Diverse Effects of Natural and Synthetic Surfactants on the Inhibition of Staphylococcus aureus Biofilm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Bacterial Strains
2.3. Biosurfactant Production
2.4. Samples Purification
2.5. LC-MS/MS
2.6. Rhamnolipids Quantification
2.7. Determination of Critical Micelle Concentration
2.8. Stability Study
2.9. Antimicrobial Activity (Co-Incubation Assay)
2.10. Cell Surface Hydrophobicity
2.11. Cell Membrane Permeability
2.12. Inhibition of Bacterial Adhesion, Biofilm Formation and Disruption of Mature Biofilm on Silicone
2.12.1. Medical-Grade Silicone
2.12.2. Biofilm Dispersal Activity (Co-Incubation Assay)
2.12.3. Anti-Adhesive Activity (Deposition Assay)
2.12.4. Anti-Biofilm Activity (Pre-Coating Assay)
2.13. Statistical Analysis
3. Results
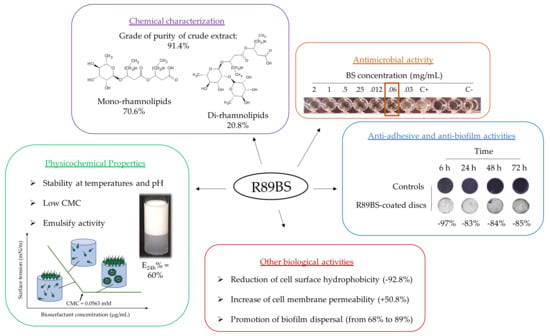
3.1. Chemical Characterization
3.2. Rhamnolipids Quantification
3.3. Determination of Critical Micelle Concentration and Stability Study
3.4. Antimicrobial Activity
3.5. Cell Surface Hydrophobicity and Cell Membrane Permeability
3.6. Biofilm Dispersal
3.7. Anti-Adhesive Activity
3.8. Anti-Biofilm Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parvizi, J.; Pawasarat, I.M.; Azzam, K.A.; Joshi, A.; Hansen, E.N.; Bozic, K. Periprosthetic joint infection: The economic impact of methicillin-resistant infections. J. Arthroplast. 2010, 25, 103–107. [Google Scholar] [CrossRef]
- Song, X.; Perencevich, E.; Campos, J.; Short, B.L.; Singh, N. Clinical and economic impact of methicillin-resistant Staphylococcus aureus colonization or infection on neonates in intensive care units. Infect. Control Hosp. Epidemiol. 2010, 31, 177–182. [Google Scholar] [CrossRef]
- DeLeo, F.R.; Otto, M.; Kreiswirth, B.N.; Chambers, H.F. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 2010, 375, 1557–1568. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.N.; Feazel, L.M.; Bessesen, M.T.; Price, C.S.; Janoff, E.N.; Pace, N.R. The human nasal microbiota and Staphylococcus aureus carriage. PLoS ONE 2010, 5, e10598. [Google Scholar] [CrossRef]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.; Kathju, S.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bereket, W.; Hemalatha, K.; Getenet, B.; Wondwossen, T.; Solomon, A.; Zeynudin, A.; Kannan, S. Update on bacterial nosocomial infections. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1039–1044. [Google Scholar]
- Otto, M. Staphylococcal infections: Mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Scali, F. Staphylococcus aureus virulence factors in evasion from innate immune defenses in human and animal diseases. Immunol. Lett. 2013, 150, 12–22. [Google Scholar] [CrossRef]
- Hogan, S.; Stevens, N.T.; Humphreys, H.; O’Gara, J.P.; O’Neill, E. Current and future approaches to the prevention and treatment of staphylococcal medical device-related infections. Curr. Pharm. Des. 2015, 21, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Archer, N.K.; Mazaitis, M.J.; Costerton, J.W.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus aureus biofilms: Properties, regulation, and roles in human disease. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef] [Green Version]
- Otto, M. Staphylococcal biofilms. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-resistant Staphylococcus aureus (MRSA): Antibiotic-resistance and the biofilm phenotype. MedChemComm 2019, 10, 1231–1241. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev. Anti Infect. Ther. 2015, 13, 1499–1516. [Google Scholar] [CrossRef] [Green Version]
