas, S.; Saura, C.; Oliveira, M.; Serra, V.; Schmid, P.; Lord, S.; Paez, D.; Perez, J.; et al. Abstract OT1-01-06: PIQHASSO: Open label, non-randomized, multicenter phase 1/2b study investigating safety and efficacy of PQR309 and eribulin combination in patients (pts) with locally advanced (LA) or metastatic HER2 (-) and triple-negative breast cancer (TNBC) (study PQR309-007). Cancer Res. 2017, 77, OT1-01. [Google Scholar] [CrossRef]
- Wang, K. TCH Versus EC-TH as Neoadjuvant Treatment for HER2-Positive Breast Cancer (neoCARH). Guangdong Provincial People’s Hospital; 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03140553 (accessed on 26 April 2021).
- Li, W.; Zhu, T.; Hu, M.; Yang, M.; Ji, F.; Gao, H.F.; Yang, C.Q.; Zhang, L.L.; Cheng, M.Y.; Xu, F.P.; et al. Comparison of the efficacy and safety of the EC-T (epirubicin/cyclophosphamide followed by docetaxel) and TCb (docetaxel/carboplatin) neoadjuvant regimens in early TOP2A-normal stage II-III breast cancer. Neoplasma 2021, 67, 1409–1415. [Google Scholar] [CrossRef]
- Tredan, O. Evaluation of Pembrolizumab in Lymphopenic Metastatic Breast Cancer Patients Treated with Metronomic Cyclophosphamide (CHEMOIMMUNE). Centre Leon Berard; 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03139851 (accessed on 26 April 2021).
- Liu, G. Combination of Cryosurgery and NK Immunotherapy for Advanced Breast Cancer; Fuda Cancer Hospital: Guangzhou, China, 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02844335 (accessed on 26 April 2021).
- Cortés, J. T-DM1 and Non-pegylated Liposomal Doxorubicin in HER2-positive Metastatic Breast Cancer. MedSIR; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02562378 (accessed on 26 April 2021).
- López-Miranda, E.; Pérez-García, J.M.; Di Cosimo, S.; Brain, E.; Ravnik, M.; Escrivá-de-Romaní, S.; Vidal, M.; Gligorov, J.; Borštnar, S.; Calabuig, L.; et al. Trastuzumab Emtansine Plus Non-Pegylated Liposomal Doxorubicin in HER2-Positive Metastatic Breast Cancer (Thelma): A Single-Arm, Multicenter, Phase Ib Trial. Cancers 2020, 12, 3509. [Google Scholar] [CrossRef]
- López-Miranda, E.; Brain, E.; Saura, C.; Gligorov, J.; Dubot, C.; Dieras, V.; Suter, T.; Aguirre, E.; Perez-García, J.; Llombart, A.; et al. Abstract OT1-02-03: Phase I multicenter clinical trial evaluating the combination of trastuzumab emtansine (T-DM1) and non-pegylated liposomal doxorubicin (NPLD) in HER2-positive metastatic breast cancer (MBC) (MEDOPP038 study). Cancer Res. 2017, 77, OT1-02. [Google Scholar] [CrossRef] [Green Version]
- Kukreja, A. Safety Study of Pertuzumab (in Combination with Trastuzumab and Docetaxel) in Indian Participants with Breast Cancer. Hoffmann-La Roche; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02445586 (accessed on 26 April 2021).
- Tiersten, A. Durvalumab and Eribulin in Her2-Negative Metastatic Breast Cancer and Recurrent Ovarian Cancer. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03430518 (accessed on 26 April 2021).
- Landry, C.A.; Guziel, J.M.; Ru, M.; Shapiro, C.L.; Fasano, J.; Bhardwaj, A.S.; Irie, H.; Bhardwaj, N.; Tiersten, A. A phase Ib study evaluating the safety and tolerability of durvalumab in combination with eribulin in patients with HER2-negative metastatic breast cancer and recurrent ovarian cancer. J. Clin. Oncol. 2018, 36, TPS3116. [Google Scholar] [CrossRef]
- Phase IIIb Study to Evaluate the Safety and Tolerability of Herceptin SC with Perjeta and Docetaxel in Patients with HER2-positive Advanced Breast Cancer. Hoffmann-La Roche; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02402712 (accessed on 26 April 2021).
- Kuemmel, S.; Tondini, C.A.; Abraham, J.; Nowecki, Z.; Itrych, B.; Hitre, E.; Karaszewska, B.; Juárez-Ramiro, A.; Morales-Vásquez, F.; Pérez-García, J.M.; et al. Subcutaneous trastuzumab with pertuzumab and docetaxel in HER2-positive metastatic breast cancer: Final analysis of MetaPHER, a phase IIIb single-arm safety study. Breast Cancer Res. Treat. 2021, 187, 467–476. [Google Scholar] [CrossRef]
- Dimery, I. A Multi-Center Study of Ibrutinib in Combination with MEDI4736 in Subjects with Relapsed or Refractory Solid Tumors. Pharmacyclics LLC; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02403271 (accessed on 26 April 2021).
- Hong, D.; Rasco, D.; Veeder, M.; Luke, J.J.; Chandler, J.; Balmanoukian, A.; George, T.; Munster, P.; Berlin, J.D.; Gutierrez, M.; et al. A Phase 1b/2 Study of the Bruton Tyrosine Kinase Inhibitor Ibrutinib and the PD-L1 Inhibitor Durvalumab in Patients with Pretreated Solid Tumors. Oncology 2019, 97, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Palbociclib in Combination with Letrozole As Treatment of Post-Menopausal Women with HR+, HER2- Advanced Breast Cancer. Pfizer; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02679755 (accessed on 26 April 2021).
- Campone, M. S 81694 Plus Paclitaxel in Metastatic Breast Cancer. Institut de Recherches Internationales Servier; 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03411161 (accessed on 26 April 2021).
- Cardona, M. Nintedanib + Letrozole in Postmenopausal Women with Breast Cancer: Clinical Trial Safety and Pharmacodynamics. Centro Nacional de Investigaciones Oncologicas CARLOS III; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02619162 (accessed on 26 April 2021).
- Quintela-Fandino, M.; Apala, J.V.; Malon, D.; Mouron, S.; Hornedo, J.; Gonzalez-Cortijo, L.; Colomer, R.; Guerra, J. Nintedanib plus letrozole in early breast cancer: A phase 0/I pharmacodynamic, pharmacokinetic, and safety clinical trial of combined FGFR1 and aromatase inhibition. Breast Cancer Res. 2019, 21, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prat, A.; Gavilá, J. Neadjuvant Multiagent Chemotherapy or Letrozole Plus Ribociclib in Luminal B/HER2-negative Breast Cancer. (CORALLEEN). SOLTI Breast Cancer Research Group; 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03248427 (accessed on 26 April 2021).
- Prat, A.; Saura, C.; Pascual, T.; Hernando, C.; Munoz, M.; Pare, L.; Farré, B.G.; Fernández, P.L.; Galván, P.; Chic, N.; et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 33–43. [Google Scholar] [CrossRef]
- Conte, P. Neoadjuvant Chemo-endocrine Therapy and Immunotherapy for Pre-menopausal Luminal B Breast Cancer Patients (GIADA). Istituto Oncologico Veneto IRCCS; 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT04659551 (accessed on 26 April 2021).
- Ueno, N. Trial of Ra-223 Dichloride in Combination with Hormonal Therapy and Denosumab in the Treatment of Patients with Hormone-Positive Bone-Dominant Metastatic Breast Cancer. M.D. Anderson Cancer Center; 2015. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02366130 (accessed on 26 April 2021).
- Ueno, N.T.; Tahara, R.K.; Fujii, T.; Reuben, J.M.; Gao, H.; Saigal, B.; Lucci, A.; Iwase, T.; Ibrahim, N.K.; Damodaran, S.; et al. Phase II study of Radium-223 dichloride combined with hormonal therapy for hormone receptor-positive, bone-dominant metastatic breast cancer. Cancer Med. 2019, 9, 1025–1032. [Google Scholar] [CrossRef]
- Chang, J.C.N. TAK-228 Plus Tamoxifen in Patients with ER-Positive, HER2-negative Breast Cancer (ANETT). 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02988986 (accessed on 26 April 2021).
- Koca, E.; Niravath, P.A.; Ensor, J.; Patel, T.A.; Li, X.; Hemati, P.; Wong, H.; Qian, W.; Boone, T.; Zhao, J.; et al. Open label, phase II trial of neoadjuvant TAK-228 plus tamoxifen in patients with estrogen receptor (ER)-positive, human epidermal growth factor receptor type 2 (HER2)-negative breast cancer-ANETT. J. Clin. Oncol. 2019, 37, 584. [Google Scholar] [CrossRef]
- Koca, E.; Niravath, P.A.; Ensor, J.; Patel, T.A.; Li, X.; Hemati, P.; Wong, H.; Qian, W.; Boone, T.; Zhao, J.; et al. ANETT: PhAse II trial of NEoadjuvant TAK-228 plus Tamoxifen in patients with hormone receptor-positive breast cancer. Breast Cancer Res. Treat. 2021, 1–7. [Google Scholar] [CrossRef]
- A Study to Evaluate the Efficacy and Safety of Pertuzumab + Trastuzumab + Docetaxel Versus Placebo + Trastuzumab + Docetaxel in Previously Untreated Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Metastatic Breast Cancer (PUFFIN). Hoffmannla Roche; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02896855 (accessed on 26 April 2021).
- Xu, B.; Li, W.; Zhang, Q.; Shao, Z.; Li, Q.; Wang, X.; Li, H.; Sun, T.; Yin, Y.; Zheng, H.; et al. Pertuzumab, trastuzumab, and docetaxel for Chinese patients with previously untreated HER2-positive locally recurrent or metastatic breast cancer (PUFFIN): A phase III, randomized, double-blind, placebo-controlled study. Breast Cancer Res. Treat. 2020, 182, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Posadas, E.M. Study of TRC105 with Abiraterone and with Enzalutamide in Prostate Cancer Patients Progressing on Therapy. Cedars-Sinai Medical Center; 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03418324 (accessed on 26 April 2021).
- Tagawa, S. Neoadjuvant J591 Treatment for Prostate Cancer. Weill Medical College of Cornell University; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02693860 (accessed on 26 April 2021).
- Beisland, C. Phase I Clinical Trial of Cryoimmunotherapy against Prostate Cancer (CryoIT). Alden Cancer Therapy II; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02423928 (accessed on 26 April 2021).
- Thomsen, L.C.V.; Honoré, A.; Reisæter, L.A.; Almås, B.; Førde, K.; Kristoffersen, E.K.; Melve, G.K.; Biermann, M.; Helle, S.I.; Azeem, W.; et al. A prospective phase I trial of dendritic cell-based cryoimmunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2020, 38, 3029. [Google Scholar] [CrossRef]
- A Study of Enzalutamide and LY3023414 in Men with Prostate Cancer. Eli Lilly and Company; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02407054 (accessed on 26 April 2021).
- Sweeney, C.; Percent, I.J.; Babu, S.; Cultrera, J.; Mehlhaff, B.A.; Goodman, O.B.; Morris, D.; Schnadig, I.D.; Albany, C.; Shore, N.D.; et al. Phase 1b/2 study of enzalutamide (ENZ) with LY3023414 (LY) or placebo (PL) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) after progression on abiraterone. J. Clin. Oncol. 2019, 37, 5009. [Google Scholar] [CrossRef]
- Safety and Tolerability of Atezolizumab (ATZ) in Combination with Radium-223 Dichloride (R-223-D) in Meta-static Castrate-Resistant Prostate Cancer (CRPC) Progressed Following Treatment with an Androgen Pathway Inhibitor. Hoffmann-La Roche; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02814669 (accessed on 26 April 2021).
- Morris, M.J.; Fong, L.; Petrylak, D.P.; Sartor, A.O.; Higano, C.S.; Pagliaro, L.C.; Alva, A.S.; Appleman, L.J.; Tan, W.; Vaishampayan, U.N.; et al. Safety and clinical activity of atezolizumab (atezo) + radium-223 dichloride (r-223) in 2L metastatic castration-resistant prostate cancer (mCRPC): Results from a phase Ib clinical trial. J. Clin. Oncol. 2020, 38, 5565. [Google Scholar] [CrossRef]
- Lee, L.S. Neoadjuvant Apalutamide (ARN509) and Radical Prostatectomy in Treatment of Intermediate to High Risk Prostate Cance. Singapore General Hospital; 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03124433 (accessed on 26 April 2021).
- A Safety and Pharmacokinetics Study of Niraparib Plus an Androgen Receptor-Targeted Therapy in Men with Metastatic Castration-Resistant Prostate Cancer (BEDIVERE). Janssen Research & Development, LLC; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02924766 (accessed on 26 April 2021).
- Saad, F.; Chi, K.N.; Shore, N.D.; Graff, J.N.; Posadas, E.M.; Espina, B.M.; Zhu, E.; Hazra, A.; Bradic, B.; Cheng, S.; et al. Phase Ib study of niraparib plus androgen receptor-targeted therapy (ART) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 122. [Google Scholar] [CrossRef]
- Saad, F.; Chi, K.N.; Shore, N.D.; Graff, J.N.; Posadas, E.M.; Lattouf, J.-B.; Espina, B.M.; Zhu, E.; Yu, A.; Hazra, A.; et al. Niraparib with androgen receptor-axis-targeted therapy in patients with metastatic castration-resistant prostate cancer: Safety and pharmacokinetic results from a phase 1b study (BEDIVERE). Cancer Chemother. Pharmacol. 2021, 88, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.D. Open Label Phase Two Study of Enzalutamide with Concurrent Administration of Radium Ra 223 Dichloride in Castration-Resistant (Hormone-Refractory) Prostate Cancer Subjects with Symptomatic Bone Metastasis. Carolina Research Professionals, LLC; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02507570 (accessed on 26 April 2021).
- Shore, N.D.; Schellhammer, P.F.; Tutrone, R.F.; Mariados, N.F.; Harrelson, S.S. Open Label Phase II Study of Enzalutamide with Concurrent Administration of Radium 223 Dichloride in Patients with Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2020, 18, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.I. PLX3397, Radiation Therapy, and Antihormone Therapy in Treating Patients with Intermediate- or High-Risk Prostate Cancer. Barbara Ann Karmanos Cancer Institute; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02472275 (accessed on 26 April 2021).
