Enhanced Bilosomal Properties Resulted in Optimum Pharmacological Effects by Increased Acidification Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microencapsulation Technology
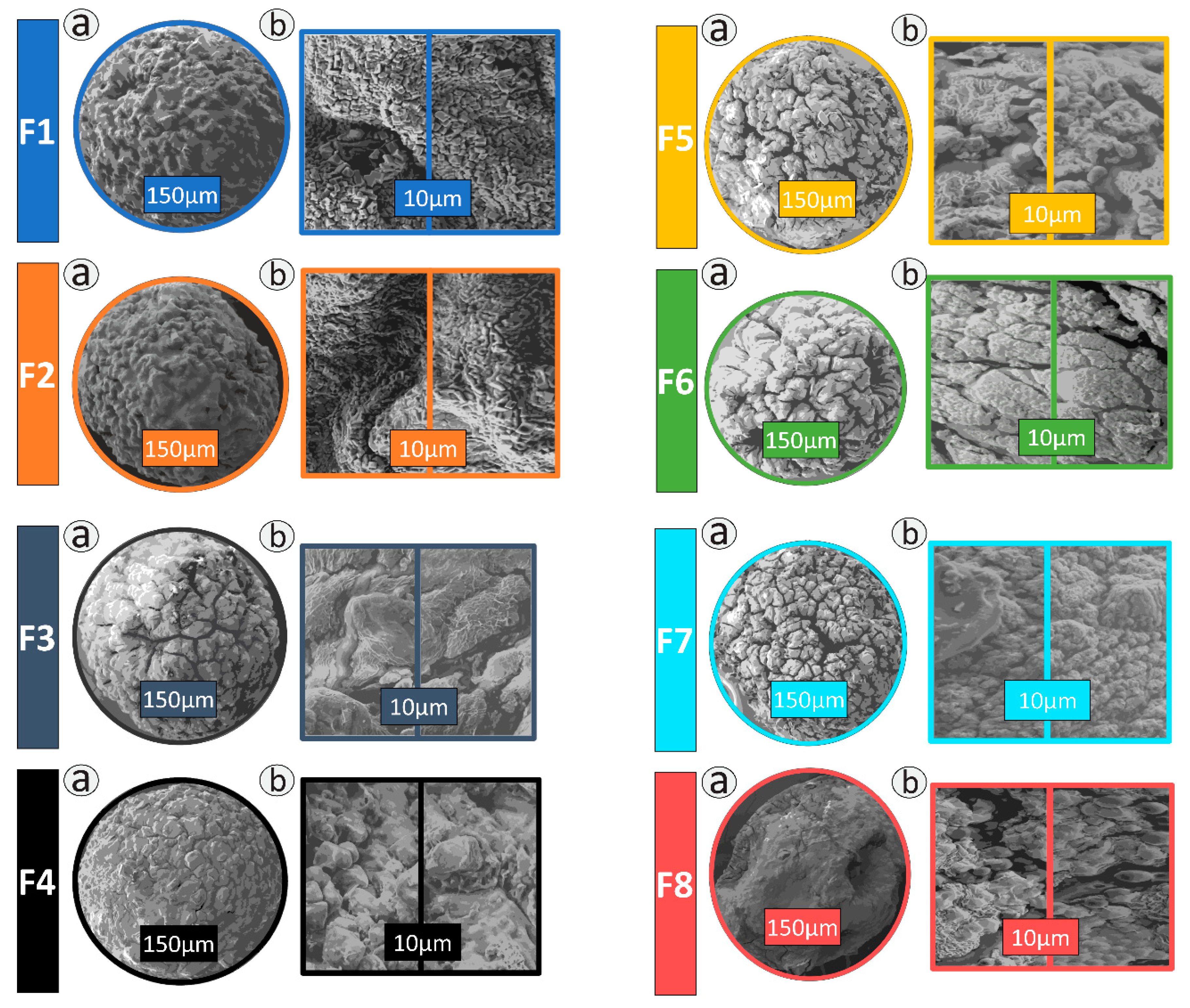
2.3. Morphology and Topography Studies
2.4. Sizing
2.5. Cell Viability Assays
2.6. Insulin Production and MTT
2.7. Inflammatory Markers
2.8. Mitochondrial Activity
3. Results
3.1. Microscopy
3.2. Insulin Production and MTT
3.3. Inflammatory Markers
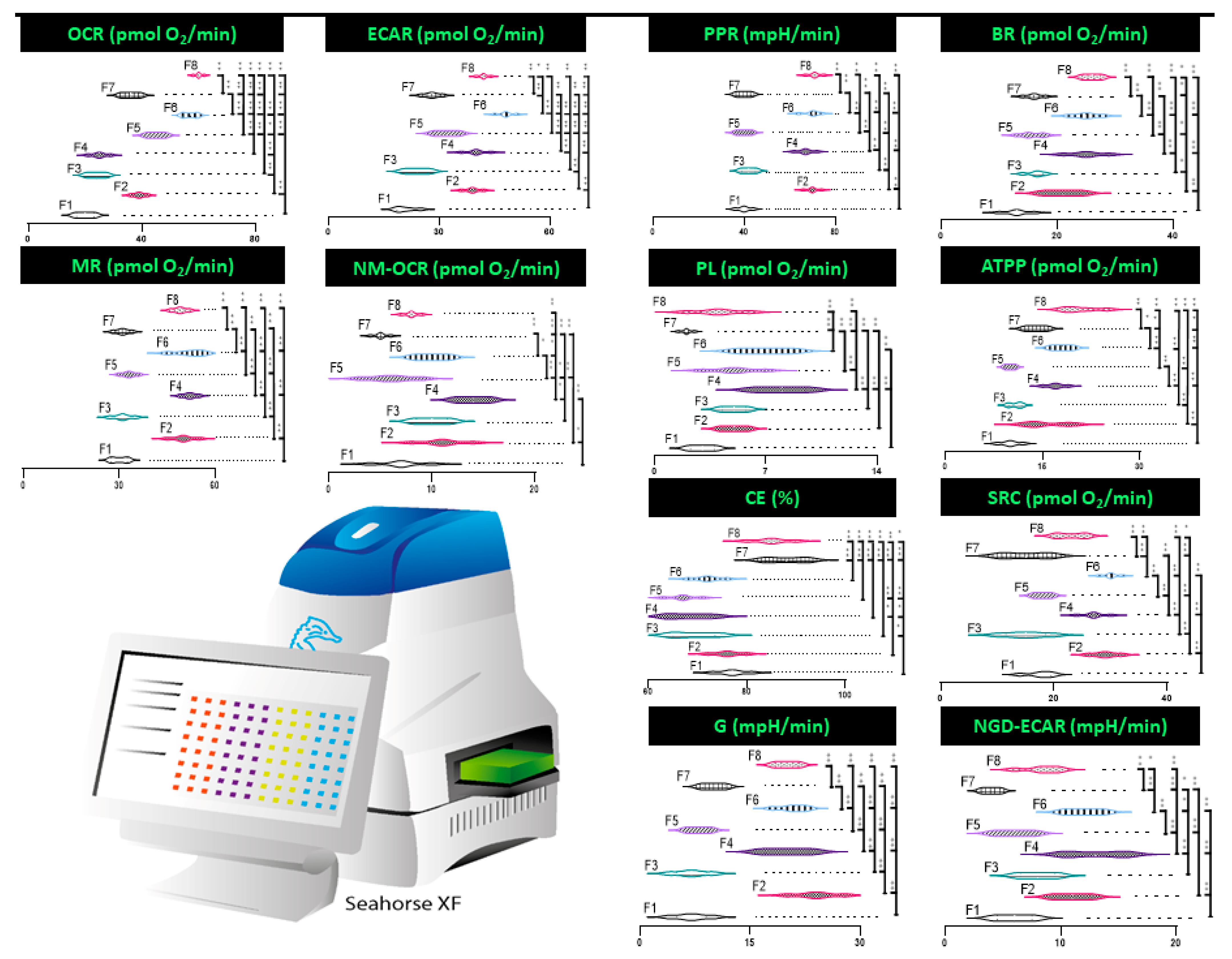
3.4. Mitochondrial Respiration, Metabolism, and Bioenergetics
4. Discussion
4.1. Insulin Production and MTT
4.2. Inflammatory Markers
4.3. Mitochondrial Respiration, Metabolism, and Bioenergetics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stumvoll, M.; Goldstein, B.J.; Van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Torpy, J.M.; Lynm, C.; Glass, R.M. Diabetes. JAMA 2009, 301, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, W.A.; Peters, K.E.; Makepeace, A.; Griffiths, S.; Bundell, C.; Grant, S.F.A.; Ellard, S.; Hattersley, A.T.; Chubb, S.A.P.; Bruce, D.G.; et al. Prevalence of diabetes in Australia: Insights from the Fremantle Diabetes Study Phase II. Intern. Med. J. 2018, 48, 803–809. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Diabetes Expenditure in Australia 2008–09; AIHW: Canberra, Australia, 2013.
- Nolan, C.J.; Damm, P.; Prentki, M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet 2011, 378, 169–181. [Google Scholar] [CrossRef]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, A. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef]
- Riaz, M.; Basit, A.; Fawwad, A.; Ahmedani, M.Y.; Rizvi, Z.A. Factors associated with non-adherence to insulin in patients with type 1 diabetes. Pak. J. Med. Sci. 1969, 30, 233–239. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. Primary bile acid chenodeoxycholic acid-based microcapsules to examine β-cell survival and the inflammatory response. BioNanoScience 2016, 6, 103–109. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Mikov, M.; Golocorbin-Kon, S.; Arfuso, F.; Al-Salami, H. Novel chenodeoxycholic acid–sodium alginate matrix in the microencapsulation of the potential antidiabetic drug, probucol. An in vitro study. J. Microencapsul. 2015, 32, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Wagle, S.R.; Walker, D.; Kovacevic, B.; Gedawy, A.; Mikov, M.; Golocorbin-Kon, S.; Mooranian, A.; Al-Salami, H. Micro-Nano formulation of bile-gut delivery: Rheological, stability and cell survival, basal and maximum respiration studies. Sci. Rep. 2020, 10, 7715. [Google Scholar] [CrossRef] [PubMed]
