Effective Actions of Ion Release from Mesoporous Bioactive Glass and Macrophage Mediators on the Differentiation of Osteoprogenitor and Endothelial Progenitor Cells
Abstract
:1. Introduction
2. Materials and Methods
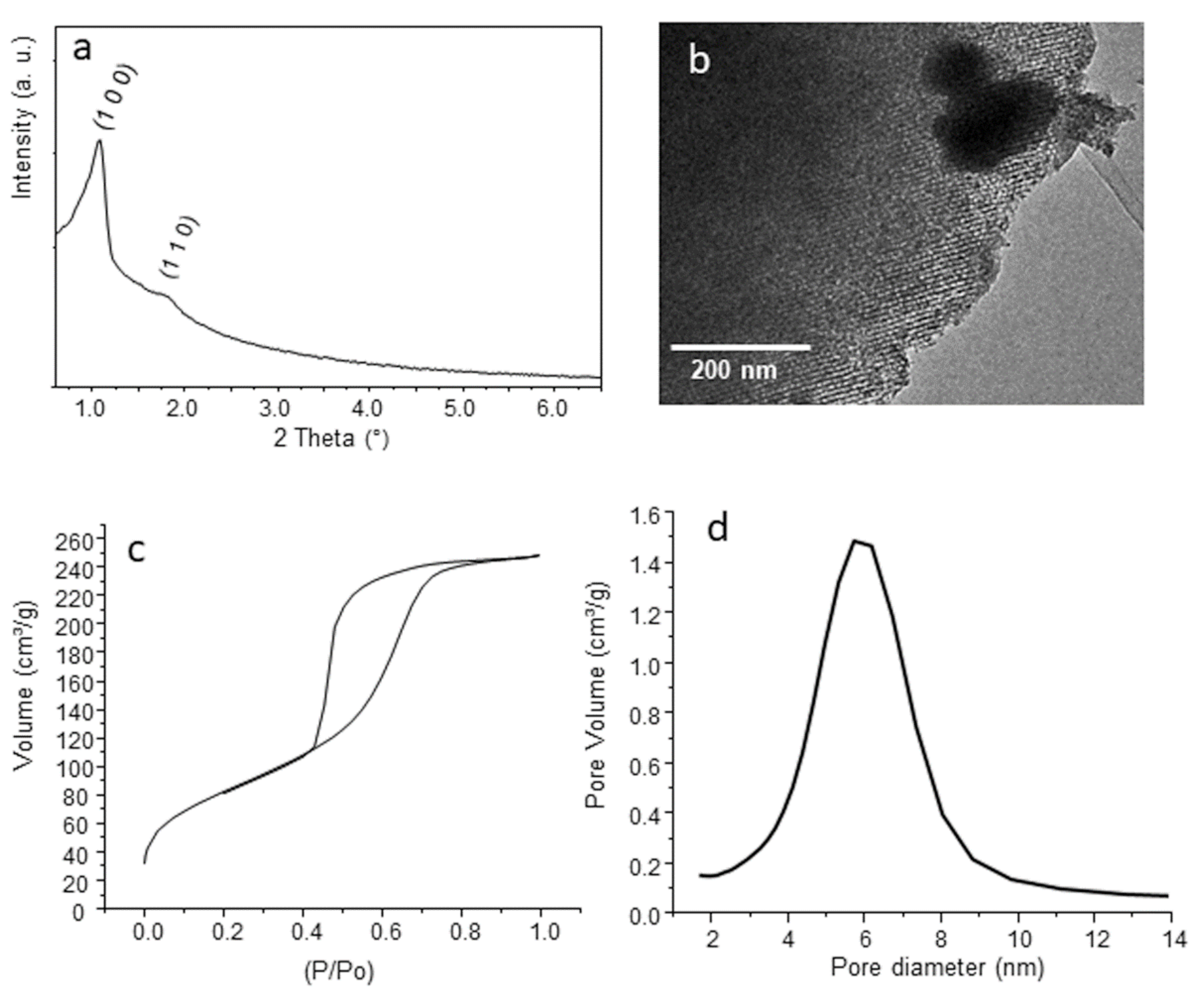
2.1. Synthesis and Characterization of MBG-75S
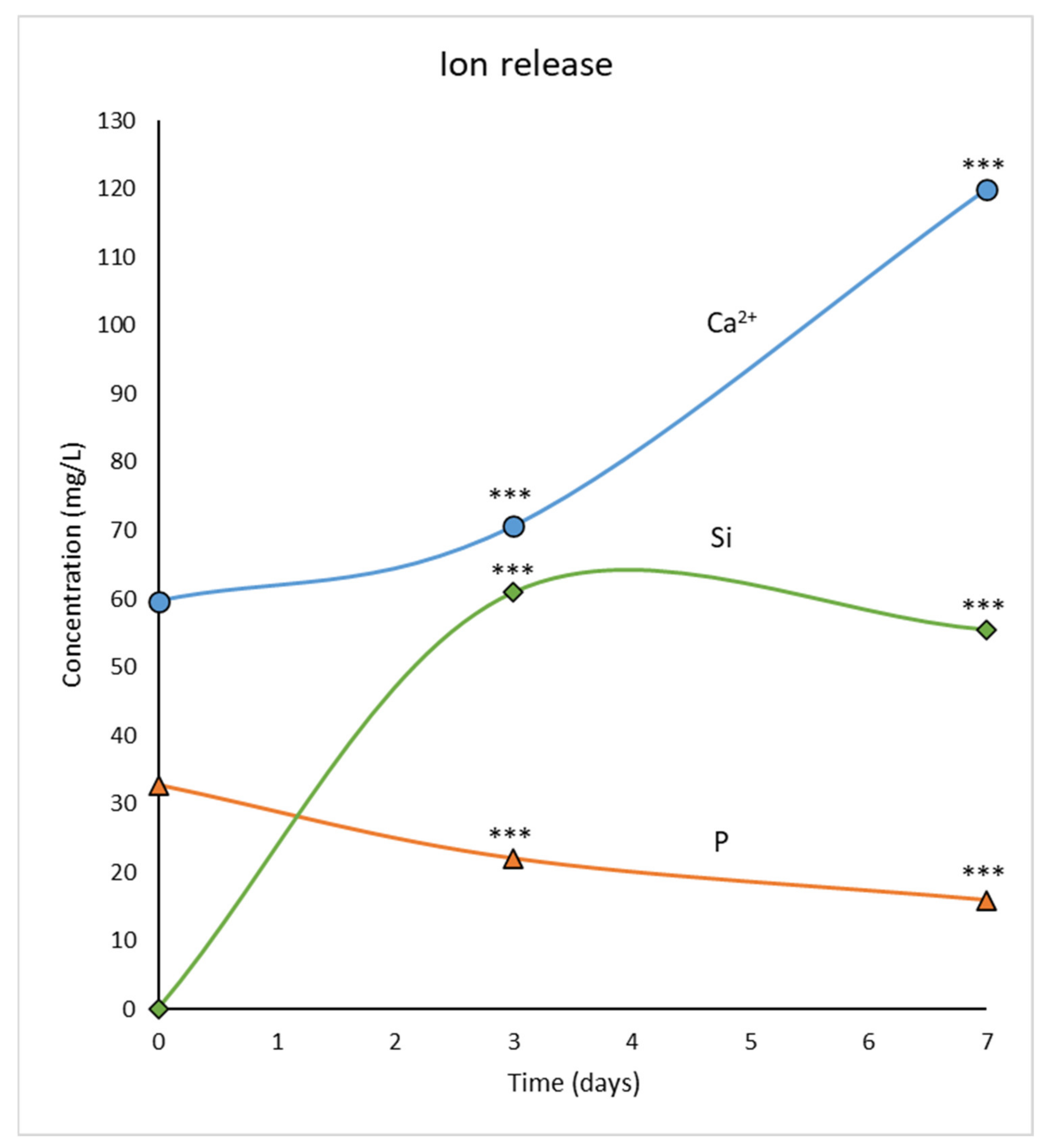
2.2. Measurement of Ion Release from MBG-75S into the Culture Medium
2.3. Culture and Differentiation of MC3T3-E1 Pre-Osteoblasts. Indirect Treatment with MBG-75S
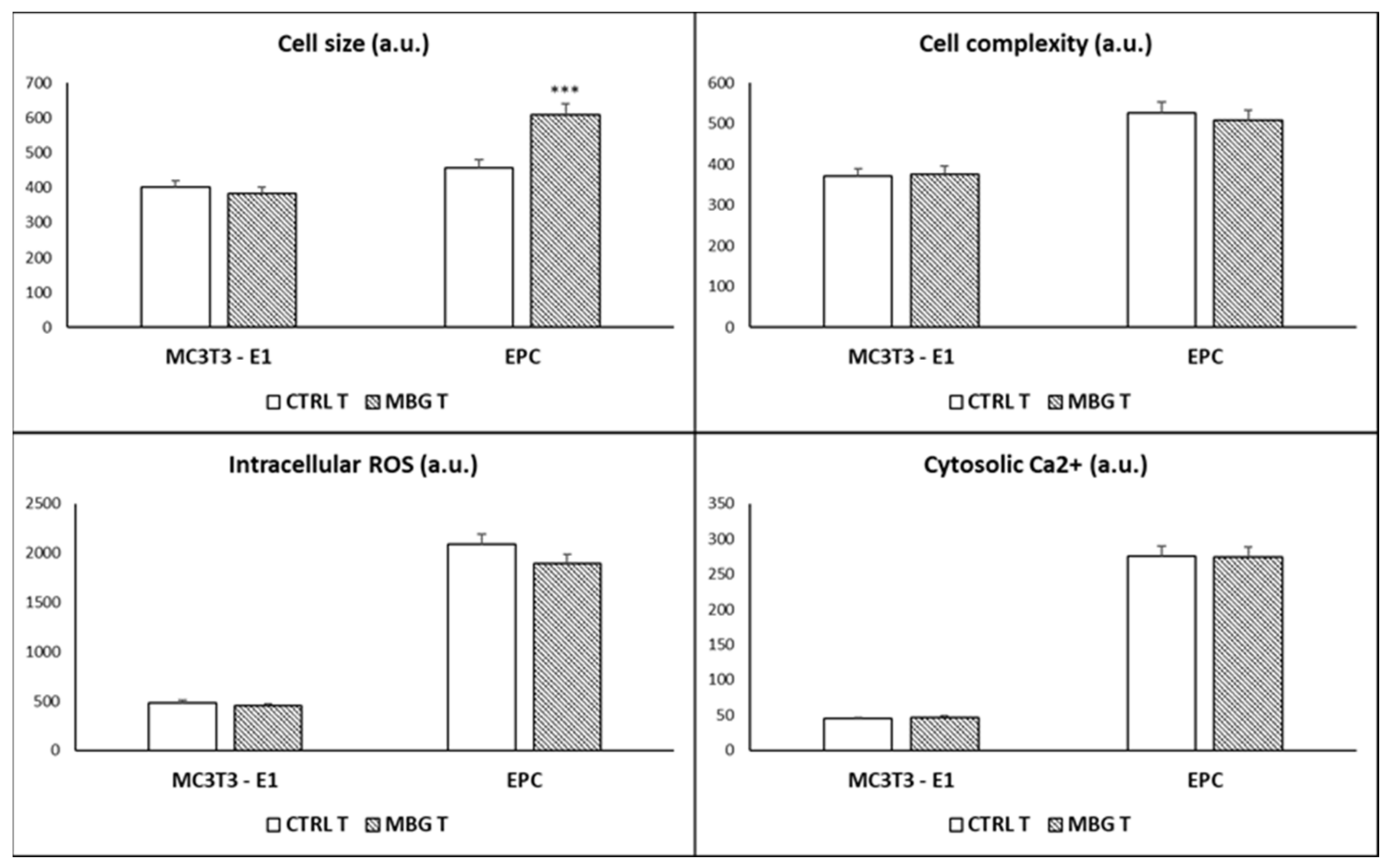
2.4. Cell Size and Complexity Analysis
2.5. Intracellular Reactive Oxygen Species (ROS) Content
2.6. Intracellular Calcium Content
2.7. Alkaline Phosphatase Activity (ALP)
2.8. Mineralization Assay
2.9. Obtention, Culture, and Characterization of Endothelial Progenitor Cells (EPCs). Indirect Treatment with MBG-75S
2.10. Culture and Indirect Treatment of RAW 264.7 Macrophages with MBG-75S. Exposure of MC3T3-E1 Pre-Osteoblasts to Macrophage Conditioned Media
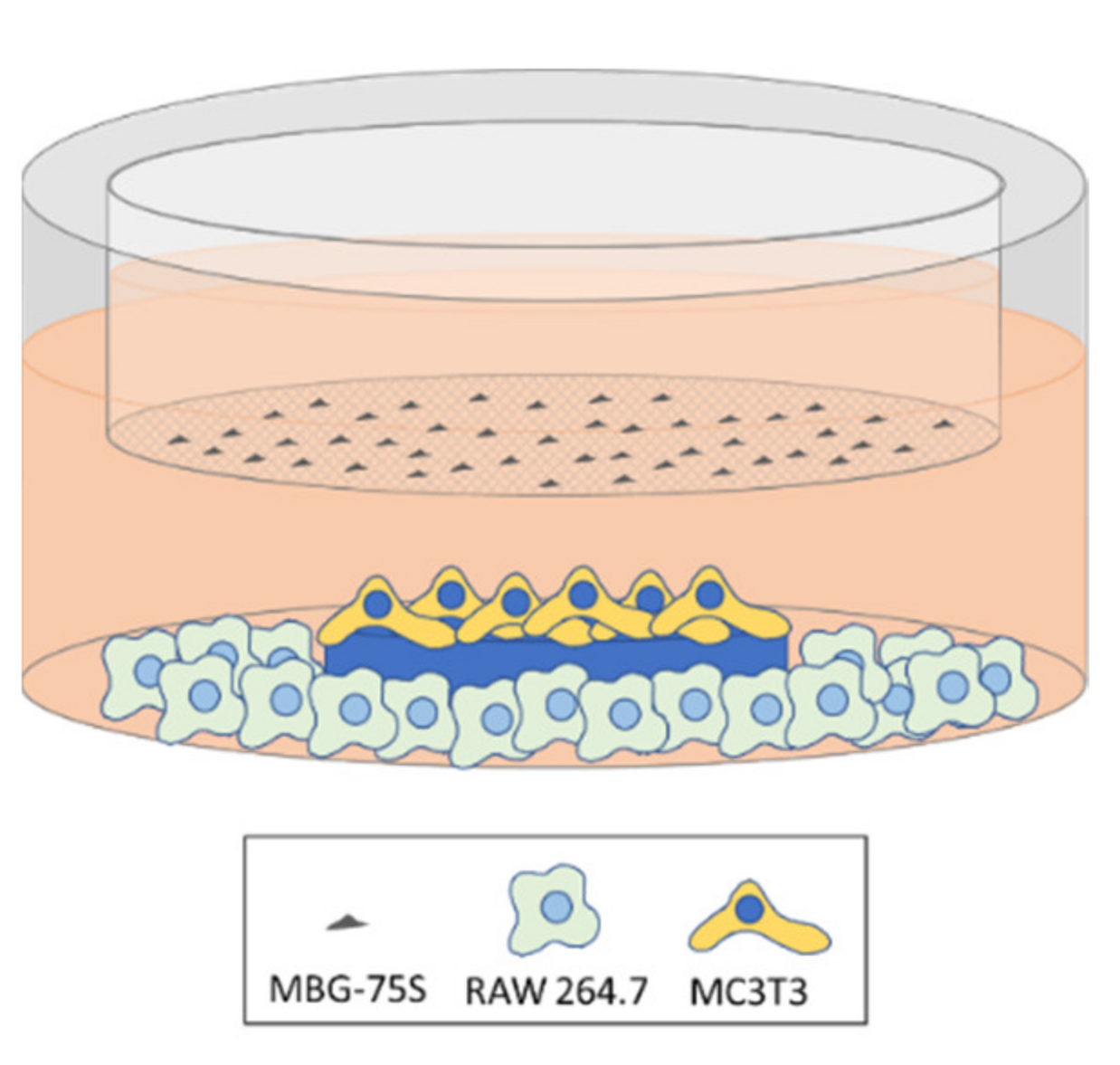
2.11. Coculture of MC3T3-E1 Pre-Osteoblasts and RAW 264.7 Macrophages. Indirect Treatment with MBG-75S and Measurement of ALP in MC3T3-E1 Cells
2.12. Coculture of EPCs and RAW 264.7 Macrophages. Indirect Treatment with MBG-75S and Measurement of VEGFR2 Expression in EPCs and CD206 Expression in RAW 264.7 Macrophages
2.13. Statistics
3. Results and Discussion
3.1. Characterization of MBG-75S
3.2. Ion Release from MBG-75S into the Culture Medium
3.3. Action of Ion Release from MBG-75S on Cell Size, Cell Complexity, Intracellular Reactive Oxygen Species, and Cytosolic Calcium of MC3T3-E1 Pre-Osteoblasts and Endothelial Progenitor Cells
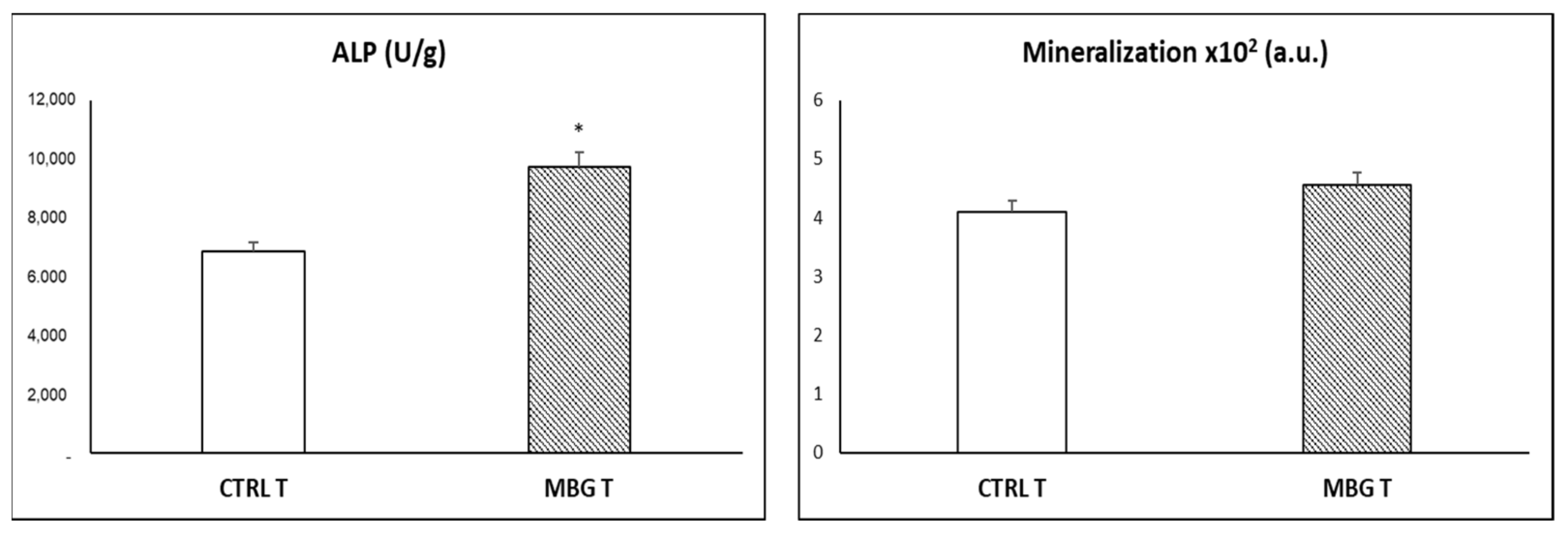
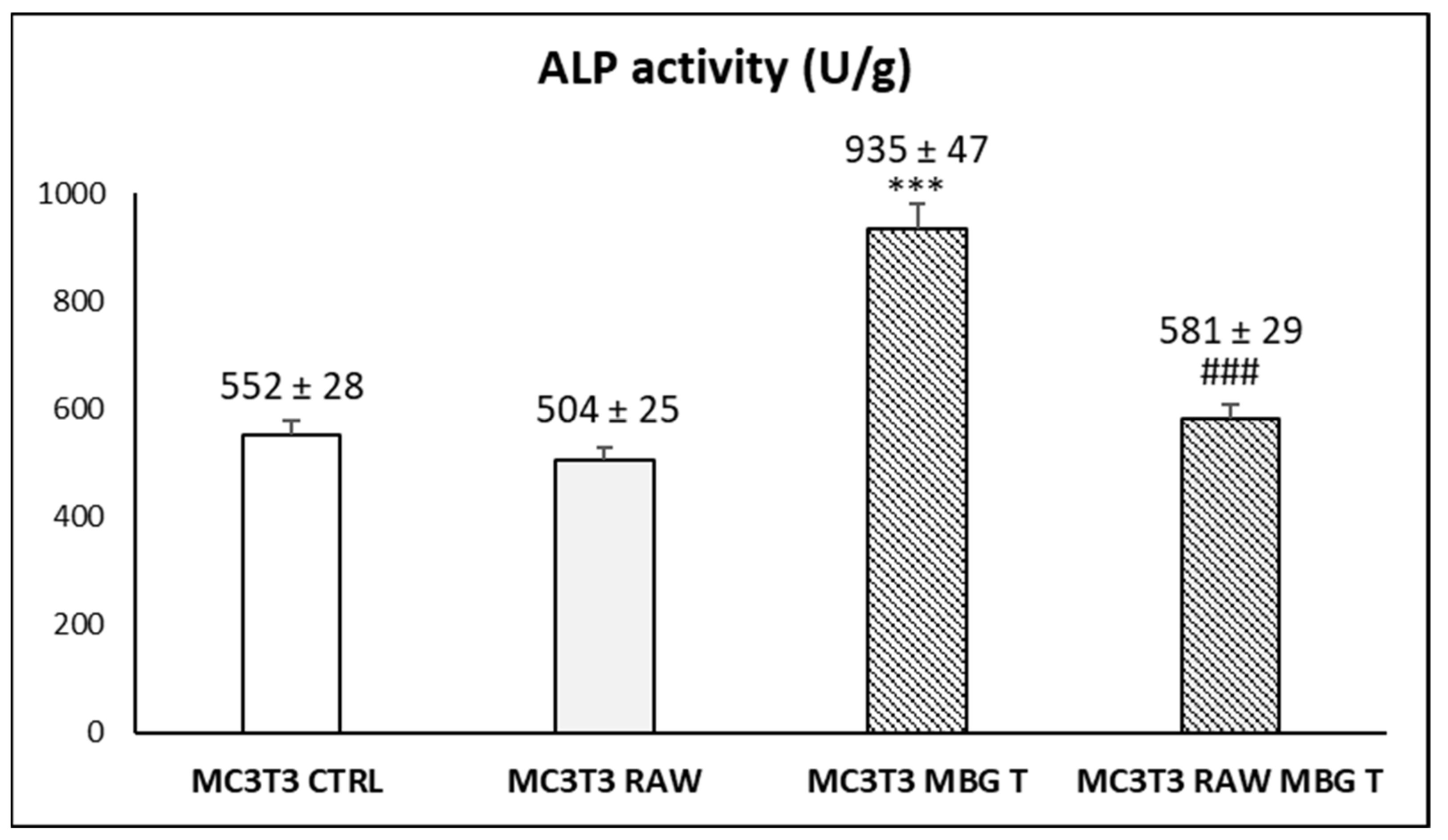
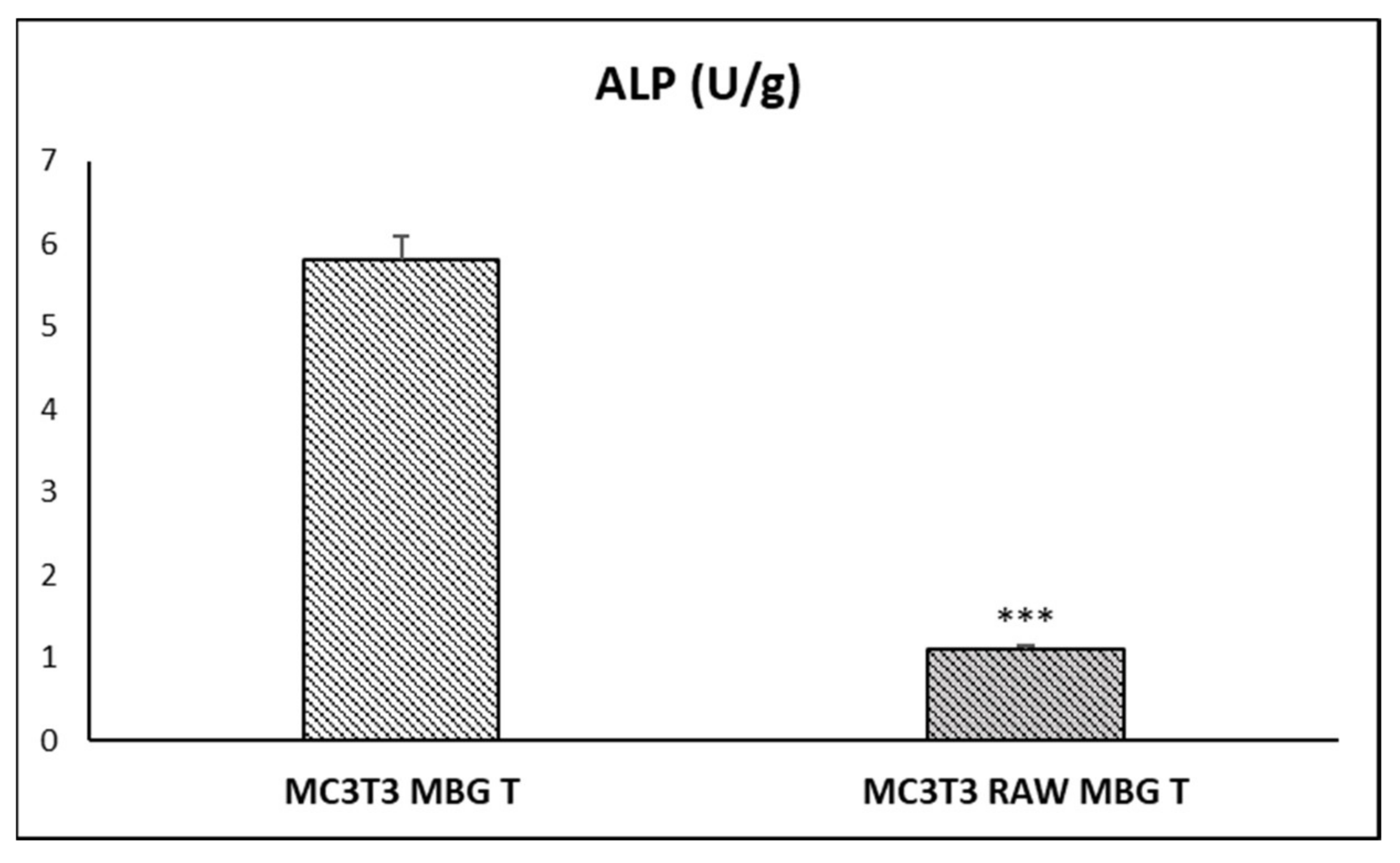
3.4. Action of Ion Release from MBG-75S on Differentiation of MC3T3-E1 Pre-Osteoblasts. Modulating Role of Macrophages
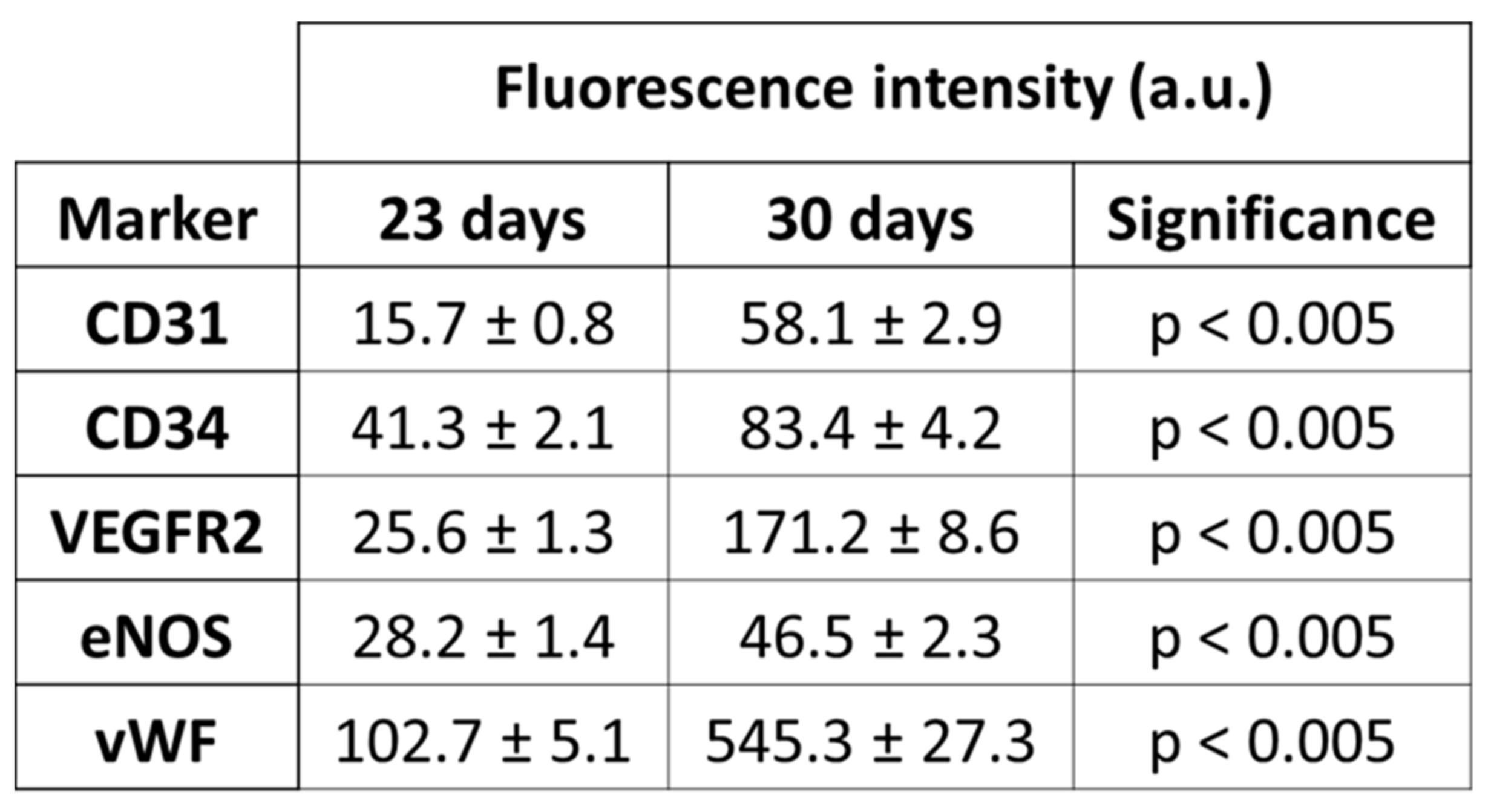
3.5. Differentiation and Phenotypic Characterization of EPCs
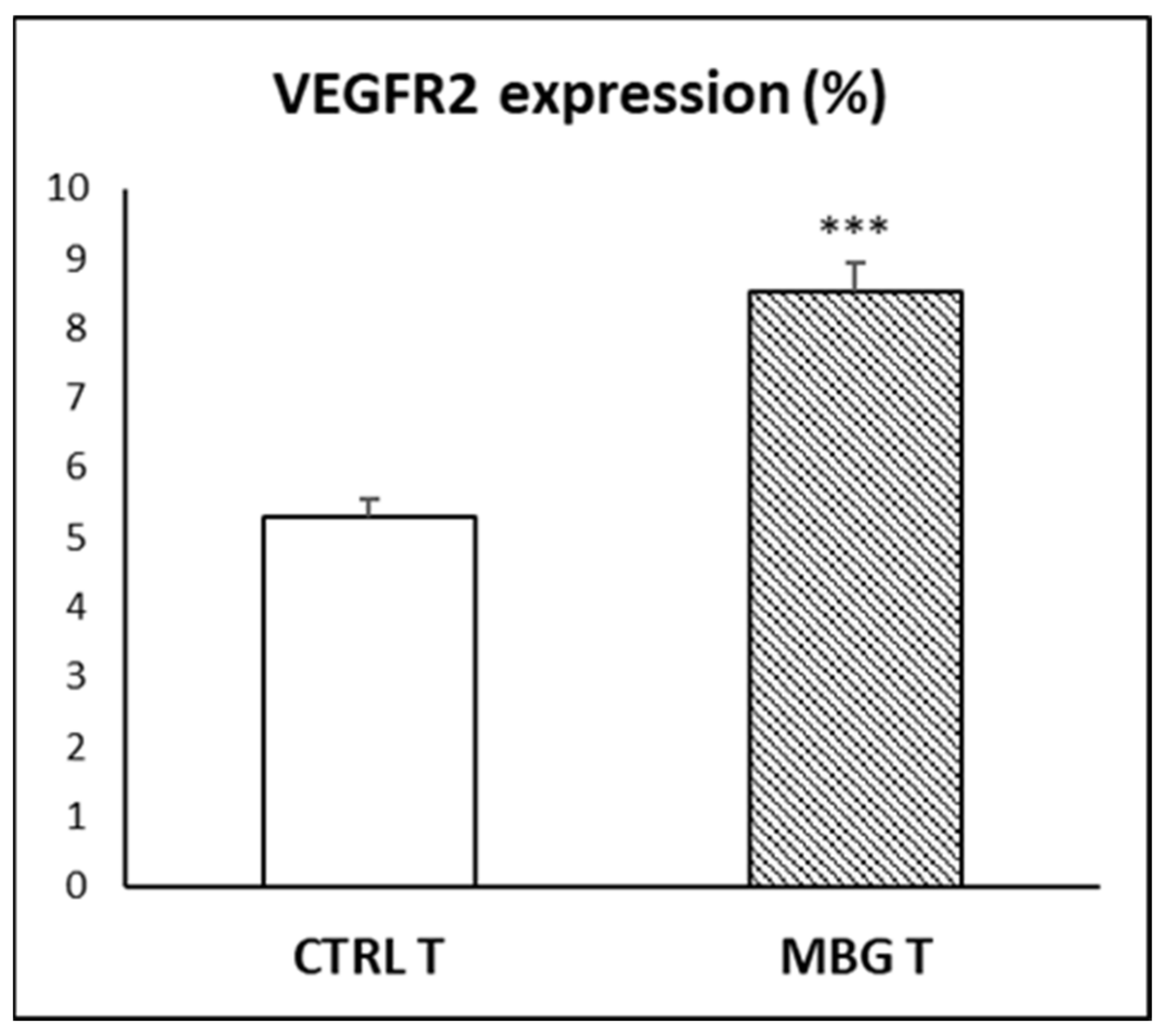
3.6. Action of Ion Release from MBG-75S on VEGFR2 Expression in EPCs
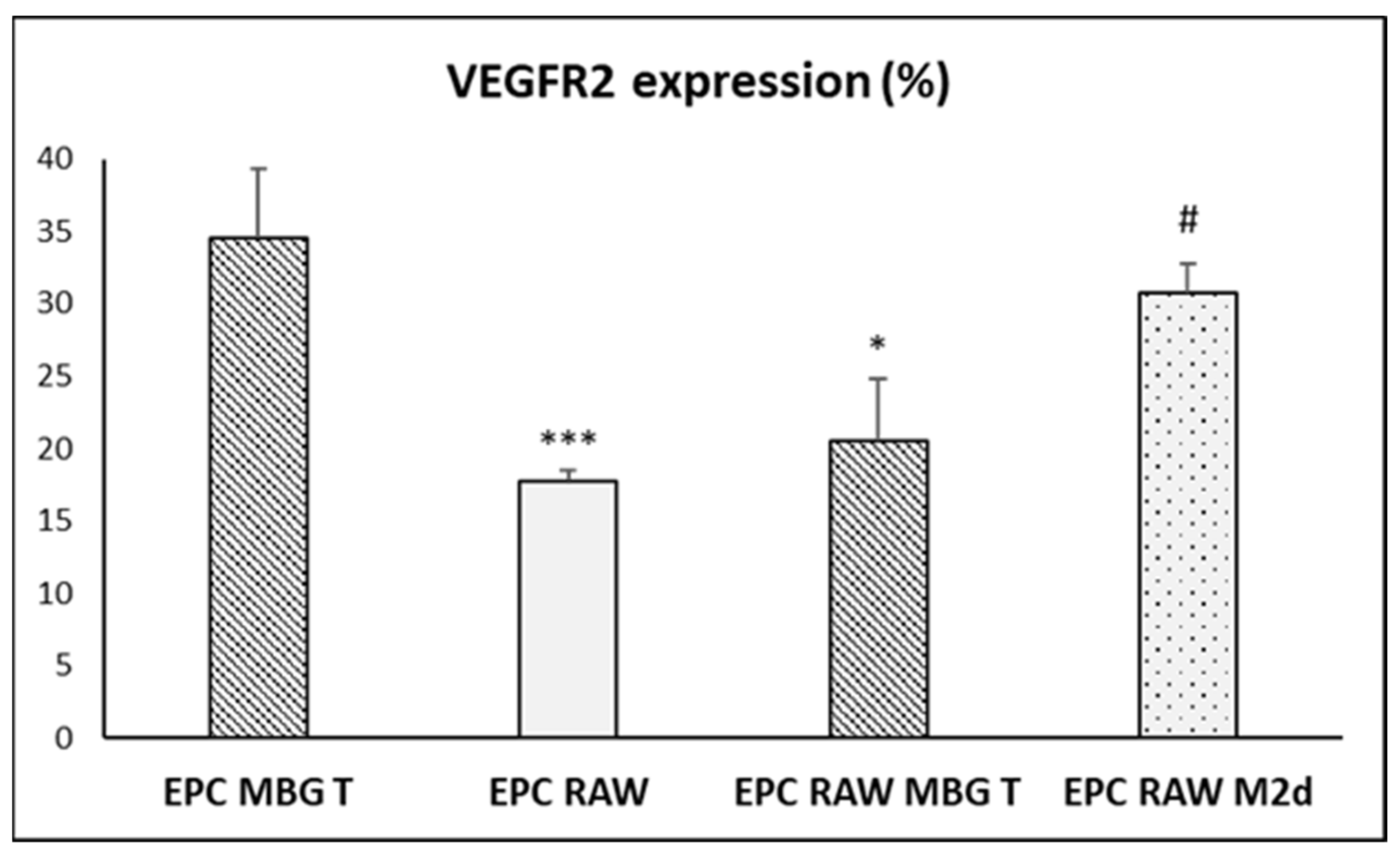
3.7. Action of Ion Release from MBG-75S on VEGFR2 and CD206 Expression in Cocultured EPCs and RAW 264.7 Macrophages, Respectively