- A Phase I/II Study for the Safety and Efficacy of Panitumumab in Combination with TAS-102 for Patients with Colorectal Cancer (APOLLON). Takeda; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02613221 (accessed on 26 April 2021).
- Kato, T.; Kagawa, Y.; Komatsu, Y.; Oki, E.; Yoshino, T.; Yamazaki, K.; Yasui, H.; Satake, H.; Shibuya, K.; Oba, K.; et al. A phase I/II study for panitumumab combined with TAS-102 in patients with RAS wild-type metastatic colorectal cancer (APOLLON study): Phase I results. J. Clin. Oncol. 2017, 35, 770. [Google Scholar] [CrossRef]
- Kuboki, Y.; Yoshino, T.; Kato, T.; Kagawa, Y.; Gamoh, M.; Yasui, H.; Yamazaki, K.; Komatsu, Y.; Satake, H.; Goto, M.; et al. APOLLON: A phase I/II study of panitumumab combined with TAS-102 in patients (pts) with RAS wild-type (wt) metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2018, 36, 3523. [Google Scholar] [CrossRef]
- O’Dwyer, P.J. Phase I/II Trial of Regorafenib, Hydroxychloroquine, and Entinostat in Metastatic Colorectal Cancer. Abramson Cancer Center of the University of Pennsylvania; 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03215264 (accessed on 26 April 2021).
- Study of Magrolimab (Hu5F9-G4) in Combination with Cetuximab in Participants with Solid Tumors and Ad-vanced Colorectal Cancer. Gilead Sciences; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02953782 (accessed on 26 April 2021).
- Fisher, G.A.; Lakhani, N.J.; Eng, C.; Hecht, J.R.; Bendell, J.C.; Philip, P.A.; O’Dwyer, P.J.; Johnson, B.; Kardosh, A.; Ippolito, T.M.; et al. A phase Ib/II study of the anti-CD47 antibody magrolimab with cetuximab in solid tumor and colorectal cancer patients. J. Clin. Oncol. 2020, 38, 114. [Google Scholar] [CrossRef]
- Aranda, E.; Gómez, A. Phase II Trial to Assess FOLFIRI+Aflibercept Efficacy in Patients with Oxaliplatin-pretreated Metastatic Colorectal Cancer with or without ACE Polymorphisms (POLAF). Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD); 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02970916 (accessed on 26 April 2021).
- Ahn, J.B. Pemetrexed and Erlotinib for Metastatic Colorectal Cancer. Yonsei University; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02723578 (accessed on 26 April 2021).
- Beom, S.-H.; Lee, K.-W.; Cho, S.-H.; Park, Y.; Kim, T.; Jung, M.; Shin, S.; Ahn, J.; Lee, K.H. A phase II study of pemetrexed and erlotinib for metastatic colorectal cancer refractory to standard chemotherapy. Ann. Oncol. 2018, 29, viii190. [Google Scholar] [CrossRef]
- Phase Ib Study of PDR001 in Combination with Regorafenib in Adult Patients with Previously Treated Metastatic Colorectal Cancer. Novartis Pharmaceuticals; 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03081494 (accessed on 26 April 2021).
- Van Custem, E. A Study Evaluating S 95005 Plus Bevacizumab and Capecitabine Plus Bevacizumab in Patients with Previously Untreated Colorectal Cancer Who Are Non-eligible for Intensive Therapy (TASCO1). Institut de Recherches Internationales Servier; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02743221 (accessed on 26 April 2021).
- Van Cutsem, E.; Danielewicz, I.; Saunders, M.; Pfeiffer, P.; Argilés, G.; Borg, C.; Glynne-Jones, R.; Punt, C.; Van de Wouw, A.; Fedyanin, M.; et al. Trifluridine/tipiracil plus bevacizumab in patients with untreated metastatic colorectal cancer ineligible for intensive therapy: The randomized TASCO1 study. Ann. Oncol. 2020, 31, 1160–1168. [Google Scholar] [CrossRef]
- Barton, J. A Study to Evaluate eFT508 Alone and in Combination with Avelumab in Subjects with MSS Colorectal Cancer. Effector Therapeutics; 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03258398 (accessed on 26 April 2021).
- Hubbard, J.M.; Patel, M.R.; Bekaii-Saab, T.S.; Falchook, G.S.; Freilich, B.L.; Dasari, A.; Knisely, B.T.; Anderson, M.; Chiang, G.G.; Webster, K.R.; et al. A phase II, open label, randomized, noncomparative study of eFT508 (tomivosertib) alone or in combination with avelumab in subjects with relapsed/refractory microsatellite stable colorectal cancer (MSS CRC). J. Clin. Oncol. 2019, 37, e14145. [Google Scholar] [CrossRef]
- Open-label, Single Arm Trial of BI 695502 in Patients with Previously Untreated Metastatic Colorectal Cancer (INVICTAN®-3). Boehringer Ingelheim; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02776683 (accessed on 26 April 2021).
- Bokemeyer, C. Phase 1 Trial to Evaluate mFOLFOX6 with Selinexor In Patients with Metastatic Colorectal Cancer (SENTINEL). GSO Global Clinical Research BV; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02384850 (accessed on 26 April 2021).
- Nilsson, S.; Stein, A.; Rolfo, C.; Kranich, A.L.; Mann, J.; Papadimitriou, K.; Theile, S.; Amberg, S.; Bokemeyer, C. Selinexor (KPT-330), an Oral Selective Inhibitor of Nuclear Export (SINE) Compound, in Combination with FOLFOX in Patients with Metastatic Colorectal Cancer (mCRC)—Final Results of the Phase I Trial SENTINEL. Curr. Cancer Drug Targets 2020, 20, 811–817. [Google Scholar] [CrossRef] [PubMed]
- A Study Evaluating TAS-102 Plus Nivolumab in Patients with MSS CRC. Taiho Oncology, Inc.; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02860546 (accessed on 26 April 2021).
- Patel, M.R.; Falchook, G.S.; Hamada, K.; Makris, L.; Bendell, J.C. A phase 2 trial of trifluridine/tipiracil plus nivolumab in patients with heavily pretreated microsatellite-stable metastatic colorectal cancer. Cancer Med. 2021, 10, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Falchook, G.S.; Hamada, K.; Makris, L.; Winkler, R.E.; Gordon, G.S.; Bendell, J.C. Results of a phase II study evaluating trifluridine/tipiracil plus nivolumab in patients with heavily pretreated microsatellite-stable (MSS) metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2019, 37, 48. [Google Scholar] [CrossRef]
- Cassier, P. Evaluation of Safety and Activity of an Anti-PDL1 Antibody (DURVALUMAB) Combined with CSF-1R TKI (PEXIDARTINIB) in Patients with Metastatic/Advanced Pancreatic or Colorectal Cancers (MEDIPLEX). Centre Leon Berard; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02777710 (accessed on 26 April 2021).
- A Study to Investigate Efficacy and Safety of Cobimetinib Plus Atezolizumab and Atezolizumab Monotherapy Versus Regorafenib in Participants with Metastatic Colorectal Adenocarcinoma (COTEZO IMblaze370). Hoffmann-La Roche; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02788279 (accessed on 26 April 2021).
- Eng, C.; Kim, T.W.; Bendell, J.; Argilés, G.; Tebbutt, N.C.; Di Bartolomeo, M.; Falcone, A.; Fakih, M.; Kozloff, M.; Segal, N.H.; et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019, 20, 849–861. [Google Scholar] [CrossRef]
- Study of Cobimetinib in Combination with Atezolizumab and Bevacizumab in Participants with Gastrointestinal and Other Tumors. Hoffmann-La Roche; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02876224 (accessed on 26 April 2021).
- Orlandi, A. Erbitux MEtastatic Colorectal Cancer Strategy Study. 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02484833 (accessed on 26 April 2021).
- Pinto, C.; Normanno, N.; Orlandi, A.; Maiello, E.; Bilancia, D.; Corsi, D.C.; Tamburini, E.; Pisconti, S.; Ferraú, F.; Di Costanzo, F.; et al. Cetuximab metastatic colorectal cancer strategy (ERMES) study: A phase III randomized two arm study with FOLFIRI + cetuximab until disease progression compared to FOLFIRI + cetuximab for 8 cycles followed by cetuximab alone until disease progression in first-line treatment of patients with RAS and BRAF wild type metastatic colorectal cancer. J. Clin. Oncol. 2017, 35, TPS810. [Google Scholar] [CrossRef]
- Pinto, C.; Normanno, N.; Orlandi, A.; Fenizia, F.; Damato, A.; Maiello, E.; Tamburini, E.; Di Costanzo, F.; Tonini, G.; Bilancia, D.; et al. Phase III study with FOLFIRI + cetuximab versus FOLFIRI + cetuximab followed by cetuximab alone in RAS and BRAF WT mCRC. Futur. Oncol. 2018, 14, 1339–1346. [Google Scholar] [CrossRef]
- Cohen, D.J. Study of Irinotecan and AZD1775, a Selective Wee 1 Inhibitor, in RAS or BRAF Mutated, Second-line Metastatic Colorectal Cancer. NYU Langone Health; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02906059 (accessed on 26 April 2021).
- Cohen, D.J.; Grabocka, E.; Bar-Sagi, D.; Godin, R.; Leichman, L.P. A phase Ib study combining irinotecan with AZD1775, a selective WEE 1 kinase inhibitor, in RAS/RAF mutated metastatic colorectal cancer patients who progressed on first line therapy. J. Clin. Oncol. 2017, 35, TPS3627. [Google Scholar] [CrossRef]
- A Study of Brontictuzumab with Chemotherapy for Subjects with Previously Treated Metastatic Colorectal Cancer. OncoMed Pharmaceuticals, Inc.; 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03031691 (accessed on 26 April 2021).
- A Phase 1a/b Dose Escalation Study of the Safety, Pharmacokinetics, and Pharmacodynamics of OMP-131R10. On-coMed Pharmaceuticals, Inc.; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02482441 (accessed on 26 April 2021).
- Safety and Efficacy Study of AMG 820 and Pembrolizumab Combination in Select Advanced Solid Tumor Cancer. Amgen; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02713529 (accessed on 26 April 2021).
- Razak, A.R.; Cleary, J.M.; Moreno, V.; Boyer, M.; Aller, E.C.; Edenfield, W.; Tie, J.; Harvey, R.D.; Rutten, A.; Shah, M.A.; et al. Safety and efficacy of AMG 820, an anti-colony-stimulating factor 1 receptor antibody, in combination with pembrolizumab in adults with advanced solid tumors. J. Immunother. Cancer 2020, 8, e001006. [Google Scholar] [CrossRef]
- Chen, J. Safety and Efficiency of IRE Plus γδ T Cell against Locally Advanced Pancreatic Cancer; Fuda Cancer Hospital: Guangzhou, China, 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03180437 (accessed on 26 April 2021).
- Lin, M.; Zhang, X.; Liang, S.; Luo, H.; Alnaggar, M.; Liu, A.; Yin, Z.; Chen, J.; Niu, L.; Jiang, Y. Irreversible electroporation plus allogenic Vγ9Vδ2 T cells enhances antitumor effect for locally advanced pancreatic cancer patients. Signal. Transduct. Target. Ther. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Niu, L. Combination of Irreversible Electroporation and NK Immunotherapy for Advanced Pancreatic Cancer; Fuda Cancer Hospital: Guangzhou, China, 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02718859 (accessed on 26 April 2021).
- Benaim, E. RX-3117 in Combination with Abraxane® in Subjects with Metastatic Pancreatic Cancer. Rexahn Phar-Maceuticals, Inc.; 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03189914 (accessed on 26 April 2021).
- Babiker, H.M.; Schlegel, P.J.; Hicks, L.G.; Bullock, A.J.; Burhani, N.; Benaim, E.; Peterson, C.; Heaton, C.; Ocean, A.J. A phase I/II study of RX-3117, an oral antimetabolite nucleoside, in combination with nab-paclitaxel (nab-pac) as first-line treatment of metastatic pancreatic cancer (met-PC): Preliminary results. J. Clin. Oncol. 2019, 37, 420. [Google Scholar] [CrossRef]
- Wilmink, J.W. Phase I/II Study of LDE225 with Gemcitabine and Nab-paclitaxel in Patients with Pancreatic Cancer (MATRIX). Academisch Medisch Centrum—Universiteit van Amsterdam (AMC-UvA); 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02358161 (accessed on 26 April 2021).
- Pijnappel, E.; Klaassen, R.; Van Der Lee, K.; Van Empel, M.P.; Richel, D.; Legdeur, M.; Nederveen, A.; Van Laarhoven, H.; Wilmink, H. Phase I/II study of LDE225 in combination with gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer. Ann. Oncol. 2019, 30, v265. [Google Scholar] [CrossRef]
- Ramanathan, R.K. BPM31510 Administered Intravenously with Gemcitabine in Advanced Pancreatic Cancer Patients. Berg, LLC; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02650804 (accessed on 26 April 2021).
- Kundranda, M.N.; Propper, D.; Ritch, P.S.; Strauss, J.; Hidalgo, M.; Gillmore, R.; Sarangarajan, R.; Narain, N.R.; Kiebish, M.A.; Rodrigues, L.O.; et al. Phase II trial of BPM31510-IV plus gemcitabine in advanced pancreatic ductal adenocarcinomas (PDAC). J. Clin. Oncol. 2020, 38, 723. [Google Scholar] [CrossRef]
- Olaptesed (NOX-A12) Alone and in Combination with Pembrolizumab in Colorectal and Pancreatic Cancer (Key-note-559). NOXXON Pharma AG; 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03168139 (accessed on 26 April 2021).
- Halama, N.; Prüfer, U.; Froemming, A.; Beyer, D.; Eulberg, D.; Jungnelius, J.; Mangasarian, A. Phase I/II study with CXCL12 inhibitor NOX-A12 and pembrolizumab in patients with microsatellite-stable, metastatic colorectal or pancreatic cancer. Ann. Oncol. 2019, 30, v231. [Google Scholar] [CrossRef]
- Halama, N.; Pruefer, U.; Frömming, A.; Beyer, D.; Eulberg, D.; Jungnelius, J.U.B.; Mangasarian, A. Experience with CXCL12 inhibitor NOX-A12 plus pembrolizumab in patients with microsatellite-stable, metastatic colorectal or pancreatic cancer. J. Clin. Oncol. 2019, 37, e14143. [Google Scholar] [CrossRef]
- Cohen, D.J. Study of Nivolumab, Cabiralizumab, and Stereotactic Body Radiotherapy (SBRT) for Locally Advanced Unresectable Pancreatic Cancer. NYU Langone Health; 2018. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03599362 (accessed on 26 April 2021).