- Hiltunen, A.K.; Savijoki, K.; Nyman, T.A.; Miettinen, I.; Ihalainen, P.; Peltonen, J.; Fallarero, A. Structural and Functional Dynamics of Staphylococcus aureus Biofilms and Biofilm Matrix Proteins on Different Clinical Materials. Microorganisms 2019, 7, 584. [Google Scholar] [CrossRef] [Green Version]
- Olmo, J.A.-D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Sáez-Martínez, V.; Vilas-Vilela, J.L. Antibacterial Coatings for Improving the Performance of Biomaterials. Coatings 2020, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 1–616. [Google Scholar]
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations. Pharmaceutics 2020, 12, 1099. [Google Scholar] [CrossRef]
- Ueda, Y.; Mashima, K.; Miyazaki, M.; Hara, S.; Takata, T.; Kamimura, H.; Takagi, S.; Jimi, S. Inhibitory effects of polysorbate 80 on MRSA biofilm formed on different substrates including dermal tissue. Sci. Rep. 2019, 9, 3128. [Google Scholar] [CrossRef]
- Toutain-Kidd, C.M.; Kadivar, S.C.; Bramante, C.T.; Bobin, S.A.; Zegans, M.E. Polysorbate 80 inhibition of Pseudomonas aeruginosa biofilm formation and its cleavage by the secreted lipase LipA. Antimicrob. Agents Chemother. 2009, 53, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Malinowski, A.M.; McClarty, B.M.; Robinson, C.; Spear, W.; Sanchez, M.; Sparkes, T.C.; Brooke, J.S. Polysorbate 80 and polymyxin B inhibit Stenotrophomonas maltophilia biofilm. Diagn. Microbiol. Infect. Dis. 2017, 87, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Joung, D.K.; Mun, S.H.; Lee, K.S.; Kang, O.H.; Choi, J.G.; Kim, S.B.; Gong, R.; Chong, M.S.; Kim, Y.C.; Lee, D.S.; et al. The Antibacterial Assay of Tectorigenin with Detergents or ATPase Inhibitors against Methicillin-Resistant Staphylococcus aureus. Evid. Based Complement Alternat. Med. 2014, 2014, 716509. [Google Scholar] [CrossRef] [Green Version]
- Pizzirusso, A.; De Nicola, A.; Sevink, G.J.A.; Correa, A.; Cascella, M.; Kawakatsu, T.; Rocco, M.; Zhao, Y.; Celino, M.; Milano, G. Biomembrane solubilization mechanism by Triton X-100: A computational study of the three stage model. Phys. Chem. Chem. Phys. 2017, 19, 29780–29794. [Google Scholar] [CrossRef] [PubMed]
- Koley, D.; Bard, A.J. Triton X-100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy (SECM). Proc. Natl. Acad. Sci. USA 2010, 107, 16783–16787. [Google Scholar] [CrossRef] [Green Version]
- Komatsuzawa, H.; Suzuki, J.; Sugai, M.; Miyake, Y.; Suginaka, H. The effect of Triton X-100 on the in-vitro susceptibility of methicillin-resistant Staphylococcus aureus to oxacillin. J. Antimicrob. Chemother. 1994, 34, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Komatsuzawa, H.; Sugai, M.; Suginaka, H. Triton X-100-induced lipoteichoic acid release is correlated with the methicillin resistance in Staphylococcus aureus. FEMS Microbiol. Lett. 2000, 182, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Bishop, P.L. Influence of nonionic surfactant on attached biofilm formation and phenanthrene bioavailability during simulated surfactant enhanced bioremediation. Environ. Sci. Technol. 2007, 41, 7107–7113. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banpurkar, A.G.; Banat, I.M.; Sangshetti, J.N.; Patil, R.H.; Gade, W.N. Multiple Roles of Biosurfactants in Biofilms. Curr. Pharm. Des. 2016, 22, 1429–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fracchia, L.; Ceresa, C.; Banat, I.M. Biosurfactants in Cosmetic, Biomedical and Pharmaceutical Industry. In Microbial Biosurfactants and Their Environmental and Industrial Applications; Banat, I.M., Thavasi, R., Eds.; CRS Press: Boca Raton, FL, USA, 2019; pp. 258–288. [Google Scholar]