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Izquierdo-Barba, I.; Arcos, D.; Sakamoto, Y.; Terasaki, O.; López-Noriega, A.; Vallet-Regí, M. High performance mesoporous bioceramics mimicking bone mineralization. Chem. Mater. 2008, 20, 3191–3198. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Control. Release 2014, 193, 282–295. [Google Scholar] [CrossRef]
- Polo, L.; Gómez-Cerezo, N.; García-Fernández, A.; Aznar, E.; Vivancos, J.L.; Arcos, D.; Vallet-Regí, M.; Martínez-Máñez, R. Mesoporous bioactive glasses equipped with stimuli-responsive molecular gates for the controlled delivery of levofloxacin against bacteria. Chem. Eur. J. 2018, 24, 18944–18951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polo, L.; Gómez-Cerezo, N.; Aznar, E.; Vivancos, J.L.; Sancenón, F.; Arcos, D.; Vallet-Regí, M.; Martínez-Máñez, R. Molecular gates in mesoporous bioactive glasses for the treatment of bone tumours and infection. Acta Biomater. 2017, 50, 114–126. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Gómez-Cerezo, N.; Verron, E.; Montouillout, V.; Fayon, F.; Lagadec, P.; Bouler, J.M.; Bujoli, B.; Arcos, D.; Vallet-Regí, M. The response of pre-osteoblasts and osteoclasts to gallium containing mesoporous bioactive glasses. Acta Biomater. 2018, 76, 333–343. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, C.; Zhang, X.; Chang, J.; Dai, K. Regulation of immune response by bioactive ions released from silicate bioceramics for bone regeneration. Acta Biomater. 2018, 66, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Forte, L.; Torricelli, P.; Boanini, E.; Gazzano, M.; Rubini, K.; Fini, M.; Bigi, A. Antioxidant and bone repair properties of quercetin-functionalized hydroxyapatite: An in vitro osteoblast-osteoclast-endothelial cell coculture study. Acta Biomater. 2016, 32, 298–308. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Incorporation and effects of mesoporous bioactive glass nanospheres loaded with ipriflavone on osteoblast/osteoclast cocultures. Eur. J. Pharm. Biopharm. 2018, 133, 258–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Cerezo, N.; Casarrubios, L.; Morales, I.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Effects of a mesoporous bioactive glass on osteoblasts, osteoclasts and macrophages. J. Colloid Interface Sci. 2018, 528, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, M.; Labbaf, S.; Karimzadeh, F.; Pinna, A.; Baharlou-Houreh, A.; Nasr-Esfahani, M.H. Mesoporous bioactive glasses for the combined application of osteosarcoma treatment and bone regeneration. Mater. Sci. Eng. C 2019, 104, 109994. [Google Scholar] [CrossRef]