- Cohen, D.J.; Medina, B.; Du, K.L.; Coveler, A.L.; Manji, G.A.; Oberstein, P.E.; Perna, S.K.; Miller, G. Phase II multi-institutional study of nivolumab (Nivo), cabiralizumab (Cabira), and stereotactic body radiotherapy (SBRT) for locally advanced unresectable pancreatic cancer (LAUPC). J. Clin. Oncol. 2019, 37, TPS4163. [Google Scholar] [CrossRef]
- Niu, L. Simultaneous Gemcitabine and Irreversible Electroporation for Locally Advanced Pancreatic Cancer; Fuda Cancer Hospital: Guangzhou, China, 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02981719 (accessed on 26 April 2021).
- Study of Pegilodecakin (LY3500518) with FOLFOX Compared to FOLFOX Alone Second-line Tx in Participants With Metastatic Pancreatic Cancer (Sequoia). Eli Lilly and Company; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02923921 (accessed on 26 April 2021).
- Hecht, J.R.; Lonardi, S.; Bendell, J.C.; Sim, H.-W.; Macarulla, T.; Lopez, C.D.; Van Cutsem, E.; Martin, A.J.M.; Park, J.O.; Greil, R.; et al. Randomized Phase III Study of FOLFOX Alone and with Pegilodecakin as Second-line Therapy in Patients with Metastatic Pancreatic Cancer (SEQUOIA). J. Clin. Oncol. 2020, 38, 637. [Google Scholar] [CrossRef]
- Wong, H.C. QUILT-2.001: ALT-803 in Patients with Advanced Pancreatic Cancer in Conjunction with Gemcitabine and Nab-Paclitaxel. Altor BioScience; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02559674 (accessed on 26 April 2021).
- Acoba, J.D.; Rock, A.; Wong, H.C. Phase Ib/II study of ALT-803 in combination with gemcitabine and nab-paclitaxel in patients with advanced pancreatic cancer. J. Clin. Oncol. 2017, 35, TPS510. [Google Scholar] [CrossRef]
- ACP-196 Alone and in Combination with Pembrolizumab in Subjects with Advanced or Metastatic Pancreatic Cancer (KEYNOTE144). Acerta Pharma BV; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02362048 (accessed on 26 April 2021).
- Overman, M.; Javle, M.; Davis, R.E.; Vats, P.; Kumar-Sinha, C.; Xiao, L.; Mettu, N.B.; Parra, E.R.; Benson, A.B.; Lopez, C.D.; et al. Randomized phase II study of the Bruton tyrosine kinase inhibitor acalabrutinib, alone or with pembrolizumab in patients with advanced pancreatic cancer. J. Immunother. Cancer 2019, 8, e000587. [Google Scholar] [CrossRef] [Green Version]
- A Study of Galunisertib (LY2157299) and Durvalumab (MEDI4736) in Participants with Metastatic Pancreatic Cancer. Eli Lilly and Company; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02734160 (accessed on 26 April 2021).
- Melisi, D.; Oh, D.-Y.; Hollebecque, A.; Calvo, E.; Varghese, A.; Borazanci, E.; Macarulla, T.; Merz, V.; Zecchetto, C.; Zhao, Y.; et al. Safety and activity of the TGFβ receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J. Immunother. Cancer 2021, 9, e002068. [Google Scholar] [CrossRef] [PubMed]
- Melisi, D.; Hollebecque, A.; Oh, D.-Y.; Calvo, E.; Varghese, A.M.; Borazanci, E.H.; Mercade, T.M.; Simionato, F.; Park, J.O.; Bendell, J.C.; et al. A phase Ib dose-escalation and cohort-expansion study of safety and activity of the transforming growth factor (TGF) β receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J. Clin. Oncol. 2019, 37, 4124. [Google Scholar] [CrossRef]
- Vonderheide, R. Study of Neo-Adjuvant RO7009789 Alone or Neo-Adjuvant RO7009789 Plus Nab-Paclitaxel and Gemcitabine Followed by Adjuvant RO7009789 Plus Nab-Paclitaxel and Gemcitabine for Patients with Newly Diagnosed Resectable Pancreatic Carcinoma. Abramson Cancer Center of the University of Pennsylvania; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02588443 (accessed on 26 April 2021).
- Kuśnierz, K. Clinical Phase II Clinical Study Evaluating the Toxicity and Efficacy of mFOLFIRINOX Associated with SBRT (Stereotactic Radiotherapy) in Patients with Unresectable Locally Advanced Pancreatic Cancer. Medical University of Silesia; 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03891472 (accessed on 26 April 2021).
- Rudloff, U. M7824 (MSB0011359C) in Combination with Gemcitabine in Adults with Previously Treated Advanced Adenocarcinoma of the Pancreas. National Cancer Institute (NCI); 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03451773 (accessed on 26 April 2021).
- A Study of Napabucasin Plus Nab-Paclitaxel with Gemcitabine in Adult Patients with Metastatic Pancreatic Adenocarcinoma (CanStem111P). Sumitomo Dainippon Pharma Oncology, Inc.; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02993731 (accessed on 26 April 2021).
- Bekaii-Saab, T.S.; Li, C.-P.; Okusaka, T.; O’Neil, B.H.; Reni, M.; Tabernero, J.; Qin, S.; Van Cutsem, E.; Borodyansky, L.; Li, C. CanStem111P trial: A phase III study of napabucasin (BBI-608) plus nab-paclitaxel (nab-PTX) with gemcitabine (gem) in adult patients with metastatic pancreatic adenocarcinoma (mPDAC). J. Clin. Oncol. 2017, 35, TPS4148. [Google Scholar] [CrossRef]
- Sonbol, M.; Ahn, D.H.; Goldstein, D.; Okusaka, T.; Tabernero, J.; Macarulla, T.; Reni, M.; Li, C.-P.; O’Neil, B.; Van Cutsem, E.; et al. CanStem111P trial: A Phase III study of napabucasin plus nab-paclitaxel with gemcitabine. Futur. Oncol. 2019, 15, 1295–1302. [Google Scholar] [CrossRef]
- Dave, D.; Desai, U.; Despande, N. Photodynamic Therapy: A View through Light. J. Orofac. Res. 2012, 2, 82–86. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Wojtunik-Kulesza, K.A.; Oniszczuk, T.; Kasprzak, K. The potential of photodynamic therapy (PDT)—Experimental investigations and clinical use. Biomed. Pharmacother. 2016, 83, 912–929. [Google Scholar] [CrossRef]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.; Childs, C.J.; Sibata, C.H.; Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.; et al. Photosensitizers in clinical PDT. Photodiagnosis Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017, 5, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahamse, H.; Hamblin, M.R. New photossensitizersfot photodynamic therapy. Biochem. J. 2017, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, J.A.R.; Medina, M.L.V.M.; Zegarra, M.L.; Rodriguez, R. Efficacy, Safety and Quality of Life After TOOKAD® Soluble VTP for Localized Prostate Cancer (PCM304). Steba Biotech S.A.; 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT01875393 (accessed on 26 April 2021).
- Emberton, M. Efficacy and Safety Study of TOOKAD® Soluble for Localised Prostate Cancer Compared to Active Surveillance. (PCM301). Steba Biotech S.A.; 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT01310894 (accessed on 26 April 2021).
- Trachtenberg, J. Study of WST09 in Prostate Cancer after Radiation: Repeat Procedure. STEBA France; 2010. Available online: https://clinicaltrials.gov/ct2/show/NCT00305929 (accessed on 26 April 2021).
- Trachtenberg, J. Phase II/III Study of WST09 in Prostate Cancer after Radiation Therapy. STEBA France; 2010. Available online: https://clinicaltrials.gov/ct2/show/NCT00312442 (accessed on 26 April 2021).
- Almeida, L. Photodynamic Therapy with LUZ11 in Advanced Head and Neck Cancer. Luzitin SA; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02070432 (accessed on 26 April 2021).
- Daniell, M.D.; Hill, J.S. A History of Photodynamic Therapy. ANZ J. Surg. 1991, 61, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The History of Photodetection and Photodynamic Therapy. Photochemistry Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Brancaleon, L.; Moseley, H.; Brancaleon, L.; Moseley, H. Laser and Non-laser Light Sources for Photodynamic Therapy. Lasers Med. Sci. 2002, 17, 173–186. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, W.; Zhang, T.; Jiang, X.; Hu, Y. Hybrid nanoparticle composites applied to photodynamic therapy: Strategies and applications. J. Mater. Chem. B 2020, 8, 4726–4737. [Google Scholar] [CrossRef]
- Fukumura, D.; Jain, R.K. Tumor microenvironment abnormalities: Causes, consequences, and strategies to normalize. J. Cell. Biochem. 2006, 101, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A. Therapeutic targeting of the tumor microenvironment. Cancer Cell 2005, 7, 513–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bristow, R.G.; Hill, R.P.; Bristow, R.G.; Hill, R.P. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef]
- Semenza, G.L. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim. et Biophys. Acta (BBA) Bioenerg. 2016, 1863, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Protsiv, M.; Ley, C.; Lankester, J.; Hastie, T.; Parsonnet, J. Decreasing human body temperature in the United States since the Industrial Revolution. eLife 2020, 9. [Google Scholar] [CrossRef]
- Geneva, I.; Cuzzo, B.; Fazili, T.; Javaid, W. Normal Body Temperature: A Systematic Review. Open Forum Infect. Dis. 2019, 6, ofz032. [Google Scholar] [CrossRef]
- Liu, Z. Clinical effects of high frequency hyperthermia-assisted irinotecan chemotherapy on patients with middle and advanced colorectal cancer and its safety assessment. Oncol. Lett. 2018, 17, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Tempel, N.V.D.; Horsman, M.; Kanaar, R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int. J. Hyperth. 2016, 32, 446–454. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit Rev. Oncol Hematol. 2002, 116, 69–75. [Google Scholar] [CrossRef]
- Guillemin, P.; Gui, L.; Lorton, O.; Zilli, T.; Crowe, L.A.; Desgranges, S.; Montet, X.; Terraz, S.; Miralbell, R.; Salomir, R.; et al. Mild hyperthermia by MR-guided focused ultrasound in an ex vivo model of osteolytic bone tumour: Optimization of the spatio-temporal control of the delivered temperature. J. Transl. Med. 2019, 17, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotr, G.A.S. Essential Facts on the History of Hyperthermia and their Connections with Electromedicine. arXiv 2017, 12, 37–40. [Google Scholar]
- Szasz, A.; Iluri, N.; Szasz, O. Local Hyperthermia in Oncology—To Choose or not to Choose? In Hyperthermia; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Seynhaeve, A.; Amin, M.; Haemmerich, D.; van Rhoon, G.; Hagen, T.T. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug Deliv. Rev. 2020, 163–164, 125–144. [Google Scholar] [CrossRef]
- MacEwan, S.R.; Callahan, D.J.; Chilkoti, A. Stimulus-responsive macromolecules and nanoparticles for cancer drug delivery. Nanomedicine 2010, 5, 793–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matteucci, M.L.; Anyarambhatla, G.; Rosner, G.; Azuma, C.; Fisher, P.E.; Dewhirst, M.W.; Needham, D.; Thrall, D.E. Hyperthermia increases accumulation of technetium-99m-labeled liposomes in feline sarcomas. Clin. Cancer Res. 2000, 6, 3748–3755. [Google Scholar]
- Rao, W.; Deng, Z.-S.; Liu, J. A Review of Hyperthermia Combined with Radiotherapy/Chemotherapy on Malignant Tumors. Crit. Rev. Biomed. Eng. 2010, 38, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Oei, A.L.; Vriend, L.E.M.; Crezee, J.; Franken, N.A.P.; Krawczyk, P.M. Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat. Oncol. 2015, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, K.; Zaidi, S.F. Treating cancer with heat: Hyperthermia as promising strategy to enhance apoptosis. J. Pak. Med. Assoc. 2013, 63, 504–508. [Google Scholar]
- Lapin, N.A.; Krzykawska-Serda, M.; Dilliard, S.; Mackeyev, Y.; Serda, M.; Wilson, L.J.; Curley, S.A.; Corr, S.J. The effects of non-invasive radiofrequency electric field hyperthermia on biotransport and biodistribution of fluorescent [60]fullerene derivative in a murine orthotopic model of breast adenocarcinoma. J. Control. Release 2017, 260, 92–99. [Google Scholar] [CrossRef]
- Huang, S.H.; Yang, K.J.; Wu, J.C.; Chang, K.J.; Wang, S.M. Effects of hyperthermia on the cytoskeleton and focal adhesion proteins in a human thyroid carcinoma cell line. J. Cell. Biochem. 1999, 75, 327–337. [Google Scholar] [CrossRef]
- Eppink, B.; Krawczyk, P.M.; Stap, J.; Kanaar, R. Hyperthermia-induced DNA repair deficiency suggests novel therapeutic anti-cancer strategies. Int. J. Hyperth. 2012, 28, 509–517. [Google Scholar] [CrossRef]
- Bouwman, P.; Jonkers, J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat. Rev. Cancer 2012, 12, 587–598. [Google Scholar] [CrossRef]
- Frey, B.; Weiss, E.-M.; Rubner, Y.; Wunderlich, R.; Ott, O.J.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Old and new facts about hyperthermia-induced modulations of the immune system. Int. J. Hyperth. 2012, 28, 528–542. [Google Scholar] [CrossRef]
- Alanazi, F.K.; Radwan, A.A.; Alsarra, I. Biopharmaceutical applications of nanogold. Saudi Pharm. J. 2010, 18, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Guo, X.; Zhang, X.; Chen, S.; Wang, Y.; Chen, T.; Huang, G.; Gao, Y.; Tian, Z.; Yang, Z. Multifunctional phototheranostic nanomedicine for cancer imaging and treatment. Mater. Today Bio 2020, 5, 100035. [Google Scholar] [CrossRef]
- Academy, B.; Gomes, A.N.A.T.P.C.; Neves, M.G.P.M.S.; Qu, C.; Naturais, P. Cancer, Photodynamic Therapy and Porphyrin-Type Derivatives. An. da Acad. Bras. de Ciências 2018, 90, 993–1026. [Google Scholar]
- Ito, H.; Tamura, M.; Matsui, H.; Majima, H.J.; Indo, H.P.; Hyodo, I. Isoflavone intake inhibits the development of 7,12 dimethylbenz(a)anthracene(DMBA) induced mammary tumors in normal andovariectomized rats. J. Clin. Biochem. Nutr. 2014, 54, 31–38. [Google Scholar] [CrossRef]