- Wagle, S.R.; Kovacevic, B.; Walker, D.; Ionescu, C.M.; Shah, U.; Stojanovic, G.; Kojic, S.; Mooranian, A.; Al-Salami, H. Alginate-based drug oral targeting using bio-micro/nano encapsulation technologies. Expert Opin. Drug Deliv. 2020, 17, 1361–1376. [Google Scholar] [CrossRef]
- Kecman, S.; Škrbić, R.; Cengic, A.B.; Mooranian, A.; Al-Salami, H.; Mikov, M.; Golocorbin-Kon, S. Potentials of human bile acids and their salts in pharmaceutical nano delivery and formulations adjuvants. Technol. Health Care 2020, 28, 325–335. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Takechi, R.; Al-Sallami, H.; Mikov, M.; Goločorbin-Kon, S.; Kovacevic, B.; Arfuso, F.; Al-Salami, H. Pharmacological effects of nanoencapsulation of human-based dosing of probucol on ratio of secondary to primary bile acids in gut, during induction and progression of type 1 diabetes. Artif. Cells Nanomed. Biotechnol. 2018, 46, S748–S754. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Zamani, N.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Al-Salami, H. Novel nano-encapsulation of probucol in microgels: Scanning electron micrograph characterizations, buoyancy profiling, and antioxidant assay analyses. Artif. Cells Nanomed. Biotechnol. 2018, 46, S741–S747. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Zamani, N.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Al-Salami, H. Eudragit®-based microcapsules of probucol with a gut-bacterial processed secondary bile acid. Ther. Deliv. 2018, 9, 811–821. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Mamo, J.; Al-Sallami, H.; Al-Salami, H. The biological effects of the hypolipidaemic drug probucol microcapsules fed daily for 4 weeks, to an insulin-resistant mouse model: Potential hypoglycaemic and anti-inflammatory effects. Drug Deliv. Transl. Res. 2018, 8, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. Viability and topographical analysis of microencapsulated β-cells exposed to a biotransformed tertiary bile acid: An ex vivo study. Int. J. Nano Biomater. 2016, 6, 74. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. Flow vibration-doubled concentric system coupled with low ratio amine to produce bile acid-macrocapsules of β-cells. Ther. Deliv. 2016, 7, 171–178. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The impact of allylamine-bile acid combinations on cell delivery microcapsules in diabetes. J. Microencapsul. 2016, 33, 569–574. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Sallami, H.S.; Fang, Z.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Watts, G.; Matthews, V.; Arfuso, F.; et al. Probucol Release from Novel Multicompartmental Microcapsules for the Oral Targeted Delivery in Type 2 Diabetes. AAPS PharmSciTech 2014, 16, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Fakhoury, M.; Al-Salami, H.; Negrulj, R.; Mooranian, A. Inflammatory bowel disease: Clinical aspects and treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Di Ciaula, A.; Garruti, G.; Baccetto, L.R.; Molina, E.-M.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2017, 16, S4–S14. [Google Scholar] [CrossRef]