- Wilkesmann, S.; Fellenberg, J.; Nawaz, Q.; Reible, B.; Moghaddam, A.; Boccaccini, A.R.; Westhauser, F. Primary osteoblasts, osteoblast precursor cells or osteoblast-like cell lines: Which human cell types are (most) suitable for characterizing 45S5-bioactive glass? J. Biomed. Mater. Res. 2020, 108, 663–674. [Google Scholar] [CrossRef]
- Addison, W.N.; Nelea, V.; Chicatun, F.; Chien, Y.C.; Tran-Khanh, N.; Buschmann, M.D.; Nazhat, S.N.; Kaartinen, M.T.; Vali, H.; Tecklemburg, M.M.; et al. Extracellular matrix mineralization in murine MC3T3-E1 osteoblast cultures: An ultrastructural, compositional and comparative analysis with mouse bone. Bone 2015, 71, 244–256. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Ralphs, J.R.; Richards, R.G.; Hayes, J.S. A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J. Biomed. Mater. Res. A 2014, 102, 2636–2643. [Google Scholar] [CrossRef]
- Przekora, A. The summary of the most important cell-biomaterial interactions that need to be considered during in vitro biocompatibility testing of bone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2019, 97, 1036–1051. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Santos-Ruiz, L.; Becerra, J.; Feito, M.J.; Fernández-Villa, D.; Serrano, M.C.; Díaz-Güemes, I.; Fernández-Tomé, B.; Enciso, S.; Sánchez-Margallo, F.M.; et al. Synergistic effect of Si-hydroxyapatite coating and VEGF adsorption on Ti6Al4V-ELI scaffolds for bone regeneration in an osteoporotic bone environment. Acta Biomater. 2019, 83, 456–466. [Google Scholar] [CrossRef]
- Feito, M.J.; Serrano, M.C.; Oñaderra, M.; Matesanz, M.C.; Sánchez-Salcedo, S.; Arcos, D.; Vallet-Regí, M.; Portolés, M.T. Effects of immobilized VEGF on endothelial progenitor cells cultured on silicon substituted and nanocrystalline hydroxyapatites. RSC Adv. 2016, 6, 92586–92595. [Google Scholar] [CrossRef] [Green Version]
- Casarrubios, L.; Gómez-Cerezo, N.; Sánchez-Salcedo, S.; Feito, M.J.; Serrano, M.C.; Saiz-Pardo, M.; Ortega, L.; de Pablo, D.; Díaz-Güemes, I.; Fernández-Tomé, B.; et al. Silicon substituted hydroxyapatite/VEGF scaffolds stimulate bone regeneration in osteoporotic sheep. Acta Biomater. 2020, 101, 544–553. [Google Scholar] [CrossRef]