- Master, A. Megan, L.; Anirban, S.G. Pustaka 1. J. Control. Release 2014, 168, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Qian, M.; Yao, Q.; Meng, Z.; Cui, H.; Zhang, L.; Li, Y.; Wu, S.; Wang, J.; Chen, Q.; et al. Synthetic infrared nano-photosensitizers with hierarchical zoom-in target-delivery functionalities for precision photodynamic therapy. J. Control. Release 2021, 334, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Yuan, A.; Xu, L.; Zhang, F.; Zhang, S.; Zhao, X.; Liu, G.; Chen, W.; Guo, H. Activatable Photodynamic Therapy for Prostate Cancer by NIR Dye/Photosensitizer Loaded Albumin Nanoparticles. J. Biomed. Nanotechnol. 2019, 15, 311–318. [Google Scholar] [CrossRef]
- Gas, P. Temperature Distribution of Human Tissue in Interstitial Microwave Hyperthermia. Przegląd Elektrotechniczny 2012, 88, 144–146. [Google Scholar]
- Petrova, N.V.; Velichko, A.K.; Razin, S.V.; Kantidze, O.L. Early S-phase cell hypersensitivity to heat stress. Cell Cycle 2016, 15, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavelsky, V.; Vais, O.; Piura, B.; Wolfson, M.; Rabinovich, A.; Fraifeld, V. The role of Hsp90 in cell response to hyperthermia. J. Therm. Biol. 2004, 29, 509–514. [Google Scholar] [CrossRef]
- Kurokawa, H.; Ito, H.; Terasaki, M.; Matsui, H. Hyperthermia enhances photodynamic therapy by regulation of HCP1 and ABCG2 expressions via high level ROS generation. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Caglar, E.; Dobrucali, A. Self-Expandable Metallic Stent Placement in the Palliative Treatment of Malignant Obstruction of Gastric Outlet and Duodenum. Clin. Endosc. 2013, 46, 59–64. [Google Scholar] [CrossRef]
- Robertson, C.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Moor, A.C. Signaling pathways in cell death and survival after photodynamic therapy. J. Photochem. Photobiol. B Biol. 2000, 57, 1–13. [Google Scholar] [CrossRef]
- Hwang, H.S.; Shin, H.; Han, J.; Na, K. Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J. Pharm. Investig. 2018, 48, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Musolino, C.; Gangemi, S.; Allegra, A.; Pioggia, G.; Tonacci, A.; Musolino, C.; Gangemi, S. Oxidative Stress and Photodynamic Therapy of Skin Cancers: Mechanisms, Challenges and Promising Developments. Antioxidants 2020, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, K.M.M.G.; Cordeiro, M.E.R.; Pereira, M.C.D.S.; Barbosa, D.; Pezzini, A.A.; Kerppers, I.I. Comparative study in photodynamic therapy using the same photosensitizer in tissue repair after second-degree burns in an experimental model. Lasers Dent. Sci. 2019, 4, 17–24. [Google Scholar] [CrossRef]
- Hou, C.-H.; Lin, F.-L.; Hou, S.-M.; Liu, J.-F. Hyperthermia Induces Apoptosis through Endoplasmic Reticulum and Reactive Oxygen Species in Human Osteosarcoma Cells. Int. J. Mol. Sci. 2014, 15, 17380–17395. [Google Scholar] [CrossRef] [Green Version]
- Christensen, T.; Wahl, A.; Smedshammer, L.; Christensen, T.; Wahl, A.; Smedshammer, L. Effects of haematoporphyrin derivative and light in combination with hyperthermia on cells in culture. Br. J. Cancer 1984, 50, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, P.; Schuetz, J.D. The role of ABCG2 and ABCB6 in porphyrin metabolism and cell survival. Curr. Pharm. Biotechnol. 2011, 12, 647–655. [Google Scholar] [CrossRef]
- Liang, L.; Bi, W.; Tian, Y. Autophagy in photodynamic therapy. Trop. J. Pharm. Res. 2016, 15, 885. [Google Scholar] [CrossRef] [Green Version]
- Oleinick, N.L. Initiation of Autophagy by Photodynamic Therapy David. Methods Enzym. 2009, 453, 1–16. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [Green Version]
- Moy, A.J.; Tunnell, J.W. Combinatorial immunotherapy and nanoparticle mediated hyperthermia. Adv. Drug Deliv. Rev. 2017, 114, 175–183. [Google Scholar] [CrossRef]
- Gordon, R.; Hines, J.; Gordon, D. Intracellular hyperthermia a biophysical approach to cancer treatment via intracellular temperature and biophysical alterations. Med. Hypotheses 1979, 5, 83–102. [Google Scholar] [CrossRef]
- Baronzio, G.; Parmar, G.; Baronzio, M.; Baronzio, G.; Parmar, G.; Baronzio, M. Overview of Methods for Overcoming Hindrance to Drug Delivery to Tumors, with Special Attention to Tumor Interstitial Fluid. Front. Oncol. 2015, 5, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher-Colombo, S.M.; Maas, A.L.; Yuan, M.; Busch, T.M. Photodynamic Therapy-Induced Angiogenic Signaling: Consequences and Solutions to Improve Therapeutic Response. Isr. J. Chem. 2012, 52, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Srivatsan, A.; Rao, K.V.R.; Chen, Y.; Wang, Y.; Batt, C.; Morgan, J.; Sen, A.; Repasky, E.; Pandey, R.K. Effect of hyperthermia on PDT and imaging. In Proceedings of the 12th World Congress of the International Photodynamic Association, Seattle, WA, USA, 11–15 June 2009. [Google Scholar] [CrossRef]
- Song, C.W.; Shakil, A.; Osborn, J.L.; Iwata, K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int. J. Hyperth. 2009, 25, 91–95. [Google Scholar] [CrossRef]
- Lv, Z.; Wei, H.; Li, Q.; Su, X.; Liu, S.; Zhang, K.Y.; Lv, W.; Zhao, Q.; Li, X.; Huang, W. Achieving efficient photodynamic therapy under both normoxia and hypoxia using cyclometalated Ru(ii) photosensitizer through type I photochemical process. Chem. Sci. 2017, 9, 502–512. [Google Scholar] [CrossRef] [Green Version]
- Hegyi, G.; Szigeti, G.P.; Szász, A. Hyperthermia versus Oncothermia: Cellular Effects in Complementary Cancer Therapy. Evid. Based Complement. Altern. Med. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Firczuk, M.; Nowis, D.; Golab, J. PDT-induced inflammatory and host responses. Photochem. Photobiol. Sci. 2011, 10, 653–663. [Google Scholar] [CrossRef]
- Gollnick, S.; Evans, S.S.; Baumann, H.; Owczarczak, B.; Maier, P.; Vaughan, L.; Wang, W.C.; Unger, E.; Henderson, B.W. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br. J. Cancer 2003, 88, 1772–1779. [Google Scholar] [CrossRef] [Green Version]
- Calderwood, S.K.; Thériault, J.R.; Gong, J. How is the immune response affected by hyperthermia and heat shock proteins? Int. J. Hyperth. 2005, 21, 713–716. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Geng, C.; Lin, T.; Wang, X.; Zhao, L.; Tang, J. Thermal ablation versus conventional regional hyperthermia has greater anti-tumor activity against melanoma in mice by upregulating CD4+ cells and enhancing IL-2 secretion. Prog. Nat. Sci. 2009, 19, 1699–1704. [Google Scholar] [CrossRef]
- Jolesch, A.; Elmer, K.; Bendz, H.; Issels, R.; Noessner, E. Hsp70, a messenger from hyperthermia for the immune system. Eur. J. Cell Biol. 2012, 91, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Taratula, O.; Dani, R.K.; Schumann, C.; Xu, H.; Wang, A.; Song, H.; Dhagat, P.; Taratula, O. Multifunctional nanomedicine platform for concurrent delivery of chemotherapeutic drugs and mild hyperthermia to ovarian cancer cells. Int. J. Pharm. 2013, 458, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Reginato, E.; Wolf, P.; Hamblin, M.R. Immune response after photodynamic therapy increases anti-cancer and anti-bacterial effects. World J. Immunol. 2014, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-G.; Mehta, K.; Cohen, P.; Guha, C. Hyperthermia on immune regulation: A temperature’s story. Cancer Lett. 2008, 271, 191–204. [Google Scholar] [CrossRef]
- Basu, S. Fever-like temperature induces maturation of dendritic cells through induction of hsp90. Int. Immunol. 2003, 15, 1053–1061. [Google Scholar] [CrossRef]
- Yan, X.; Xiu, F.; An, H.; Wang, X.; Wang, J.; Cao, X. Fever range temperature promotes TLR4 expression and signaling in dendritic cells. Life Sci. 2007, 80, 307–313. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Wakabayashi, T.; Yoshida, J.; Kobayashi, T.; Ito, A.; Shinkai, M.; Honda, H.; Wakabayashi, T.; et al. Augmentation of MHC class I antigen presentation via heat shock protein expression by hyperthermia. Cancer Immunol. Immunother. 2001, 50, 515–522. [Google Scholar] [CrossRef]
- Han, W.; Zhang, S.; Deng, R.; Du, Y.; Qian, J.; Zheng, X.; Xu, B.; Xie, Z.; Yan, F.; Tian, W. Self-assembled nanostructured photosensitizer with aggregation-induced emission for enhanced photodynamic anticancer therapy. Sci. China Mater. 2019, 63, 136–146. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.S.; Zheng, G. Liposomal nanostructures for photosensitizer delivery. Lasers Surg. Med. 2011, 43, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Seidi, K.; Neubauer, H.; Moriggl, R.; Jahanban-Esfahlan, R.; Javaheri, T. Tumor target amplification: Implications for nano drug delivery systems. J. Control. Release 2018, 275, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Chen, S.; Tiwari, S.; Shi, K.; Zhang, S.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jo, Y.-U.; Na, K. Photodynamic therapy with smart nanomedicine. Arch. Pharmacal Res. 2020, 43, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ambasta, R.K.; Sharma, A.; Kumar, P.; Ambasta, R.K.; Sharma, A.; Kumar, P. Nanoparticle mediated targeting of VEGFR and cancer stem cells for cancer therapy. Vasc. Cell 2011, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Eldar-Finkelman, H. Glycogen synthase kinase 3: An emerging therapeutic target. Trends Mol. Med. 2002, 8, 126–132. [Google Scholar] [CrossRef]
- Barańska, E.; Wiecheć-Cudak, O.; Rak, M.; Bienia, A.; Mrozek-Wilczkiewicz, A.; Krzykawska-Serda, M.; Serda, M. Interactions of a Water-Soluble Glycofullerene with Glucose Transporter 1. Analysis of the Cellular Effects on a Pancreatic Tumor Model. Nanomaterials 2021, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 1–40. [Google Scholar] [CrossRef]
- Ahirwar, S.; Mallick, S.; Bahadur, D. Photodynamic therapy using graphene quantum dot derivatives. J. Solid State Chem. 2020, 282, 121107. [Google Scholar] [CrossRef]
- Chen, J.; Wu, W.; Zhang, F.; Zhang, J.; Liu, H.; Zheng, J.; Guo, S.; Zhang, J. Graphene quantum dots in photodynamic therapy. Nanoscale Adv. 2020. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Lin, L.; Slatkin, D.N.; Dilmanian, F.A.; Vadas, T.M.; Smilowitz, H.M. Gold nanoparticle hyperthermia reduces radiotherapy dose. Nanomedicine 2014, 10, 1609–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levi-Polyachenko, N.; Jacob, R.; Day, C.; Kuthirummal, N. Chitosan wound dressing with hexagonal silver nanoparticles for hyperthermia and enhanced delivery of small molecules. Colloids Surfaces B Biointerfaces 2016, 142, 315–324. [Google Scholar] [CrossRef]
- Meneses-Brassea, B.P.; Borrego, E.A.; Blazer, D.S.; Sanad, M.F.; Pourmiri, S.; Gutierrez, D.A.; Varela-Ramirez, A.; Hadjipanayis, G.C.; El-Gendy, A.A. Ni-Cu Nanoparticles and Their Feasibility for Magnetic Hyperthermia. Nanomaterials 2020, 10, 1988. [Google Scholar] [CrossRef]
- Vetrone, F.; Naccache, R.; Zamarrón, A.; De La Fuente, A.J.; Sanz-Rodríguez, F.; Maestro, L.M.; Rodríguez, E.M.; Jaque, D.; Solé, J.G.; Capobianco, J.A. Temperature Sensing Using Fluorescent Nanothermometers. ACS Nano 2010, 4, 3254–3258. [Google Scholar] [CrossRef]
- Yagawa, Y.; Tanigawa, K.; Kobayashi, Y.; Yamamoto, M. Cancer immunity and therapy using hyperthermia with immunotherapy, radiotherapy, chemotherapy, and surgery. J. Cancer Metastasis Treat. 2017, 3, 218. [Google Scholar] [CrossRef]
- De Almeida, D.R.Q.; Terra, L.F.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Aroldi, F.; Lord, S.R.; Aroldi, F.; Lord, S.R. Window of opportunity clinical trial designs to study cancer metabolism. Br. J. Cancer 2019, 122, 45–51. [Google Scholar] [CrossRef]
- Vujaskovic, Z.; Song, C.W. Physiological mechanisms underlying heat-induced radiosensitization. Int. J. Hyperth. 2004, 20, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Tschoep-Lechner, K.E.; Milani, V.; Berger, F.; Dieterle, N.; Abdel-Rahman, S.; Salat, C.; Issels, R. Gemcitabine and cisplatin combined with regional hyperthermia as second-line treatment in patients with gemcitabine-refractory advanced pancreatic cancer. Int. J. Hyperth. 2012, 29, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Westermann, A.M.; Jones, E.L.; Schem, B.C.; Van Der Steen-Banasik, E.M.; Koper, P.; Mella, O.; Uitterhoeve, A.L.J.; De Wit, R.; Van Der Velden, J.; Burger, C.; et al. First results of triple-modality treatment combining radiotherapy, chemotherapy, and hyperthermia for the treatment of patients with Stage IIB, III, and IVA cervical carcinoma. Cancer. 2005, 104, 763–770. [Google Scholar] [CrossRef]
- Westermann, A.; Mella, O.; Van Der Zee, J.; Jones, E.L.; Van Der Steen-Banasik, E.; Koper, P.; Uitterhoeve, A.L.; De Wit, R.; Van Der Velden, J.; Burger, C.; et al. Long-term survival data of triple modality treatment of stage IIB–III–IVA cervical cancer with the combination of radiotherapy, chemotherapy and hyperthermia—An update. Int. J. Hyperth. 2012, 28, 549–553. [Google Scholar] [CrossRef]
- Weinberg, B.D.; Allison, R.R.; Sibata, C.; Parent, T.; Downie, G. Results of combined photodynamic therapy (PDT) and high dose rate brachytherapy (HDR) in treatment of obstructive endobronchial non-small cell lung cancer (NSCLC). Photodiagnosis Photodyn. Ther. 2010, 7, 50–58. [Google Scholar] [CrossRef]
- Zschaeck, S. Moderate Whole Body Hyperthermia for Patients Undergoing Re-irradiation for Head and Neck Cancer -Influence on the Tumor Microenvironment (GKH-TMM); Charite University: Berlin, Germany, 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03547388 (accessed on 26 April 2021).