- Negrulj, R.; Mooranian, A.; Al-Salami, H. Potentials and limitations of bile acids in type 2 diabetes mellitus: Applications of microencapsulation as a novel oral delivery system. J. Endocrinol. Diabetes Mellit. 2013, 1, 49–59. [Google Scholar]
- Mooranian, A.; Zamani, N.; Takechi, R.; Luna, G.; Mikov, M.; Goločorbin-Kon, S.; Kovacevic, B.; Arfuso, F.; Al-Salami, H. Modulatory nano/micro effects of diabetes development on pharmacology of primary and secondary bile acids concentrations. Curr. Diabetes Rev. 2020, 16, 900–909. [Google Scholar] [CrossRef]
- Prawitt, J.; Caron, S.; Staels, B. Bile acid metabolism and the pathogenesis of type 2 diabetes. Curr. Diabetes Rep. 2011, 11, 160–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Salami, H.; Mamo, J.C.; Mooranian, A.; Negrulj, R.; Lam, V.; Elahy, M.; Takechi, R. Long-term supplementation of microencapsulated ursodeoxycholic acid prevents hypertension in a mouse model of insulin resistance. Exp. Clin. Endocrinol. Diabetes 2016, 125, 28–32. [Google Scholar] [CrossRef]
- Negrulj, R.; Mooranian, A.; Chen-Tan, N.; Al-Sallami, H.S.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Watts, G.; Arfuso, F.; Al-Salami, H. Swelling, mechanical strength, and release properties of probucol microcapsules with and without a bile acid, and their potential oral delivery in diabetes. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1290–1297. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Biological assessments of encapsulated pancreatic β-Cells: Their potential transplantation in diabetes. Cell. Mol. Bioeng. 2016, 9, 530–537. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Fakhoury, M.; Arfuso, F.; Jones, F.; Al-Salami, H. Advanced bile acid-based multi-compartmental microencapsulated pancreatic β-cells integrating a polyelectrolyte-bile acid formulation, for diabetes treatment. Artif. Cells Nanomed. Biotechnol. 2014, 44, 588–595. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. Multicompartmental, multilayered probucol microcapsules for diabetes mellitus: Formulation characterization and effects on production of insulin and inflammation in a pancreatic β-cell line. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1642–1653. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. Characterization of a novel bile acid-based delivery platform for microencapsulated pancreatic β-cells. Artif. Cells Nanomed. Biotechnol. 