- Ceresa, C.; Fracchia, L.; Fedeli, E.; Porta, C.; Banat, I.M. Recent Advances in Biomedical, Therapeutic and Pharmaceutical Applications of Microbial Surfactants. Pharmaceutics 2021, 13, 466. [Google Scholar] [CrossRef]
- Hajfarajollah, H.; Eslami, P.; Mokhtarani, B.; Akbari Noghabi, K. Biosurfactants from probiotic bacteria: A review. Biotechnol. Appl. Biochem. 2018, 65, 768–783. [Google Scholar] [CrossRef]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5, 697. [Google Scholar] [CrossRef] [Green Version]
- Olasanmi, I.O.; Thring, R.W. The Role of Biosurfactants in the Continued Drive for Environmental Sustainability. Sustainability 2018, 10, 4817. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.L.; Penfold, J.; Thomas, R.K.; Smyth, T.J.P.; Perfumo, A.; Marchant, R.; Banat, I.M.; Stevenson, P.; Parry, A.; Tucker, I.; et al. Mixing behavior of the biosurfactant, rhamnolipid, with a conventional anionic surfactant, sodium dodecyl benzene sulfonate. Langmuir 2010, 26, 17958–17968. [Google Scholar] [CrossRef]
- Chen, M.L.; Penfold, J.; Thomas, R.K.; Smyth, T.J.P.; Perfumo, A.; Marchant, R.; Banat, I.M.; Stevenson, P.; Parry, A.; Tucker, I.; et al. Solution self-assembly and adsorption at the air-water interface of the monorhamnose and dirhamnose rhamnolipids and their mixtures. Langmuir 2010, 26, 18281–18292. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.M.; Hausmann, R. Regulatory and metabolic network of rhamnolipid biosynthesis: Traditional and advanced engineering towards biotechnological production. Appl. Microbiol. Biotechnol. 2011, 91, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N. Environmental applications for biosurfactants. Environ. Pollut. 2005, 133, 183–198. [Google Scholar] [CrossRef]
- Costa, S.; Nitschke, M.; Lépine, F.; Déziel, E.; Contiero, J. Structure, properties and applications of rhamnolipids produced by Pseudomonas aeruginosa L2-1 from cassava wastewater. Process Biochem. 2010, 45, 1511–1516. [Google Scholar] [CrossRef]
- Zgoła-Grześkowiak, A.; Kaczorek, E. Isolation, preconcentration and determination of rhamnolipids in aqueous samples by dispersive liquid-liquid microextraction and liquid chromatography with tandem mass spectrometry. Talanta 2011, 83, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Rudden, M.; Tsauosi, K.; Marchant, R.; Banat, I.M.; Smyth, T.J. Development and validation of an ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method for the quantitative determination of rhamnolipid congeners. Appl. Microbiol. Biotechnol. 2015, 99, 9177–9187. [Google Scholar] [CrossRef]
- Behrens, B.; Engelen, J.; Tiso, T.; Blank, L.M.; Hayen, H. Characterization of rhamnolipids by liquid chromatography/mass spectrometry after solid-phase extraction. Anal. Bioanal. Chem. 2016, 408, 2505–2514. [Google Scholar] [CrossRef]