- Shirota, T.; He, H.; Yasui, H.; Matsuda, T. Human endothelial progenitor cell-seeded hybrid graft: Proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng. 2003, 9, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T. Isolation of putative progenitor endotelial cells for angiogenesis. Science 1997, 275, 964–966. [Google Scholar] [CrossRef]
- Case, J.; Ingram, D.A.; Haneline, L.S. Oxidative stress impairs endothelial progenitor cell function. Antioxid. Redox Signal. 2008, 10, 1895–1906. [Google Scholar] [CrossRef]
- Serrano, M.C.; Pagani, R.; Vallet-Regí, M.; Peña, J.; Comas, J.V.; Portolés, M.T. Nitric oxide production by endothelial cells derived from blood progenitors cultured on NaOH-treated polycaprolactone films: A biofunctionality study. Acta Biomater. 2009, 5, 2045–2053. [Google Scholar] [CrossRef]
- Stout, R.D.; Stuttles, J. Functional plasticity of macrophages: Reversible adaptation to changing microenvironments. J. Leukoc. Biol. 2004, 76, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivanshkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKenzie, K.F.; Pattison, M.J.; Arthur, J.S. Transcriptional regulation of IL-10 and its cell-specific role in vivo. Crit. Rev. Immunol. 2014, 34, 315–345. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Martínez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Selfi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Ferrante, C.J.; Pinhal-Einfield, G.; Elson, G.; Cronstein, B.N.; Hasko, G.; Outram, S.; Leibovich, S.J. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation 2013, 36, 921–931. [Google Scholar] [CrossRef]
- Pinhal-Einfield, G.; Ramanathan, M.; Hasko, G.; Vogel, S.N.; Salzman, A.L.; Boons, G.J.; Leibovich, S.J. An angiogenic switch in macrophages involving synergy between toll-like receptors 2, 4, 7, and 9 and adenosine A2a receptors. Am. J. Pathol. 2003, 163, 711–721. [Google Scholar] [CrossRef] [Green Version]
- Brinker, C.J.; Lu, Y.; Sellinger, A.; Fan, H. Evaporation-induced self-assembly: Nanostructures made easy. Adv. Mater. 1999, 11, 579–585. [Google Scholar] [CrossRef]
- Vilmaraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar]