2014, 44, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Al-Salami, H.; Morahan, G.; Jamieson, E. Designing anti-diabetic β-cells microcapsules using polystyrenic sulfonate, polyallylamine, and a tertiary bile acid: Morphology, bioenergetics, and cytokine analysis. Biotechnol. Prog. 2016, 32, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The incorporation of water-soluble gel matrix into bile acid-based microcapsules for the delivery of viable β-cells of the pancreas, in diabetes treatment: Biocompatibility and functionality studies. Drug Deliv. Transl. Res. 2015, 6, 17–23. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The Influence of Stabilized Deconjugated Ursodeoxycholic Acid on Polymer-Hydrogel System of Transplantable NIT-1 Cells. Pharm. Res. 2016, 33, 1182–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. Alginate-deoxycholic acid interaction and its impact on pancreatic β-cells and insulin secretion and potential treatment of type 1 diabetes. J. Pharm. Innov. 2016, 11, 156–161. [Google Scholar] [CrossRef]
- Zekorn, T.; Siebers, U.; Horcher, A.; Schnettler, R.; Zimmermann, U.; Bretzel, R.G.; Federlin, K. Alginate coating of islets of Langerhans: In vitro studies on a new method for microencapsulation for immuno-isolated transplantation. Acta Diabetol. 1992, 29, 41–45. [Google Scholar] [CrossRef] [PubMed]
- De Vos, P.; De Haan, B.J.; Wolters, G.H.J.; Strubbe, J.H.; Van Schilfgaarde, R. Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia 1997, 40, 262–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duvivier-Kali, V.F.; Omer, A.; Parent, R.J.; O’Neil, J.J.; Weir, G.C. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes 2001, 50, 1698–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calafiore, R. Alginate microcapsules for pancreatic islet cell graft immunoprotection: Struggle and progress towards the final cure for type 1 diabetes mellitus. Exp. Opin. Biol. Ther. 2003, 3, 201–205. [Google Scholar] [CrossRef]
- De Vos, P.; Faas, M.M.; Strand, B.; Calafiore, R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 2006, 27, 5603–5617. [Google Scholar] [CrossRef]
- Jacobs-Tulleneers-Thevissen, D.; Chintinne, M.M.; Ling, Z.; Gillard, P.; Schoonjans, L.L.; Delvaux, G.; Strand, B.L.; Gorus, F.; Keymeulen, B.; Pipeleers, D. Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia 2013, 56, 1605–1614. [Google Scholar] [CrossRef] [Green Version]
- Arola, S.; Ansari, M.; Oksanen, A.; Retulainen, E.; Hatzikiriakos, S.G.; Brumer, H. The sol–gel transition of ultra-low solid content TEMPO-cellulose nanofibril/mixed-linkage β-glucan bionanocomposite gels. Soft Matter 2018, 14, 9393–9401. [Google Scholar] [CrossRef]