- Ceresa, C.; Tessarolo, F.; Maniglio, D.; Tambone, E.; Carmagnola, I.; Fedeli, E.; Caola, I.; Nollo, G.; Chiono, V.; Allegrone, G.; et al. Medical-Grade Silicone Coated with Rhamnolipid R89 Is Effective against Staphylococcus spp. Biofilms. Molecules 2019, 24, E3843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceresa, C.; Rinaldi, M.; Chiono, V.; Carmagnola, I.; Allegrone, G.; Fracchia, L. Lipopeptides from Bacillus subtilis AC7 inhibit adhesion and biofilm formation of Candida albicans on silicone. Antonie Van Leeuwenhoek 2016, 109, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- Ceresa, C.; Rinaldi, M.; Tessarolo, F.; Maniglio, D.; Fedeli, E.; Tambone, E.; Caciagli, P.; Banat, I.M.; Diaz De Rienzo, M.A.; Fracchia, L. Inhibitory Effects of Lipopeptides and Glycolipids on C. albicans-Staphylococcus spp. Dual-Species Biofilms. Front. Microbiol. 2021, 11, 545654. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.M.; Hörmann, B.; Kugel, M.; Syldatk, C.; Hausmann, R. Evaluation of rhamnolipid production capacity of Pseudomonas aeruginosa PAO1 in comparison to the rhamnolipid over-producer strains DSM 7108 and DSM 2874. Appl. Microbiol. Biotechnol. 2011, 89, 585–592. [Google Scholar] [CrossRef]
- Eslami, P.; Hajfarajollah, H.; Bazsefidpar, S. Recent advancements in the production of rhamnolipid bio-surfactants by Pseudomonas aeruginosa. RSC Adv. 2020, 10, 34014–34032. [Google Scholar] [CrossRef]
- Griffin, W.C. Classification of Surface-Active Agents by “HLB”. J. Cosmet. Sci. 1949, 1, 311–326. [Google Scholar]
- Diaz De Rienzo, M.A.; Kamalanathan, I.; Martin, P.J. Comparative study of the production of rhamnolipid biosurfactants by B. thailandensis E264 and P. aeruginosa ATCC 9027 using foam fractionation. Process Biochem. 2016, 51, 820–827. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, Q.; Qian, Y.; Song, Y.; Yao, J.; Choi, M.M. Microcalorimetric investigation of the effect of non-ionic surfactant on biodegradation of pyrene by PAH-degrading bacteria Burkholderia cepacia. Ecotox. Environ. Saf. 2013, 98, 361–367. [Google Scholar] [CrossRef]
- Ghosh, I.; Mukherji, S. Diverse effect of surfactants on pyrene biodegradation by a Pseudomonas strain utilizing pyrene by cell surface hydrophobicity induction. Int. Biodeterior. Biodegrad. 2016, 108, 67–75. [Google Scholar] [CrossRef]
- Manivasagan, P.; Sivasankar, P.; Venkatesan, J.; Sivakumar, K.; Kim, S. Optimization, production and characterization of glycolipid biosurfactant from the marine actinobacterium, Streptomyces sp. MAB36. Bioprocess Biosyst. Eng. 2014, 37, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Marzban, A.; Ebrahimipour, G.; Danesh, A. Bioactivity of a novel glycolipid produced by a Halophilic Buttiauxella sp. and improving submerged fermentation using a response surface method. Molecules 2016, 21, 1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nashida, J.; Nishi, N.; Takahashi, Y.; Hayashi, C.; Igarashi, M.; Takahashi, D.; Toshima, K. Systematic and Stereoselective Total Synthesis of Mannosylerythritol Lipids and Evaluation of Their Antibacterial Activity. J. Org. Chem. 2018, 83, 7281–7289. [Google Scholar] [CrossRef]
- Silveira, V.A.I.; Freitas, C.A.U.Q.; Celligoi, M.A.P.C. Antimicrobial Applications of Sophorolipid from Candida bombicola: A Promising Alternative to Conventional Drugs. J. Appl. Biol. Biotechnol. 2018, 6, 87–90. [Google Scholar]
- Mani, P.; Dineshkumar, G.; Jayaseelan, T.; Deepalakshmi, K.; Ganesh Kumar, C.; Senthil Balan, S. Antimicrobial activities of a promising glycolipid biosurfactant from a novel marine Staphylococcus saprophyticus SBPS 15. 3 Biotech 2016, 6, 163. [Google Scholar] [CrossRef] [Green Version]