- Rogasch, J.; Beck, M.; Stromberger, C.; Hofheinz, F.; Ghadjar, P.; Wust, P.; Budach, V.; Amthauer, H.; Tinhofer, I.; Furth, C.; et al. PET measured hypoxia and MRI parameters in re-irradiated head and neck squamous cell carcinomas: Findings of a prospective pilot study. F1000Research 2020, 9, 1350. [Google Scholar] [CrossRef] [PubMed]
- Zschaeck, S.; Weingärtner, J.; Ghadjar, P.; Wust, P.; Mehrhof, F.; Kalinauskaite, G.; Ehrhardt, V.H.; Hartmann, V.; Tinhofer, I.; Heiland, M.; et al. Fever range whole body hyperthermia for re-irradiation of head and neck squamous cell carcinomas: Final results of a prospective study. Oral Oncol. 2021, 116, 105240. [Google Scholar] [CrossRef] [PubMed]
- Senthil, M. Adjuvant Hyperthermic Intraperitoneal Chemotherapy for Locally Advanced Gastric Cancer. Loma Linda University; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02672865 (accessed on 26 April 2021).
- Vujaskovic, Z.; Inman, B.A. Mitomycin C with Hyperthermia and Intravesical Mitomycin C to Treat Recurrent Bladder Cancer. Mark Dewhirst; 2008. Available online: https://clinicaltrials.gov/ct2/show/NCT00734994 (accessed on 26 April 2021).
- Inman, B.A.; Stauffer, P.; Craciunescu, O.A.; Maccarini, P.F.; Dewhirst, M.W.; Vujaskovic, Z. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. Int. J. Hyperth. 2014, 30, 171–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, J.H. Intraperitoneal Hyperthermic Perfusion with Oxaliplatin in Treating Patients with Stage IV Peritoneal Cancer Due to Appendix Cancer or Colorectal Cancer. Wake Forest University Health Sciences; 2007. Available online: https://clinicaltrials.gov/ct2/show/NCT00458809 (accessed on 26 April 2021).
- Iv, J.H.S.; Shen, P.; Russell, G.; Rn, J.F.; Bs, L.M.; Ms, F.M.C.; Levine, K.; Jones, B.T.; Levine, E.A. A Phase I Trial of Oxaliplatin for Intraperitoneal Hyperthermic Chemoperfusion for the Treatment of Peritoneal Surface Dissemination from Colorectal and Appendiceal Cancers. Ann. Surg. Oncol. 2008, 15, 2137–2145. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, T.M. Hyperthermic Intraperitoneal Oxaliplatin for Peritoneal Malignancies. Masonic Cancer Center, University of Minnesota; 2008. Available online: https://clinicaltrials.gov/ct2/show/NCT00625092 (accessed on 26 April 2021).
- Schrump, D.S. Paclitaxel and Hyperthermic Perfusion in Treating Patients with Lung Cancer or Lung Metastases That Cannot Be Re moved By Surgery; NCT00020007 National Cancer Institute (NCI): Bethesda, MD, USA, 2003. Available online: https://clinicaltrials.gov/ct2/show/NCT00020007 (accessed on 26 April 2021).
- Middleton, M.R. Targeted Chemotherapy Using Focused Ultrasound for Liver Tumours (TARDOX). University of Oxford; 2014. Available online: https://clinicaltrials.gov/ct2/show/NCT02181075 (accessed on 26 April 2021).
- Lyon, P.C.; Griffiths, L.F.; Lee, J.; Chung, D.; Carlisle, R.; Wu, F.; Middleton, M.R.; Gleeson, F.V.; Coussios, C.C. Clinical trial protocol for TARDOX: A phase I study to investigate the feasibility of targeted release of lyso-thermosensitive liposomal doxorubicin (ThermoDox®) using focused ultrasound in patients with liver tumours. J. Ther. Ultrasound 2017, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gray, M.D.; Lyon, P.C.; Mannaris, C.; Folkes, L.K.; Stratford, M.; Campo, L.; Chung, D.Y.F.; Scott, S.; Anderson, M.; Goldin, R.; et al. Focused Ultrasound Hyperthermia for Targeted Drug Release from Thermosensitive Liposomes: Results from a Phase I Trial. Radiology 2019, 291, 232–238. [Google Scholar] [CrossRef]
- Lyon, P.C.; Gray, M.D.; Mannaris, C.; Folkes, L.K.; Stratford, M.; Campo, L.; Chung, D.Y.F.; Scott, S.; Anderson, M.; Goldin, R.; et al. Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): A single-centre, open-label, phase 1 trial. Lancet Oncol. 2018, 19, 1027–1039. [Google Scholar] [CrossRef] [Green Version]
- Dose Escalation of Cisplatin Hyperthermic Intraperitoneal Chemotherapy after Surgery in Patients with Unresec-table Stage IIIC Ovarian, Tube or Peritoneal Primary Adenocarcinoma (CHIPASTIN). Gustave Roussy, Cancer Campus, Grand Paris; 2014. Available online: https://clinicaltrials.gov/ct2/show/NCT02217956 (accessed on 26 April 2021).
- Gouy, S.; Ferron, G.; Glehen, O.; Bayar, A.; Marchal, F.; Pomel, C.; Quenet, F.; Bereder, J.; Le Deley, M.-C.; Morice, P. Results of a multicenter phase I dose-finding trial of hyperthermic intraperitoneal cisplatin after neoadjuvant chemotherapy and complete cytoreductive surgery and followed by maintenance bevacizumab in initially unresectable ovarian cancer. Gynecol. Oncol. 2016, 142, 237–242. [Google Scholar] [CrossRef]
- Ou, J. Safety and Efficacy of Vitamin C Infusion in Combination with Local mEHT to Treat. Non Small Cell Lung Cancer (VCONSCLC); Clifford Hospital: Guangzhou, China, 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02655913 (accessed on 26 April 2021).
- Ou, J.; Zhu, X.; Lu, Y.; Zhao, C.; Zhang, H.; Wang, X.; Gui, X.; Wang, J.; Zhang, X.; Zhang, T.; et al. The safety and pharmacokinetics of high dose intravenous ascorbic acid synergy with modulated electrohyperthermia in Chinese patients with stage III-IV non-small cell lung cancer. Eur. J. Pharm. Sci. 2017, 109, 412–418. [Google Scholar] [CrossRef]
- Ou, J.; Zhu, X.; Chen, P.; Du, Y.; Lu, Y.; Peng, X.; Bao, S.; Wang, J.; Zhang, X.; Zhang, T.; et al. A randomized phase II trial of best supportive care with or without hyperthermia and vitamin C for heavily pretreated, advanced, refractory non-small-cell lung cancer. J. Adv. Res. 2020, 24, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, K.L. Temperature-Sensitive Liposomal Doxorubicin and Hyperthermia in Treating Women with Locally Recurrent Breast Cancer. Duke University; 2006. Available online: https://clinicaltrials.gov/ct2/show/NCT00346229 (accessed on 26 April 2021).
- Borys, N. Phase 1/2 Study of ThermoDox with Approved Hyperthermia in Treatment of Breast Cancer Recurrence at the Chest Wall (DIGNITY). Celsion; 2009. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00826085 (accessed on 26 April 2021).
- Zagar, T.M.; Vujaskovic, Z.; Formenti, S.; Rugo, H.; Muggia, F.; O’Connor, B.; Myerson, R.; Stauffer, P.; Hsu, I.-C.; Diederich, C.; et al. Two phase I dose-escalation/pharmacokinetics studies of low temperature liposomal doxorubicin (LTLD) and mild local hyperthermia in heavily pretreated patients with local regionally recurrent breast cancer. Int. J. Hyperth. 2014, 30, 285–294. [Google Scholar] [CrossRef]
- Deraco, M. Adjuvant HIPEC to Prevent Colorectal Peritoneal Metastases in High-risk Patients; Fondazione IRCCS Istituto Nazionale dei Tumori: Milano, Italy, 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02575859 (accessed on 26 April 2021).
- Baratti, D.; Kusamura, S.; Iusco, D.; Gimondi, S.; Pietrantonio, F.; Milione, M.; Guaglio, M.; Bonomi, S.; Grassi, A.; Virzì, S.; et al. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) at the Time of Primary Curative Surgery in Patients with Colorectal Cancer at High Risk for Metachronous Peritoneal Metastases. Ann. Surg. Oncol. 2016, 24, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Treatment of Primary Peritoneal Carcinosis of Digestive Origin Using Cytoreductive Surgery and Hyperthermic Intraoperative Peritoneal Chemotherapy with Mitomycin C and Irinotecan. Hospices Civils de Lyon; 2010. Available online: https://clinicaltrials.gov/ct2/show/NCT01226550 (accessed on 26 April 2021).
- Jones, E.L.; Vujaskovic, Z. MRI Sarcoma Non Invasive Thermometry. Mark Dewhirst; 2004. Available online: https://clinicaltrials.gov/ct2/show/NCT00093509 (accessed on 26 April 2021).
- Craciunescu, O.I.; Stauffer, P.R.; Soher, B.J.; Wyatt, C.R.; Arabe, O.; Maccarini, P.; Das, S.K.; Cheng, K.-S.; Wong, T.Z.; Jones, E.L.; et al. Accuracy of real time noninvasive temperature measurements using magnetic resonance thermal imaging in patients treated for high grade extremity soft tissue sarcomas. Med. Phys. 2009, 36, 4848–4858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerger, J.; Goffin, F. Feasibility Study of HIPEC for Patients with Stage III or Only Pleural Stage IV Ovarian Carcinoma in First Line Therapy. Jules Bordet Institute; 2012. Available online: https://clinicaltrials.gov/ct2/show/NCT01709487 (accessed on 26 April 2021).
- D’Hondt, V.; Goffin, F.; Roca, L.L.; Dresse, D.D.; Leroy, C.; Kerger, J.; Cordier, L.L.; De Forges, H.H.; Veys, I.; Liberale, G. Interval Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in First-Line Treatment for Advanced Ovarian Carcinoma: A Feasibility Study. Int. J. Gynecol. Cancer 2016, 26, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B. Radical Colorectal Resection and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Locally Advanced Colorectal Cancer. Wuhan University; 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02830139 (accessed on 26 April 2021).
- Xiong, B. Radical Gastrectomy and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Locally Advanced Gastric Cancer. Wuhan University; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02528110 (accessed on 26 April 2021).
- Preoperative Radiochemotherapy with Hyperthermia for Locally Advanced Rectal Cancer (HT01). University Hospital Tuebingen; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02353858 (accessed on 26 April 2021).
- Gani, C.; Lamprecht, U.; Ziegler, A.; Moll, M.; Gellermann, J.; Heinrich, V.; Wenz, S.; Fend, F.; Königsrainer, A.; Bitzer, M.; et al. Deep regional hyperthermia with preoperative radiochemotherapy in locally advanced rectal cancer, a prospective phase II trial. Radiother. Oncol. 2021, 159, 155–160. [Google Scholar] [CrossRef]
- Badgwell, B. Laparoscopic Hyperthermic Intraperitoneal Chemoperfusion (HIPEC) for Metastatic Gastric Cancer. M.D. Anderson Cancer Center; 2014. Available online: https://clinicaltrials.gov/ct2/show/NCT02092298 (accessed on 26 April 2021).
- Badgwell, B.; Blum, M.; Das, P.; Estrella, J.; Wang, X.; Fournier, K.; Royal, R.; Mansfield, P.; Ajani, J. Lessons learned from a phase II clinical trial of laparoscopic HIPEC for gastric cancer. Surg. Endosc. 2017, 32, 512. [Google Scholar] [CrossRef] [Green Version]
- Badgwell, B.; Blum, M.; Das, P.; Estrella, J.; Wang, X.; Ho, L.; Fournier, K.; Royal, R.; Mansfield, P.; Ajani, J. Phase II Trial of Laparoscopic Hyperthermic Intraperitoneal Chemoperfusion for Peritoneal Carcinomatosis or Positive Peritoneal Cytology in Patients with Gastric Adenocarcinoma. Ann. Surg. Oncol. 2017, 24, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, I.D. Hyperthermia Plus Radiation Therapy in Treating Patients with Nonmetastatic Advanced Prostate Cancer. Dana-Farber Cancer Institute; 2003. Available online: https://clinicaltrials.gov/ct2/show/NCT00003045 (accessed on 26 April 2021).