- Serrano, M.C.; Pagani, R.; Ameer, G.A.; Vallet-Regí, M.; Portolés, M.T. Endothelial cells derived from circulating progenitors as an effective source to functional endothelialization of NaOH-treated poly(epsilon-caprolactone) films. J. Biomed. Mater. Res. A 2008, 87, 964–971. [Google Scholar] [CrossRef]
- Allen, J.; Khan, S.; Serrano, M.C.; Ameer, G. Characterization of porcine circulating progenitor cells: Toward a functional endothelium. Tissue Eng. 2008, 14, 183–194. [Google Scholar] [CrossRef]
- van Mourik, J.A.; Leeksma, O.C.; Reinders, J.H.; de Groot, P.G.; Zandbergen-Spaargaren, J. Vascular endothelial cells synthesize a plasma membrane protein indistinguishable from the platelet membrane glycoprotein IIa. J. Biol. Chem. 1985, 260, 11300–11306. [Google Scholar] [CrossRef]
- Wood, H.B.; May, G.; Healy, L.; Enver, T.; Morriss-Kay, G.M. CD34 expression patterns during early mouse development are related to modes of blood vessel formation and reveal additional sites of hematopoiesis. Blood 1997, 90, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- Kuhlencordt, P.J.; Rosel, E.; Gerszten, R.E.; Morales-Ruiz, M.; Dombkowski, D.; Atkinson, W.J.; Han, F.; Preffer, F.; Rosenzweig, A.; Sessa, W.C.; et al. Role of endothelial nitric oxide synthase in endothelial activation: Insights from eNOS knockout endothelial cells. Am. J. Physiol. Cell Physiol. 2004, 286, 1195–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanetta, L.; Marcys, S.G.; Vasile, J.; Dobryansky, M.; Cohen, H.; Eng, K.; Shamamian, P.; Mignatti, P. Expression of Von Willebrand factor, an endothelial cell marker, is upregulated by angiogenesis factors: A potential method for objective assessment of tumor angiogenesis. Int. J. Cancer 2000, 85, 281–288. [Google Scholar] [CrossRef]
- Ferrara, N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am. J. Physiol. Cell Physiol. 2001, 280, 1358–1366. [Google Scholar] [CrossRef]
- Yan, X.X.; Yu, C.Z.; Zhou, X.F.; Tang, J.W.; Zhao, D.Y. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef]
- López-Noriega, A.; Arcos, D.; Izquierdo-Barba, I.; Sakamoto, Y.; Terasaki, O.; Vallet-Regí, M. Oredered mesoporous bioactive glasses for bone tissue regeneration. Chem. Mater. 2006, 18, 3137–3144. [Google Scholar] [CrossRef]