- Valotteau, C.; Banat, I.M.; Mitchell, C.A.; Lydon, H.; Marchant, R.; Babonneau, F.; Pradier, C.-M.; Baccile, N.; Humblot, V. Antibacterial properties of sophorolipid-modified gold surfaces against Gram-positive and Gram-negative pathogens. Colloids Surf. B Biointerfaces 2017, 157, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef] [Green Version]
- De Freitas Ferreira, J.; Vieira, E.A.; Nitschke, M. The antibacterial activity of rhamnolipid biosurfactant is pH dependent. Food Res. Int. 2019, 116, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.Z.V.; Nitschke, M. Evaluation of rhamnolipids surfactants as agents to reduce the adhesion of Staphylococcus aureus to polystyrene surfaces. Lett. Appl. Microbiol. 2012, 49, 960–965. [Google Scholar]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz De Rienzo, M.A.; Stevenson, P.; Marchant, R.; Banat, I.M. Antibacterial properties of biosurfactants against selected Gram-positive and-negative bacteria. FEMS Microbiol. Lett. 2015, 363, fnv224. [Google Scholar] [CrossRef] [Green Version]
- Boles, B.R.; Thoendel, M.; Singh, P.K. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol. Microbiol. 2005, 57, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Ibacache-Quiroga, C.; Ojeda, J.; Espinoza-Vergara, G.; Olivero, P.; Cuellar, M.; Dinamarca, M.A. Thehydrocarbon-degrading marine bacterium Cobetia sp. strain MM1IDA2H-1 produces a biosurfactant that interferes with quorum sensing of fish pathogens by signal hijacking. Microb. Biotechnol. 2013, 6, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Bonnichsen, L.; Bygvraa Svenningsen, N.; Rybtke, M.; de Bruijn, I.; Raaijmakers, J.M.; Tolker-Nielsen, T.; Nybroe, O. Lipopeptide biosurfactant viscosin enhances dispersal of Pseudomonas fluorescens SBW25 biofilms. Microbiology (Reading). 2015, 161, 2289–2297. [Google Scholar] [CrossRef]
- Haque, F.; Alfatah, M.; Ganesan, K.; Bhattacharyya, M.S. Inhibitory effect of sophorolipid on Candida albicans biofilm formation and hyphal growth. Sci. Rep. 2016, 6, 23575. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, W.; Zhu, X.; Zhao, H.; Lu, Y.; Zhang, C.; Lu, Z. Surfactin effectively inhibits Staphylococcus aureus adhesion and biofilm formation on surfaces. Appl. Microbiol. Biotechnol. 2019, 103, 4565–4574. [Google Scholar] [CrossRef] [PubMed]
- Elshikh, M.; Funston, S.; Chebbi, A.; Ahmed, S.; Marchant, R.; Banat, I.M. Rhamnolipids from non-pathogenic Burkholderia thailandensis E264: Physicochemical characterization, antimicrobial and antibiofilm efficacy against oral hygiene related pathogens. New Biotechnol. 2017, 36, 26–36. [Google Scholar] [CrossRef]
- Elshikh, M.; Moya-Ramírez, I.; Moens, H.; Roelants, S.; Soetaert, W.; Marchant, R.; Banat, I.M. Rhamnolipids and lactonic sophorolipids: Natural antimicrobial surfactants for oral hygiene. J. Appl. Microbiol. 2017, 123, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.R.; Banat, I.M.; van der Mei, H.C.; Teixeira, J.A.; Oliveira, R. Interference in adhesion of bacteria and yeasts isolated from explanted voice prostheses to silicone rubber by rhamnolipid biosurfactants. J. Appl. Microbiol. 2006, 100, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Elshikh, M.; Marchant, R.; Banat, I.M. Biosurfactants: Promising bioactive molecules for oral-related health applications. FEMS Microbiol. Lett. 2016, 363, fnw213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janek, T.; Krasowska, A.; Czyznikowska, Z.; Łukaszewicz, M. Trehalose Lipid biosurfactant reduces adhesion of microbial pathogens to polystyrene and silicone surfaces: An experimental and computational approach. Front. Microbiol. 2018, 9, 2441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceresa, C.; Fracchia, L.; Williams, M.; Banat, I.M.; Díaz De Rienzo, M.A. The effect of sophorolipids against microbial biofilms on medical-grade silicone. J. Biotechnol. 2020, 309, 34–43. [Google Scholar] [CrossRef]