- Peng, B.; Luo, Y.; Hu, X.; Song, L.; Yang, J.; Zhu, J.; Wen, Y.; Yu, R. Isolation, structural characterization, and immunostimulatory activity of a new water-soluble polysaccharide and its sulfated derivative from Citrus medica L. var. sarcodactylis. Int. J. Biol. Macromol. 2019, 123, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. The effect of molecular weights of microencapsulating polymers on viability of mouse-cloned pancreatic β-cells: Biomaterials, osmotic forces and potential applications in diabetes treatment. Pharm. Dev. Technol. 2017, 23, 145–150. [Google Scholar] [CrossRef] [PubMed]
- De Vos, P.; Van Straaten, J.F.; Nieuwenhuizen, A.; De Groot, M.; Ploeg, R.J.; De Haan, B.J.; Van Schilfgaarde, R. Why do microencapsulated islet grafts fail in the absence of fibrotic overgrowth? Diabetes 1999, 48, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Del Guerra, S.; Bracci, C.; Nilsson, K.; Belcourt, A.; Kessler, L.; Lupi, R.; Marselli, L.; de Vos, P.; Marchetti, P. Entrapment of dispersed pancreatic islet cells in CultiSpher-S macroporous gelatin microcarriers: Preparation, in vitro characterization, and microencapsulation. Biotechnol. Bioeng. 2001, 75, 741–744. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Influence of biotechnological processes, speed of formulation flow and cellular concurrent stream-integration on insulin production from β-cells as a result of co-encapsulation with a highly lipophilic bile acid. Cell. Mol. Bioeng. 2017, 11, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Zamani, N.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Kovacevic, B.; Al-Salami, H. Bio Micro-Nano Technologies of Antioxidants Optimised Their Pharmacological and Cellular Effects, ex vivo, in Pancreatic β-Cells. Nanotechnol. Sci. Appl. 2020, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Zamani, N.; Kovacevic, B.; Ionescu, C.M.; Luna, G.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Kojic, S.; Al-Salami, H. Pharmacological effects of secondary bile acid microparticles in diabetic murine model. Curr. Diabetes Rev. 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Ionescu, C.M.; Takechi, R.; Luna, G.; Mikov, M.; Goločorbin-Kon, S.; Kovacevic, B.; Al-Salami, H. Oral gavage of nano-encapsulated conjugated acrylic acid-bile acid formulation in type 1 diabetes altered pharmacological profile of bile acids, and improved glycaemia and suppressed inflammation. Pharmacol. Rep. 2020, 72, 368–378. [Google Scholar] [CrossRef]