- Udall, J.M.; Moscicki, R.A.; Preffer, F.I.; Ariniello, P.D.; Carter, E.A.; Bhan, A.K.; Bloch, K.J. Flow cytometry: A new approach to the isolation and characterization of Kupffer cells. Adv. Exp. Med. Biol. 1987, 216, 821–827. [Google Scholar]
- Cicuéndez, M.; Izquierdo-Barba, I.; Portolés, M.T.; Vallet-Regí, M. Biocompatibility and levofloxacin delivery of mesoporous materials. Eur. J. Pharm. Biopharm. 2013, 84, 115–124. [Google Scholar] [CrossRef]
- Tilocca, A. Structural models of bioactive glasses from molecular dynamics simulations. Proc. R. Soc. A 2009, 465, 1003–1027. [Google Scholar] [CrossRef]
- Arcos, D.; Greenspan, D.; Vallet-Regí, M. A new quantitative method to evaluate the in vitro bioactivity of melt and sol-gel-derived silicate glasses. J. Biomed. Mater. Res. 2003, 65, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Toyooka, T.; Ibuki, Y. Simple and easy method to evaluate uptake potential of nanoparticles in mammalian cells using a flow cytometric light scatter analysis. Environ. Sci. Technol. 2007, 41, 3018–3024. [Google Scholar] [CrossRef] [PubMed]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J. Bone Miner. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Lian, J.B. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr. Rev. 1993, 14, 424–442. [Google Scholar] [CrossRef]
- Gómez-Cerezo, N.; Casarrubios, L.; Saiz-Pardo, M.; Ortega, L.; de Pablo, D.; Díaz-Güemes, I.; Fernández-Tomé, B.; Enciso, S.; Sánchez-Margallo, F.M.; Portolés, M.T.; et al. Mesoporous bioactive glass/e-polycaprolactone scaffolds promote bone regeneration in osteoporotic sheep. Acta Biomater. 2019, 90, 393–402. [Google Scholar] [CrossRef]
- Shi, M.; Zhou, Y.; Shao, J.; Chen, Z.; Song, B.; Chang, J.; Wua, C.; Xiao, Y. Stimulation of osteogenesis and angiogenesis of hBMSCs by delivering Si ions and functional drug from mesoporous silica nanospheres. Acta Biomater. 2015, 21, 178–189. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Ipriflavone-loaded mesoporous nanospheres with potential applications for periodontal treatment. Nanomaterials 2020, 10, 2573. [Google Scholar] [CrossRef]