- Hurwitz, M.D.; Ms, J.L.H.; Prokopios-Davos, S.; Manola, J.; Wang, Q.; Bornstein, B.A.; Hynynen, K.; Kaplan, I.D. Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer. Cancer 2010, 117, 510–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes-Jordan, A. Pilot Study of Hyperthermic Peritoneal Perfusion (HIPEC) for Adolescent and Young Adults with Desmoplastic Small Round Cell Tumor. M.D. Anderson Cancer Center; 2011. Available online: https://clinicaltrials.gov/ct2/show/NCT01277744 (accessed on 26 April 2021).
- Hayes-Jordan, A.A.; Coakley, B.A.; Green, H.L.; Xiao, L.; Fournier, K.F.; Herzog, C.E.; Ludwig, J.A.; McAleer, M.F.; Anderson, P.M.; Huh, W.W. Desmoplastic Small Round Cell Tumor Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Results of a Phase 2 Trial. Ann. Surg. Oncol. 2018, 25, 872–877. [Google Scholar] [CrossRef]
- Mahteme, H. Neo-adjuvant Chemo + Peritonectomy + Hyperthermic Intraperitoneal Chemo in Peritoneal Carcinomatosis From Gastric Cancer. Uppsala University; 2011. Available online: https://clinicaltrials.gov/ct2/show/NCT01379482 (accessed on 26 April 2021).
- Hultman, B.; Lind, P.; Glimelius, B.; Sundbom, M.; Nygren, P.; Haglund, U.; Mahteme, H. Phase II study of patients with peritoneal carcinomatosis from gastric cancer treated with preoperative systemic chemotherapy followed by peritonectomy and intraperitoneal chemotherapy. Acta Oncol. 2012, 52, 824–830. [Google Scholar] [CrossRef] [Green Version]
- In, H. Single Arm Study Treating Patients of Peritoneal Surface Malignancy (Colorectal, Appendical, Pseudomyxoma, Gastric) with Cytoreductive Surgery and Hyperthermic Intraperitoneal Mitomycin-C. Albert Einstein College of Medicine; 2014. Available online: https://clinicaltrials.gov/ct2/show/NCT02040142 (accessed on 26 April 2021).
- Davis, J.L. Surgery and Heated Chemotherapy for Adrenocortical Carcinoma. National Cancer Institute (NCI); 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT01833832 (accessed on 26 April 2021).
- Hughes, M.S.; Lo, W.M.; Beresnev, T.; Merino, M.; Shutack, Y.; Ripley, R.T.; Hernandez, J.M.; Davis, J.L. A Phase II Trial of Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy for Recurrent Adrenocortical Carcinoma. J. Surg. Res. 2018, 232, 383–388. [Google Scholar] [CrossRef]
- Shen, P. Thalidomide in Treating Patients Who Have Undergone Surgery and Chemotherapy for Cancer That Has Spread Throughout the Abdomen Due to Colorectal Cancer or Appendix Cancer. Wake Forest University Health Sciences; 2006. Available online: https://clinicaltrials.gov/ct2/show/NCT00310076 (accessed on 26 April 2021).
- Shen, P.; Thomas, C.R.; Fenstermaker, J.; Aklilu, M.; McCoy, T.P.; Levine, E.A. Phase II trial of adjuvant oral thalidomide following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface disease from colorectal/appendiceal cancer. J. Gastrointest. Cancer 2014, 45, 268–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robins, H.I. Whole-Body Hyperthermia Plus Chemotherapy in Treating Patients with Advanced Sarcoma; University of Wisconsin: Madison, WI, USA. Available online: https://clinicaltrials.gov/ct2/show/NCT00002974 (accessed on 26 April 2021).
- Robins, H.I. Melphalan and Whole-Body Hyperthermia in Treating Patients with Advanced Melanoma; University of Wisconsin: Madison, WI, USA, 2004. Available online: https://clinicaltrials.gov/ct2/show/NCT00002973 (accessed on 26 April 2021).
- Park, S.-Y. Intraoperative Hyperthermic Intraperitoneal Chemotherapy with Ovarian Cancer; National Cancer Center: Korea, 2010. Available online: https://clinicaltrials.gov/ct2/show/NCT01091636 (accessed on 26 April 2021).
- Lim, M.C.; Chang, S.-J.; Yoo, H.J.; Nam, B.-H.; Bristow, R.; Park, S.-Y. Randomized trial of hyperthermic intraperitoneal chemotherapy (HIPEC) in women with primary advanced peritoneal, ovarian, and tubal cancer. J. Clin. Oncol. 2017, 35, 5520. [Google Scholar] [CrossRef]
- Żółciak-Siwińska, A. Hyperthermia Combined Brachytherapy in CCU. Maria Sklodowska-Curie Institute—Oncology Center; 2011. Available online: https://clinicaltrials.gov/ct2/show/NCT01474356 (accessed on 26 April 2021).
- Żółciak-Siwińska, A.; Piotrkowicz, N.; Jonska-Gmyrek, J.; Nicke-Psikuta, M.; Michalski, W.; Kawczyńska, M.; Bijok, M.; Bujko, K. HDR brachytherapy combined with interstitial hyperthermia in locally advanced cervical cancer patients initially treated with concomitant radiochemotherapy—A phase III study. Radiother. Oncol. 2013, 109, 194–199. [Google Scholar] [CrossRef]
- Quenet, F. Systemic Chemotherapy with or without Intraperitoneal Chemohyperthermia in Treating Patients Undergoing Surgery for Peritoneal Carcinomatosis From Colorectal Cancer. UNICANCER; 2008. Available online: https://clinicaltrials.gov/ct2/show/NCT00769405 (accessed on 26 April 2021).
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- van Driel, W.J. Secondary Debulking Surgery +/- Hyperthermic Intraperitoneal Chemotherapy in Stage III Ovarian Cancer. The Netherlands Cancer Institute; 2007. Available online: https://clinicaltrials.gov/ct2/show/NCT00426257 (accessed on 26 April 2021).
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Van Leeuwen, J.H.S.; Schreuder, H.W.; Hermans, R.H.; De Hingh, I.H.; Van Der Velden, J.; Arts, H.J.; Massuger, L.F.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Koole, S.N.; Van Lieshout, C.; Van Driel, W.J.; Van Schagen, E.; Sikorska, K.; Kieffer, J.M.; Van Leeuwen, J.H.S.; Schreuder, H.W.; Hermans, R.H.; De Hingh, I.H.; et al. Cost Effectiveness of Interval Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy in Stage III Ovarian Cancer on the Basis of a Randomized Phase III Trial. J. Clin. Oncol. 2019, 37, 2041–2050. [Google Scholar] [CrossRef]
- Van Driel, W.; Sikorska, K.; Van Leeuwen, J.S.; Schreuder, H.; Hermans, R.; De Hingh, I.; Van Der Velden, J.; Arts, H.J.; Leon, M.; Aalbers, A.; et al. A phase 3 trial of hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer. J. Clin. Oncol. 2017, 35, 5519. [Google Scholar] [CrossRef]
- Issels, R.D. Combination Chemotherapy with or without Hyperthermia Therapy in Treating Patients with Soft Tissue Sarcoma. European Organisation for Research and Treatment of Cancer—EORTC; 2003. Available online: https://clinicaltrials.gov/ct2/show/NCT00003052 (accessed on 26 April 2021).
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients with Localized High-Risk Soft Tissue Sarcoma. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Knösel, T.; Altendorf-Hofmann, A.; Lindner, L.; Issels, R.; Hermeking, H.; Schuebbe, G.; Gibis, S.; Siemens, H.; Kampmann, E.; Kirchner, T. Loss of p16(INK4a) is associated with reduced patient survival in soft tissue tumours, and indicates a senescence barrier. J. Clin. Pathol. 2014, 67, 592–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, I.C. Pilot Study of a Catheter-Based Ultrasound Hyperthermia System; University of California: San Francisco, CA, USA, 2009. Available online: https://clinicaltrials.gov/ct2/show/NCT00911079 (accessed on 26 April 2021).
- Diederich, C.; Wootton, J.; Prakash, P.; Salgaonkar, V.; Juang, T.; Scott, S.; Chen, X.; Cunha, A.; Pouliot, J.; Hsu, I.C.; et al. A Pilot Study of Catheter-Based Ultrasound Hyperthermia with HDR Brachytherapy for Treatment of Locally Advanced Cancer of the Prostate and Cervix. AIP Conf. Proc. 1359 2011, 195–199. [Google Scholar] [CrossRef]
- Demirtas, A. Intravesical Thermochemotherapy with Mitomycinc. TC Erciyes University; 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03694535 (accessed on 26 April 2021).
- Demirtaş, A.; Tombul, T.; Sönmez, G.; Demirleğen, A.; Demirtaş, T.; Tatlışen, A. Intravesical Mitomycin-C with Bladder Wall Hyperthermia in Intermediate and High-risk Non-muscle Invasive Bladder Cancers: Prospective Clinical Trial with a Singletreatment Arm. Bull. Urooncology 2020, 19, 182–185. [Google Scholar] [CrossRef]
- Marsh, M. Photodynamic Therapy and Vismodegib for Multiple Basal Cell Carcinomas (PDT-Vismo). University of Arizona; 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02639117 (accessed on 26 April 2021).
- Rizzo, J.M.; Segal, R.J.; Zeitouni, N.C. Combination vismodegib and photodynamic therapy for multiple basal cell carcinomas. Photodiagnosis Photodyn. Ther. 2018, 21, 58–62. [Google Scholar] [CrossRef]
- DeWitt, J.M. Ultrasound-Guided Photodynamic Therapy with Photofrin & Gemcitabine for Patients with Locally Advanced Pancreatic Cancer. 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT01770132 (accessed on 26 April 2021).
- DeWitt, J.M.; Sandrasegaran, K.; O’Neil, B.; House, M.G.; Zyromski, N.J.; Sehdev, A.; Perkins, S.M.; Flynn, J.; McCranor, L.; Shahda, S. Phase 1 study of EUS-guided photodynamic therapy for locally advanced pancreatic cancer. Gastrointest. Endosc. 2019, 89, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Nwogu, C. Photodynamic Therapy During Surgery in Treating Patients with Non-small Cell Lung Cancer That Can Be Removed by Surgery. Roswell Park Cancer Institute; 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT01854684 (accessed on 26 April 2021).
- Arshad, H. Photodynamic Therapy Using HPPH in Treating Patients Undergoing Surgery for Primary or Recurrent Head and Neck Cancer. Roswell Park Cancer Institute; 2007. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00470496 (accessed on 26 April 2021).
- Rigual, N.R.; Shafirstein, G.; Frustino, J.; Seshadri, M.; Cooper, M.; Wilding, G.; Sullivan, M.A.; Henderson, B. Adjuvant Intraoperative Photodynamic Therapy in Head and Neck Cancer. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 706. [Google Scholar] [CrossRef]
- Loewen, G.M. Photodynamic Therapy Plus Brachytherapy in Treating Patients with Lung Cancer. Roswell Park Cancer Institute; 2004. Available online: https://clinicaltrials.gov/ct2/show/NCT00014066 (accessed on 26 April 2021).
- Wang, K.K. Endoscopic Therapy of Early Cancer in Barretts Esophagus. Mayo Clinic; 2005. Available online: https://clinicaltrials.gov/ct2/show/NCT00217087 (accessed on 26 April 2021).
- Demmy, T.L. Surgery and Photodynamic Therapy in Treating Patients with Malignant Mesothelioma. Roswell Park Cancer Institute; 2003. Available online: https://clinicaltrials.gov/ct2/show/NCT00054002 (accessed on 26 April 2021).
- Oseroff, A.R. Photodynamic Therapy in Treating Patients with Skin Cancer. Roswell Park Cancer Institute; 2003. Available online: https://clinicaltrials.gov/ct2/show/NCT00002963 (accessed on 26 April 2021).
- Park, D.H. S-1 and Photodynamic Therapy in Cholangiocarcinoma. Asan Medical Center, 209AD. Available online: https://clinicaltrials.gov/ct2/show/NCT00869635 (accessed on 26 April 2021).