- Ceresa, C.; Hutton, S.; Lajarin-Cuesta, M.; Heaton, R.; Hargreaves, I.; Fracchia, L.; Díaz De Rienzo, M.A. Production of Mannosylerythritol Lipids (MELs) to be used as antimicrobial agents against S. aureus ATCC 6538. Curr. Microbiol. 2020, 77, 1373–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontes, C.; Alves, M.; Santos, C.; Ribeiro, M.H.; Gonçalves, L.; Bettencourt, A.F.; Ribeiro, I.A. Can Sophorolipids prevent biofilm formation on silicone catheter tubes? Int. J. Pharm. 2016, 513, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Tambone, E.; Bonomi, E.; Ghensi, P.; Maniglio, D.; Ceresa, C.; Agostinacchio, F.; Caciagli, P.; Nollo, G.; Piccoli, F.; Caola, I.; et al. Rhamnolipid coating reduces microbial biofilm formation on titanium implants: An in vitro study. BMC Oral Health 2021, 21, 49. [Google Scholar] [CrossRef]








| Rhamnolipids Congeners |
Rt min |
[M-H]− m/z |
MS/MS (nce% 1) m/z |
Abundance % |
|---|---|---|---|---|
| mR89BS (fraction I) | ||||
| Rha - C10 - C8/C8 - C10 | 10.18 | 475 | 305, 333, 311 (27) | 2.8 |
| Rha - C10 - C10 | 14.54 | 503 | 333, 339 (27) | 90.1 |
| Rha - C12 - C10/C10 - C12 | 18.32 | 531 | 333, 361, 367 (27) | 7.1 |
| Total | 100 | |||
| dR89BS (fraction II) | ||||
| Rha - Rha - C10 - C10 | 12.12 | 649 | 339, 479 (28) | 96.9 |
| Rha - Rha - C10 - C12/C12 - C10 | 15.65 | 677 | 507, 479, 367 (29) | 3.1 |
| Total | 100 |
|
Rhamnolipids
Congeners |
3 Days
mg/g (sd 1) |
5 Days
mg/g (sd) |
6 Days
mg/g (sd) |
|---|---|---|---|
| Mono-rhamnolipids | |||
| Rha-C10-C8/C8-C10 | 9.22 (0.93) | 19.53 (1.93) | 14.93 (1.34) |
| Rha-C10-C10 | 299.93 (15.12) | 635.88 (33.70) | 485.48 (23.64) |
| Rha-C12-C10/C10-C12 | 2.84 (0.25) | 50.55 (5.35) | 38.59 (3.72) |
| Total | 311.99 | 705.96 | 539.00 |
| Di-rhamnolipids | |||
| Rha-Rha-C10-C10 | 217.80 (17.21) | 201.67 (16.34) | 205.20 (16.22) |
| Rha-Rha-C10-C12/C12-C10 | 7.21 (0.71) | 6.67 (0.88) | 24.13 (2.97) |
| Total | 225.01 | 208.34 | 229.33 |
| Total rhamnolipids | 537.00 | 914.30 | 768.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegrone, G.; Ceresa, C.; Rinaldi, M.; Fracchia, L. Diverse Effects of Natural and Synthetic Surfactants on the Inhibition of Staphylococcus aureus Biofilm. Pharmaceutics 2021, 13, 1172. https://doi.org/10.3390/pharmaceutics13081172
Allegrone G, Ceresa C, Rinaldi M, Fracchia L. Diverse Effects of Natural and Synthetic Surfactants on the Inhibition of Staphylococcus aureus Biofilm. Pharmaceutics. 2021; 13(8):1172. https://doi.org/10.3390/pharmaceutics13081172
Chicago/Turabian StyleAllegrone, Gianna, Chiara Ceresa, Maurizio Rinaldi, and Letizia Fracchia. 2021. "Diverse Effects of Natural and Synthetic Surfactants on the Inhibition of Staphylococcus aureus Biofilm" Pharmaceutics 13, no. 8: 1172. https://doi.org/10.3390/pharmaceutics13081172
APA StyleAllegrone, G., Ceresa, C., Rinaldi, M., & Fracchia, L. (2021). Diverse Effects of Natural and Synthetic Surfactants on the Inhibition of Staphylococcus aureus Biofilm. Pharmaceutics, 13(8), 1172. https://doi.org/10.3390/pharmaceutics13081172