- Wang, C.; Lin, K.; Chang, J.; Sun, J. Osteogenesis and angiogenesis induced by porous beta-CaSiO(3)/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials 2013, 34, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Mocquot, C.; Colon, P.; Fernando, D.; Jackson, P.; Pradelle-Plasse, N.; Grosgogeat, B.; Attik, N. The influence of experimental bioactive glasses on pulp cells behavior in vitro. Dent. Mater. 2020, 36, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Shi, J.; Zhi, Y.; Yang, X.; Yuan, Y.; Si, J.; Shen, S.G.F. Improved BMP2-CPC-stimulated osteogenesis in vitro and in vivo via modulation of macrophage polarization. Mater. Sci. Eng. C 2021, 118, 111471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, B.; Yuan, Y.; Zhang, X.; Gou, Y.; Gong, P.; Xiang, L. CGRP-modulated M2 macrophages regulate osteogenesis of MC3T3-E1 via Yap1. Arch. Biochem. Biophys. 2021, 697, 108697. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Nagy, J.A.; Feng, D.; Brown, L.F.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr. Top. Microbiol. Immunol. 1999, 237, 97–132. [Google Scholar]
- Shibuya, M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Street, J.; Bao, M.; Bunting, S.; Peale, F.V.; Ferrara, N.; Steinmetz, H.; Hoeffel, L.; Cleland, J.L.; Daugherty, A.; van Bruggen, N. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef] [Green Version]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.K.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.J.; West, J.L. Vascularization of engineered tissues: Approaches to promote angiogenesis in biomaterials. Curr. Top. Med. Chem. 2008, 8, 300–310. [Google Scholar] [PubMed]
- Ngo, M.T.; Harley, B.A.C. Angiogenic biomaterials to promote therapeutic regeneration and investigate disease progression. Biomaterials 2020, 255, 120207. [Google Scholar] [CrossRef] [PubMed]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of bioactive glasses on angiogenesis: A review of in vitro and in vivo evidences. Tissue Eng. Part B 2010, 16, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive glasses: Sprouting angiogenesis in tissue engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro. Tissue Eng. 2005, 11, 768–777. [Google Scholar] [CrossRef]
- Aguirre, A.; González, A.; Planell, J.A.; Engel, E. Extracellular calcium modulates in vitro bone marrow-derived Flk-1+ CD34+ progenitor cell chemotaxis and differentiation through a calcium-sensing receptor. Biochem. Biophys. Res. Commun. 2010, 393, 156–161. [Google Scholar] [CrossRef]