- Mooranian, A.; Wagle, S.R.; Kovacevic, B.; Takechi, R.; Mamo, J.; Lam, V.; Watts, G.F.; Mikov, M.; Golocorbin-Kon, S.; Stojanovic, G.; et al. Bile acid bio-nanoencapsulation improved drug targeted-delivery and pharmacological effects via cellular flux: 6-months diabetes preclinical study. Sci. Rep. 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Mathavan, S.; Ionescu, C.M.; Kovacevic, B.; Mikov, M.; Golocorbin-Kon, S.; Mooranian, A.; Dass, C.R.; Al-Salami, H. Histological effects of pharmacologically active human bile acid nano/micro-particles in Type-1 diabetes. Ther. Deliv. 2020, 11, 157–171. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Takechi, R.; Luna, G.; Mikov, M.; Goločorbin-Kon, S.; Elnashar, M.; Arfuso, F.; Al-Salami, H. An in vivo pharmacological study: Variation in tissue-accumulation for the drug probucol as the result of targeted microtechnology and matrix-acrylic acid optimization and stabilization techniques. PLoS ONE 2019, 14, e0214984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooranian, A.; Zamani, N.; Takechi, R.; Al-Sallami, H.; Mikov, M.; Goločorbin-Kon, S.; Kovacevic, B.; Arfuso, F.; Al-Salami, H. Probucol-poly(meth)acrylate-bile acid nanoparticles increase IL-10, and primary bile acids in prediabetic mice. Ther. Deliv. 2019, 10, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Zamani, N.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Al-Salami, H. Stability and biological testing of taurine-conjugated bile acid antioxidant microcapsules for diabetes treatment. Ther. Deliv. 2019, 10, 99–106. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Luna, G.; Al-Sallami, H.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Kovacevic, B.; Al-Salami, H. Bile acid-polymer-probucol microparticles: Protective effect on pancreatic β-cells and decrease in type 1 diabetes development in a murine model. Pharm. Dev. Technol. 2019, 24, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Mathavan, S.; Ionescu, C.M.; Kovacevic, B.; Mikov, M.; Golocorbin-Kon, S.; Mooranian, A.; Dass, C.R.; Al-Salami, H. Formulation buoyancy of nanoencapsulated gliclazide using primary, conjugated and deconjugated bile acids. Ther. Deliv. 2019, 10, 573–583. [Google Scholar] [CrossRef]
- Mamo, J.C.; Lam, V.; Brook, E.; Mooranian, A.; Al-Salami, H.; Fimognari, N.; Nesbit, M.; Takechi, R. Probucol prevents blood–brain barrier dysfunction and cognitive decline in mice maintained on pro-diabetic diet. Diabetes Vasc. Dis. Res. 2018, 16, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Electrokinetic potential-stabilization by bile acid-microencapsulating formulation of pancreatic β-cells cultured in high ratio poly-L-ornithine-gel hydrogel colloidal dis-persion: Applications in cell-biomaterials, tissue engineering and biotechnological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1156–1162. [Google Scholar]