- Park, D.H.; Lee, S.S.; Park, S.E.; Lee, J.L.; Choi, J.H.; Choi, H.J.; Jang, J.W.; Kim, H.J.; Eum, J.B.; Seo, D.-W.; et al. Randomised phase II trial of photodynamic therapy plus oral fluoropyrimidine, S-1, versus photodynamic therapy alone for unresectable hilar cholangiocarcinoma. Eur. J. Cancer 2014, 50, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Krzykawska-Serda, M.; Agha, M.S.; Ho, J.C.-S.; Ware, M.J.; Law, J.J.; Newton, J.M.; Nguyen, L.; Curley, S.A.; Corr, S.J. Chemotherapy and Radiofrequency-Induced Mild Hyperthermia Combined Treatment of Orthotopic Pancreatic Ductal Adenocarcinoma Xenografts. Transl. Oncol. 2018, 11, 664–671. [Google Scholar] [CrossRef]
- Krzykawska-Serda, M.; Ho, J.C.-S.; Ware, M.J.; Law, J.J.; Newton, J.M.; Nguyen, L.; Agha, M.; Curley, S.A.; Corr, S.J. Ultrasound Doppler as an Imaging Modality for Selection of Murine 4T1 Breast Tumors for Combination Radiofrequency Hyperthermia and Chemotherapy. Transl. Oncol. 2018, 11, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Brodin, N.P.; Guha, C.; Tomé, W.A. Photodynamic Therapy and Its Role in Combined Modality Anticancer Treatment. Technol. Cancer Res. Treat. 2014, 14, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.; Liu, T.; Chen, J.; Zheng, G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Sharma, P.K.; Malviya, R. Hyperthermia: Role and Risk Factor for Cancer Treatment. Achiev. Life Sci. 2016, 10, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.; Ortel, B.J.; Pereira, S.; Hasan, T.; Maytin, E.V. Biomodulatory approaches to photodynamic therapy for solid tumors. Cancer Lett. 2012, 326, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; Ter Haar, G.; Wust, P.; Crezee, J. Heating technology for malignant tumors: A review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef]
- Yanovsky, R.L.; Bartenstein, D.W.; Rogers, G.S.; Isakoff, S.J.; Chen, S.T. Photodynamic therapy for solid tumors: A review of the literature. Photodermatol. Photoimmunol. Photomed. 2019, 35, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosst, R.A.; Linnemans, W.A.M. The effects of hyperthermia on the cytoskeleton. Int. J. Hyperth. 1996, 12, 173–196. [Google Scholar] [CrossRef]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res. 2000, 60, 4440–4445. [Google Scholar] [PubMed]
- Mokwena, M.G.; Kruger, C.A.; Ivan, M.-T.; Heidi, A. A review of nanoparticle photosensitizer drug delivery uptake systems for photodynamic treatment of lung cancer. Photodiagnosis Photodyn. Ther. 2018, 22, 147–154. [Google Scholar] [CrossRef]
- Crescenzi, E.; Chiaviello, A.; Canti, G.; Reddi, E.; Veneziani, B.M.; Palumbo, G. Low doses of cisplatin or gemcitabine plus Photofrin/photodynamic therapy: Disjointed cell cycle phase-related activity accounts for synergistic outcome in metastatic non–small cell lung cancer cells (H1299). Mol. Cancer Ther. 2006, 5, 776–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, Y.-W.; Chi, K.-H.; Huang, C.-C.; Chi, M.-S.; Chiang, H.-C.; Yang, K.-L.; Li, W.-T.; Wang, Y.-S. Modulated electro-hyperthermia-enhanced liposomal drug uptake by cancer cells. Int. J. Nanomed. 2019, 14, 1269–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Xie, P.; Ye, C.; Wu, C.; Han, W.; Huang, M.; Wang, S.; Chen, H. Outside-in synthesis of mesoporous silica/molybdenum disulfide nanoparticles for antitumor application. Chem. Eng. J. 2018, 351, 157–168. [Google Scholar] [CrossRef]
- Markezana, A.; Ahmed, M.; Kumar, G.; Zorde-Khvalevsky, E.; Rozenblum, N.; Galun, E.; Goldberg, S.N.; Markezana, A.; Ahmed, M.; Kumar, G.; et al. Moderate hyperthermic heating encountered during thermal ablation increases tumor cell activity. Int. J. Hyperth. 2020, 37, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Wagnieres, G.; Bergh, H.V.D.; Griffioen, A.W. Angiostasis-induced vascular normalization can improve photodynamic therapy. Cell. Mol. Life Sci. 2010, 67, 1559–1560. [Google Scholar] [CrossRef] [Green Version]
- Fabbrini, M.; Trachsel, E.; Soldani, P.; Bindi, S.; Alessi, P.; Bracci, L.; Kosmehl, H.; Zardi, L.; Neri, D.; Neri, P. Selective occlusion of tumor blood vessels by targeted delivery of an antibody-photosensitizer conjugate. Int. J. Cancer 2005, 118, 1805–1813. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.-Y.; Shiau, A.-L.; Chen, Y.-H.; Chang, C.-J.; Chen, H.H.-W.; Wu, C.-L. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci. 2008, 99, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, C.A.; Habib, A.H.; Miller, K.; Collier, K.N.; Ondeck, C.L.; McHenry, M.E. Modeling of temperature profile during magnetic thermotherapy for cancer treatment. J. Appl. Phys. 2009, 105, 7. [Google Scholar] [CrossRef]
- Cardinal, J.; Klune, J.R.; Chory, E.; Jeyabalan, G.; Kanzius, J.S.; Nalesnik, M.; Geller, D.A. Non-Invasive Radiofrequency Ablation of Cancer Targe. Surgery 2008, 144, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Kelleher, D.K.; Bastian, J.; Thews, O.; Vaupel, P.; Kelleher, D.K.; Bastian, J.; Thews, O.; Vaupel, P. Enhanced effects of aminolaevulinic acid-based photodynamic therapy through local hyperthermia in rat tumours. Br. J. Cancer 2003, 89, 405–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelleher, D.K.; Thews, O.; Scherz, A.; Salomon, Y.; Vaupel, P. Combined hyperthermia and chlorophyll-based photodynamic therapy: Tumour growth and metabolic microenvironment. Br. J. Cancer 2003, 89, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, K.; Zuo, S.; Xu, Y.; Zha, Z.; Ke, W.; Chen, H.; Ge, Z. Light-Triggered Clustered Vesicles with Self-Supplied Oxygen and Tissue Penetrability for Photodynamic Therapy against Hypoxic Tumor. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nat. Cell Biol. 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herskind, C.; Ma, L.; Liu, Q.; Zhang, B.; Schneider, F.; Veldwijk, M.R.; Wenz, F. Biology of high single doses of IORT: RBE, 5 R’s, and other biological aspects. Radiat. Oncol. 2017, 12, 24. [Google Scholar] [CrossRef] [Green Version]
- Hall, N.; Rouge, B. The physics of proton therapy. Phys. Med. Biol. 2015, 60. [Google Scholar] [CrossRef]
- Strojan, P. Role of radiotherapy in melanoma management. Radiol. Oncol. 2010, 44, 1–12. [Google Scholar] [CrossRef]
- Dewey, W.C. Arrhenius relationships from the molecule and cell to the clinic. Int. J. Hyperth. 2009, 25, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, A. Assessment of the Evolution of Cancer Treatment Therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [Green Version]
- Morgan, N.Y.; Kramer-Marek, G.; Smith, P.D.; Camphausen, K.; Capala, J. Nanoscintillator Conjugates as Photodynamic Therapy-Based Radiosensitizers: Calculation of Required Physical Parameters. Radiat. Res. 2009, 171, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Schaffer, M.; Ertl-Wagner, B.; Kulka, U.; Jori, G.; Duhmke, E.; Hofstetter, A.; Schaffer, P.M. The Application of Photofrin II® as a Sensitizing Agent for Ionizing Radiation-A New Approach in Tumor Therapy? Curr. Med. Chem. 2005, 12, 1209–1215. [Google Scholar] [CrossRef]
- Luksiene, Z.; Kalvelyte, A.; Supino, R. On the combination of photodynamic therapy with ionizing radiation. J. Photochem. Photobiol. B Biol. 1999, 52, 35–42. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2018, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Phung, D.C.; Nguyen, H.T.; Tran, T.T.P.; Jin, S.G.; Yong, C.S.; Truong, D.H.; Tran, T.H.; Kim, J.O. Combined hyperthermia and chemotherapy as a synergistic anticancer treatment. J. Pharm. Investig. 2019, 49, 519–526. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res. 1984, 44, 4721s–4730s. [Google Scholar] [PubMed]
- Issels, R.D. Hyperthermia adds to chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Averill-Bates, D.A. Thermotolerance induced at a fever temperature of 40 °C protects cells against hyperthermia-induced apoptosis mediated by death receptor signalling. Biochem. Cell Biol. 2008, 86, 521–538. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Scoggins, C.R.; Zorzi, D.; Abdalla, E.K.; Andres, A.; Eng, C.; Curley, S.A.; Loyer, E.M.; Muratore, A.; Mentha, G.; et al. Effect of Surgical Margin Status on Survival and Site of Recurrence After Hepatic Resection for Colorectal Metastases. Ann. Surg. 2005, 241, 715–724. [Google Scholar] [CrossRef]
- Hemming, A.W.; Reed, A.; Langham, M.; Fujita, S.; Howard, R.J. Combined Resection of the Liver and Inferior Vena Cava for Hepatic Malignancy. Ann. Surg. 2004, 239, 712–721. [Google Scholar] [CrossRef]
- Ware, M.; Nguyen, L.P.; Law, J.J.; Krzykawska-Serda, M.; Taylor, K.M.; Cao, H.S.T.; Anderson, A.O.; Pulikkathara, M.; Newton, J.M.; Ho, J.C.; et al. A new mild hyperthermia device to treat vascular involvement in cancer surgery. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, K.; Gibbins, S.; Moghissi, K. A Surgical View of Photodynamic Therapy in Oncology: A Review. Surg. J. 2015, 1, e1–e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghissi, K.; Stringer, M.; Dixon, K. Fluorescence photodiagnosis in clinical practice. Photodiagnosis Photodyn. Ther. 2008, 5, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Eljamel, M.S. Fluorescence image-guided surgery of brain tumors: Explained step-by-step. Photodiagnosis Photodyn. Ther. 2008, 5, 260–263. [Google Scholar] [CrossRef]
- Höckel, M.; Vaupel, P. Tumor Hypoxia: Definitions and Current Clinical, Biologic, and Molecular Aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Span, P.N.; Bussink, J. Biology of hypoxia. Semin. Nucl. Med. 2015, 45, 101–109. [Google Scholar] [CrossRef]
- Epel, B.; Maggio, M.C.; Barth, E.D.; Miller, R.C.; Pelizzari, C.A.; Krzykawska-Serda, M.; Sundramoorthy, S.V.; Aydogan, B.; Weichselbaum, R.R.; Tormyshev, V.M.; et al. Oxygen-Guided Radiation Therapy. Int. J. Radiat. Oncol. 2019, 103, 977–984. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Carnero, A.; Lleonart, M. The hypoxic microenvironment: A determinant of cancer stem cell evolution. Insid. Cell 2015, 1, 96–105. [Google Scholar] [CrossRef]
- Helmlinger, G.; Yuan, F.; Dellian, M.; Jain, R.K. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat. Med. 1997, 3, 177–182. [Google Scholar] [CrossRef]
- Elming, P.B.; Sørensen, B.S.; Oei, A.L.; Franken, N.A.; Crezee, J.; Overgaard, J.; Horsman, M.R. Hyperthermia: The Optimal Treatment to Overcome Radiation Resistant Hypoxia. Cancers 2019, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Sierra, H.; Cordova, M.; Chen, C.-S.J.; Rajadhyaksha, M. Confocal Imaging–Guided Laser Ablation of Basal Cell Carcinomas: An Ex Vivo Study. J. Investig. Dermatol. 2015, 135, 612–615. [Google Scholar] [CrossRef] [Green Version]
- Azad, M.; Chen, Y.; Henson, E.S.; Cizeau, J.; McMillan-Ward, E.; Israels, S.J.; Gibson, S.B. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy 2008, 4, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.G.; Shin, J.H.; Shim, H.S.; Lee, C.Y.; Kim, D.J.; Kim, Y.S.; Chung, K.Y. Autophagy contributes to the chemo-resistance of non-small cell lung cancer in hypoxic conditions. Respir. Res. 2015, 16, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harhaji-Trajkovic, L.; Vilimanovich, U.; Kravic-Stevovic, T.; Bumbasirevic, V.; Trajkovic, V. AMPK-mediated autophagy inhibits apoptosis in cisplatin-treated tumour cells. J. Cell. Mol. Med. 2009, 13, 3644–3654. [Google Scholar] [CrossRef] [PubMed]
- Govaert, K.M.; Emmink, B.L.; Nijkamp, M.W.; Cheung, Z.J.; Steller, E.J.A.; Fatrai, S.; de Bruijn, M.T.; Kranenburg, O.; Rinkes, I.H.M.B. Hypoxia After Liver Surgery Imposes an Aggressive Cancer Stem Cell Phenotype on Residual Tumor Cells. Ann. Surg. 2014, 259, 750–759. [Google Scholar] [CrossRef]
- Rey, S.; Schito, L.; Koritzinsky, M.; Wouters, B.G. Molecular targeting of hypoxia in radiotherapy. Adv. Drug Deliv. Rev. 2017, 109, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.J.; Richardson, R.A.; Dewhirst, M.W. Hypoxia and radiotherapy: Opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev. 2007, 26, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Nowis, D.; Golab, J.; Agostinis, P. Photodynamic therapy: Illuminating the road from cell death towards anti-tumour immunity. Apoptosis 2010, 15, 1050–1071. [Google Scholar] [CrossRef]
- Chen, B.; Roskams, T.; de Witte, P. Enhancing the antitumoral effect of hypericin-mediated photodynamic therapy by hyperthermia. Lasers Surg. Med. 2002, 31, 158–163. [Google Scholar] [CrossRef]


| Cancer | Combination Therapy Scheme | Trial Design | Clinical Stage | Median OS * (Months) | Median PFS * (Months) | ClinicalTrial.gov ID | Ref. |
|---|---|---|---|---|---|---|---|
| Non-small cell lung cancer stage III | Chemoradiotherapy and immunotherapy | cis-/carboplatin + vinorelbine + etoposide/pemetrexed, radiation, and Nivolumab | II stage | 38.8 | 12.7 | NCT02434081 [23] | [24,25] |
| Unresectable non-small cell lung cancer | Chemotherapy and radiotherapy | Vinorelbine, Cisplatin, and radiation | II stage | 35.6 | 11.5 | NCT02709720 [26] | [27] |