- Huang, S.; Tang, Y.; Peng, X.; Cai, X.; Wa, Q.; Ren, D.; Li, Q.; Luo, J.; Li, L.; Zou, X.; et al. Acidic extracellular pH promotes prostate cancer bone metastasis by enhancing PC-3 stem cell characteristics, cell invasiveness and VEGF-induced vasculogenesis of BM-EPCs. Oncol. Rep. 2016, 36, 2025–2032. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Xu, J.; Duan, D.; Wu, L. Endothelial cells provide an instructive niche for the differentiation and functional polarization of M2-like macrophages. Blood 2012, 120, 3152–3162. [Google Scholar] [CrossRef] [Green Version]
- Castaño, I.M.; Raftery, R.M.; Chen, G.; Cavanagh, B.; Quinn, B.; Duffy, G.P.; O’Brien, F.J.; Curtin, C.M. Rapid bone repair with the recruitment of CD206+ M2-like macrophages using non-viral scaffold-mediate d miR-133a inhibition of host cells. Acta Biomater. 2020, 109, 267–279. [Google Scholar] [CrossRef]



| Oxide | Molar % |
|---|---|
| SiO2 | 71.4 |
| CaO | 25.2 |
| P2O5 | 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polo-Montalvo, A.; Casarrubios, L.; Serrano, M.C.; Sanvicente, A.; Feito, M.J.; Arcos, D.; Portolés, M.T. Effective Actions of Ion Release from Mesoporous Bioactive Glass and Macrophage Mediators on the Differentiation of Osteoprogenitor and Endothelial Progenitor Cells. Pharmaceutics 2021, 13, 1152. https://doi.org/10.3390/pharmaceutics13081152
Polo-Montalvo A, Casarrubios L, Serrano MC, Sanvicente A, Feito MJ, Arcos D, Portolés MT. Effective Actions of Ion Release from Mesoporous Bioactive Glass and Macrophage Mediators on the Differentiation of Osteoprogenitor and Endothelial Progenitor Cells. Pharmaceutics. 2021; 13(8):1152. https://doi.org/10.3390/pharmaceutics13081152
Chicago/Turabian StylePolo-Montalvo, Alberto, Laura Casarrubios, María Concepción Serrano, Adrián Sanvicente, María José Feito, Daniel Arcos, and María Teresa Portolés. 2021. "Effective Actions of Ion Release from Mesoporous Bioactive Glass and Macrophage Mediators on the Differentiation of Osteoprogenitor and Endothelial Progenitor Cells" Pharmaceutics 13, no. 8: 1152. https://doi.org/10.3390/pharmaceutics13081152
APA StylePolo-Montalvo, A., Casarrubios, L., Serrano, M. C., Sanvicente, A., Feito, M. J., Arcos, D., & Portolés, M. T. (2021). Effective Actions of Ion Release from Mesoporous Bioactive Glass and Macrophage Mediators on the Differentiation of Osteoprogenitor and Endothelial Progenitor Cells. Pharmaceutics, 13(8), 1152. https://doi.org/10.3390/pharmaceutics13081152