| Small cell lung cancer | Chemotherapy and immunotherapy | Paclitaxel and Pembrolizumab | II stage | 9.2 | 5.0 | NCT02551432 [28] | [29,30] |
| Non-small Cell Lung Cancer | Chemotherapy and immunotherapy | Carboplatin/Paclitaxel and SB8 (A proposed bevacizumab biosimilar) Carboplatin/Paclitaxel and Bevacizumab | III stage | 14.90 15.80 | 8.5 7.9 | NCT02754882 [31] | [32] |
| Non-small Cell Lung Cancer | Chemotherapy and immunotherapy | Nivolumab and Ipilimumab EGFR Nivolumab plus Ipilimumab ALK Experimental: Nivolumab + Carboplatin + Pemetrexed with EGFR chemo-naive Nivolumab + Carboplatin + Pemetrexed ALK chemo-naive | II stage | 22.3 7.6 7.75 5.9 | 1.3 0.7 4.65 2.8 | NCT03256136 [33] | — |
| Small cell lung cancer | Immunology and radiotherapy | Durvalumab and Tremelimumab Durvalumab and Tremelimumab and hypofractionated radiotherapy/stereotactic body radiation therapy | II stage | 2.8 5.7 | 2.1 3.3 | NCT02701400 [34] | [35] |
| Non-small cell lung cancer | Target therapy and Immunotherapy | Ensartinib and Durvalumab | I/II stage | — | — | NCT02898116 [36] | — |
| Non-small cell lung cancer | Surgery and immunotherapy | Cryosurgery and NK Immunotherapy | I/II stage | — | — | NCT02843815 [37] | [38]. |
| Lung neoplasms | Cryotherapy and target therapy | Cryotherapy and Icotinib | IV stage | — | — | NCT02744664 [39] | — |
| Non-small cell lung cancer | Chemotherapy and immunotherapy | Atezolizumab and Carboplatin and Paclitaxel (APC) Atezolizumab, Bevacizumab, Carboplatin, and Paclitaxel (ABCP) Bevacizumab, Carboplatin, and Paclitaxel (BCP) | III stage | In the ITT * population: ACP = 19.5 vs. ABCP = 19.8 vs. BCP = 14.9 | In the ITT * population: ACP = - vs. ABCP = 8.4 vs. BCP = 6.8 | NCT02366143 [40] | [41,42,43,44] |
| Small cell lung cancer | Chemotherapy and immunotherapy | Irinotecan and Dinutuximab | II/III stage | 6.9 | 3.5 | NCT03098030 [45] | [46] |
| Breast cancer | Chemotherapy and immunotherapy | Paclitaxel and Durvalumab | I/II stage | — | — | NCT02628132 [47] | — |
| Breast cancer | Chemotherapy and immunotherapy | Nab-paclitaxel and Durvalumab (MEDI4736) Epirubicin and Cyclophosphamide and Durvalumab (MEDI4736) | II stage | — | — | NCT02685059 [48] | [49] |
| Breast cancer | Chemotherapy and target therapy | Eribulin and PQR309 | I/II stage | — | — | NCT02723877 [50] | [51] |
| Breast cancer | Chemotherapy and immunotherapy | Docetaxel and carboplatin and trastuzumab Epirubicin and cyclophosphamide followed by docetaxel and trastuzumab | II stage | — | — | NCT03140553 [52] | [53] |
| Breast cancer | Chemotherapy and immunotherapy | Cyclophosphamide and Pembrolizumab | II stage | — | — | NCT03139851 [54] | — |
| Breast cancer | Surgery and immunotherapy | Cryosurgery and NK immunotherapy | I/II stage | — | — | NCT02844335 [55] | — |
| Breast cancer | Chemotherapy and immunotherapy | Non-pegylated liposomal Doxorubicin and Trastuzumab | I stage | — | 7.2 | NCT02562378 [56] | [57,58] |
| Breast cancer | Chemotherapy and immunotherapy | Docetaxel and Pertuzumab and Trastuzumab | IV stage | — | 23.0 | NCT02445586 [59] | — |
| Breast cancer | Chemotherapy and immunotherapy | Eribulin and Durvalumab | I stage | — | — | NCT03430518 [60] | [61] |
| Breast cancer | Chemotherapy and immunotherapy | Docetaxel and Pertuzumab (Perjeta) and Trastuzumab (Herceptin) | III stage | — | 18.7 | NCT02402712 [62] | [63] |
| Breast cancer | Target therapy and immunotherapy | Ibrutinib and Durvalumab | I/II stage | 4.2 | 1.7 | NCT02403271 [64] | [65] |
| Breast cancer | Hormone therapy and targeted therapy | Letrozole and Palbociclib | IV stage | — | — | NCT02679755 [66] | — |
| Metastatic Breast Cancer | Chemotherapy and target therapy | Paclitaxel and S81694 | I/II stage | — | — | NCT03411161 [67] | — |
| Breast cancer | Hormone therapy and targeted therapy | Letrozole and Nintedanib | I stage | — | — | NCT02619162 [68] | [69] |
| Breast cancer | Hormone therapy and targeted therapy | Letrozole and Ribociclib | II stage | — | — | NCT03248427 [70] | [71] |
| Breast Cancer | Chemotherapy and immunotherapy and hormone therapy | Epirubicin, Cyclophosphamide, Nivolumab, Triptorelin, Exemestane | II stage | — | — | NCT04659551 [72] | — |
| Breast Cancer Bone-dominant metastatic breast cancer | Immunotherapy and hormone therapy and radiopharmaceutical drug | Denosumab and Tamoxifen/Fulvestrant and Ra-223 dichloride | II stage | — | 7.4 or 16 (bone-dominant metastases) | NCT02366130 [73] | [74] |
| Estrogen receptor positive breast cancer | Hormone therapy and targeted therapy | Tamoxifen and TAK-228 | II stage | — | — | NCT02988986 [75] | [76,77] |
| Breast cancer | Chemotherapy and immunotherapy | Docetaxel and Pertuzumab and Trastuzumab | III stage | NA | 14.5 | NCT02896855 [78] | [79] |
| Prostate cancer | Hormone therapy and immunotherapy | Abiraterone and TRC105 Enzalutamide and TRC105 | II stage | — | — | NCT03418324 [80] | |
| Prostate cancer | Immunotherapy and surgery | huJ591 and 89Zr-J591 and radical prostatectomy | I stage | — | — | NCT02693860 [81] | — |
| Castration resistant prostate cancer | Chemotherapy and cryoimmunotherapy and immunotherapy | Cyclophosphamide and Dendritic cell-based cryoimmunotherapy and Ipilimumab | I stage | — | 5 (150 days) | NCT02423928 [82] | [83] |
| Prostate cancer | Hormone therapy and targeted therapy | Enzalutamide and LY3023414 | II stage | — | 7.5 | NCT02407054 [84] | [85] |
| Castrate-resistant prostate cancer | Immunotherapy and radiopharmaceutical drug | Atezolizumab and Radium-223 Dichloride | I stage | 16.3 | 3.0 | NCT02814669 [86] | [87] |
| Prostate cancer | Hormone therapy and surgery | Apalutamide and Radical Prostatectomy | II stage | — | — | NCT03124433 [88] | — |
| Prostate cancer | Hormone therapy and target therapy | Prednisone and Apalutamide/Abiraterone Acetate and Niraparib | I stage | — | — | NCT02924766 [89] | [90,91] |
| Prostate carcinoma metastatic to the bone | Hormone therapy and radiopharmaceutical drug | Enzalutamide and Radium-223 Dichloride | II stage | — | — | NCT02507570 [92] | [93] |
| Prostate cancer | Hormone therapy and target therapy and radiotherapy | Leuprolide acetate/Goserelin acetate/Degarelix, PLX3397, Radiation Therapy | I stage | — | — | NCT02472275 [94] | — |
| Colon cancer | Chemotherapy and immunotherapy | TAS-102 (Trifluridine/tipiracil) and Panitumumab | I/II stage | — | 5.8 | NCT02613221 [95] | [96,97] |
| Colorectal cancer | Chemotherapy and target therapy | Hydroxychloroquine, Entinostat, Regorafenib | I/II stage | — | — | NCT03215264 [98] | — |
| Solid tumor Colorectal cancer | Immunotherapy and target therapy | Magrolimab and Cetuximab | I/II stage | 9.5 7.6 | 3.6 1.9 | NCT02953782 [99] | [100] |
| Metastatic colorectal cancer | Chemotherapy and target therapy | FOLFIRI and Aflibercept | II stage | 12.6 | 7.4 | NCT02970916 [101] | — |
| Metastatic colorectal cancer | Chemotherapy and target therapy | Pemetrexed and Erlotinib | II stage | 7.3 | 2.5 | NCT02723578 [102] | [103] |
| Metastatic colorectal cancer | Immunotherapy and target therapy | Spartalizumab and Regorafenib | I stage | — | — | NCT03081494 [104] | — |
| Metastatic colorectal cancer | Chemotherapy and immunotherapy | TAS-102 (Trifluridine/tipiracil) and Bevacizumab Capecitabine and Bevacizumab | II stage | 18.0 16.2 | 9.2 7.8 | NCT02743221 [105] | [106] |
| Microsatellite stable relapsed or refractory colorectal cancer | Immunotherapy and target therapy | Avelumab and Tomivosertib (eFT508) | II stage | — | — | NCT03258398 [107] | [108] |
| Colorectal neoplasms | Chemotherapy and immunotherapy | mFOLFOX6 and BI 695502 | III stage | 19.4 | 10.5 | NCT02776683 [109] | — |
| Colorectal neoplasm | Chemotherapy and target therapy | mFOLFOX6 and Selinexor | I stage | — | — | NCT02384850 [110] | [111] |
| Refractory metastatic colorectal cancer | Chemotherapy and immunotherapy | TAS-102 (Trifluridine/tipiracil) and Nivolumab | II stage | — | 2.2 | NCT02860546 [112] | [113,114] |
| Colorectal cancer | Immunotherapy and target therapy | Durvalumab and Pexidartinib | I stage | — | — | NCT02777710 [115] | — |
| Colorectal cancer | Immunotherapy and target therapy | Atezolizumab and Cobimetinib | III stage | 8.87 | 1.91 | NCT02788279 [116] | [117] |
| Colorectal cancer | Immunotherapy and target therapy | Atezolizumab and Bevacizumab and Cobimetinib | I stage | NCT02876224 [118] | — | ||
| Colorectal neoplasms | Chemotherapy and target therapy | FOLFIRI and Cetuximab | III stage | — | 11.4 | NCT02484833 [119] | [120,121] |
| Metastatic colorectal cancer | Chemotherapy and immunotherapy | Irinotecan and AZD1775 | I stage | — | — | NCT02906059 [122] | [123] |
| Metastatic colorectal cancer | Chemotherapy and target therapy | TAS-102 (Trifluridine/tipiracil) and Brontictuzumab | I stage | — | — | NCT03031691 [124] | — |
| Metastatic colorectal cancer | Chemotherapy and target therapy | FOLFIRI and OMP-131R10 | I stage | — | — | NCT02482441 [125] | — |
| Colorectal cancer | Immunotherapy and target therapy | Pembrolizumab and AMG820 | I/II stage | 38.963 | 5.396 | NCT02713529 [126] | [127] |
| Pancreatic adenocarcinoma (PDAC) | Local ablative therapy and immunotherapy | Irreversible electroporation (IRE) and allogeneic γδ T cells | I/II stage | 14.5 | 11 | NCT03180437 [128] | [129] |
| Pancreatic neoplasms | Local ablative therapy and immunotherapy | Irreversible electroporation (IRE) and NK cells | I/II stage | — | — | NCT02718859 [130] | — |
| Metastatic pancreatic cancer | Target therapy and Chemotherapy | RX-3117 (Fluorocyclopentenylcytosine) and Abraxane | I/II stage | — | — | NCT03189914 [131] | [132] |
| Advanced/ metastasized pancreatic cancer | Chemotherapy and target therapy | Gemcitabine, nab-paclitaxel, LED225 (Sonidegib) | I/II stage | 6.0 | — | NCT02358161 [133] | [134] |
| Pancreatic cancer | Chemotherapy and target therapy | Gemcitabine and BP31510 (Ubidecarenone, USP) | I stage | — | — | NCT02650804 [135] | [136] |
| Pancreatic cancer | Immunotherapy and target therapy | Pembrolizumab and Olaptesed pegol | I/II stage | — | 1.87 | NCT03168139 [137] | [138,139] |
| Pancreatic cancer | Immunotherapy and radiotherapy | Nivolumab and Cabiralizumab and Stereotactic Body Radiotherapy (SBRT) | II stage | — | — | NCT03599362 [140] | [141] |
| Pancreatic cancer | Chemotherapy and irreversible electroporation | Gemcitabine and Irreversible electroporation (IRE) | I stage | — | — | NCT02981719 [142] | — |
| Pancreatic cancer | Chemotherapy and immunotherapy | FOLFOX and Pegilodecakin | III stage | 5.8 | 2.1 | NCT02923921 [143] | [144] |
| Pancreatic cancer | Chemotherapy and immunotherapy | nab-Paclitaxel, Gemcitabine, ALT-803 | I/II stage | — | — | NCT02559674 [145] | [146] |
| Metastatic pancreatic cancer | Immunotherapy and target therapy | Pembrolizumab and Acalabrutinib | II stage | — | 1.4 | NCT02362048 [147] | [148] |
| Metastatic pancreatic cancer | Immunotherapy and target therapy | Durvalumab and Galunisertib | I stage | 5.72 | 1.87 | NCT02734160 [149] | [150,151] |
| Pancreatic cancer | Chemotherapy and immunotherapy | nab-Paclitaxel, Gemcitabine, Selicrelumab | I stage | — | — | NCT02588443 [152] | — |
| Pancreatic cancer | Chemotherapy and radiotherapy | mFOLFIRINOX and Stereotactic Body Radiotherapy (SBRT) | II stage | — | — | NCT03891472 [153] | — |
| Pancreatic cancer | Chemotherapy and immunotherapy | Gemcitabine and M7824 | I/II stage | — | — | NCT03451773 [154] | — |
| Pancreatic neoplasms | Chemotherapy and target therapy | nab-Paclitaxel, Gemcitabine, Napabucasin | III stage | — | — | NCT02993731 [155] | [156,157] |
| Temperature Range (°C) | Selected Area in the Human Body |
|---|---|
| 36.32–37.76 | rectal |
| 35.76–37.52 | tympanic |
| 35.61–37.61 | urine |
| 35.73–37.41 | oral |
| 35.01–36.93 | axillary |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bienia, A.; Wiecheć-Cudak, O.; Murzyn, A.A.; Krzykawska-Serda, M. Photodynamic Therapy and Hyperthermia in Combination Treatment—Neglected Forces in the Fight against Cancer. Pharmaceutics 2021, 13, 1147. https://doi.org/10.3390/pharmaceutics13081147
Bienia A, Wiecheć-Cudak O, Murzyn AA, Krzykawska-Serda M. Photodynamic Therapy and Hyperthermia in Combination Treatment—Neglected Forces in the Fight against Cancer. Pharmaceutics. 2021; 13(8):1147. https://doi.org/10.3390/pharmaceutics13081147
Chicago/Turabian StyleBienia, Aleksandra, Olga Wiecheć-Cudak, Aleksandra Anna Murzyn, and Martyna Krzykawska-Serda. 2021. "Photodynamic Therapy and Hyperthermia in Combination Treatment—Neglected Forces in the Fight against Cancer" Pharmaceutics 13, no. 8: 1147. https://doi.org/10.3390/pharmaceutics13081147
APA StyleBienia, A., Wiecheć-Cudak, O., Murzyn, A. A., & Krzykawska-Serda, M. (2021). Photodynamic Therapy and Hyperthermia in Combination Treatment—Neglected Forces in the Fight against Cancer. Pharmaceutics, 13(8), 1147. https://doi.org/10.3390/pharmaceutics13081147