- Hamaguchi, K.; Gaskins, H.R.; Leiter, E.H. NIT-1, a pancreatic β-cell line established from a transgenic NOD/Lt mouse. Diabetes 1991, 40, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The MTT assay. Cancer Cell Cult. 2011, 731, 237–245. [Google Scholar]
- Al-Salami, H.; Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Watts, G.; Arfuso, F. An optimized probucol microencapsulated formulation integrating a secondary bile acid (deoxycholic acid) as a permeation enhancer. Drug Des. Dev. Ther. 2014, 8, 1673–1683. [Google Scholar] [CrossRef] [Green Version]
- Pavlović, N.; Goločorbin-Kon, S.; Đanić, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile Acids and their derivatives as potential modifiers of drug release and pharmacokinetic profiles. Front. Pharmacol. 2018, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.S.; Moses, A.C.; Silver, R.D.; Flier, J.S.; Carey, M.C. Nasal absorption of insulin: Enhancement by hydrophobic bile salts. Proc. Natl. Acad. Sci. USA 1985, 82, 7419–7423. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, K.; Kumar, T.S.; Lee, J.; Kim, S.K.; Lee, D.Y.; Lee, Y.-K.; Byun, Y. Synthesis and biological properties of insulin−deoxycholic acid chemical conjugates. Bioconjug. Chem. 2005, 16, 615–620. [Google Scholar] [CrossRef]
- Parks, D.J.; Blanchard, S.G.; Bledsoe, R.K.; Chandra, G.; Consler, T.G.; Kliewer, S.A.; Stimmel, J.B.; Willson, T.M.; Zavacki, A.M.; Moore, D.D.; et al. Bile acids: Natural ligands for an orphan nuclear receptor. Science 1999, 284, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, B.; Jones, S.A.; Price, R.R.; Watson, M.A.; McKee, D.D.; Moore, L.B.; Galardi, C.; Wilson, J.G.; Lewis, M.C.; Roth, M.E.; et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 2000, 6, 517–526. [Google Scholar] [CrossRef]
- Renga, B.; Mencarelli, A.; Vavassori, P.; Brancaleone, V.; Fiorucci, S. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipriani, S.; Mencarelli, A.; Palladino, G.; Fiorucci, S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J. Lipid Res. 2010, 51, 771–784. [Google Scholar] [CrossRef] [Green Version]
- Syring, K.E.; Cyphert, T.J.; Beck, T.; Flynn, C.; Mignemi, N.A.; McGuinness, O.P. Systemic bile acids induce insulin resistance in a TGR5-independent manner. Am. J. Physiol. Metab. 2019, 316, E782–E793. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.; Kim, Y.M.; Kim, Y.W.; Kim, S.G. Farnesoid X receptor activation by chenodeoxycholic acid induces detoxifying enzymes through AMP-activated protein kinase and extracellular signal-regulated kinase 1/2-mediated phosphorylation of CCAAT/enhancer binding protein β. Drug Metab. Dispos. 2011, 39, 1451–1459. [Google Scholar] [CrossRef] [Green Version]
- Dyrszka, H.; Chen, T.; Salen, G.; Mosbach, E.H. Toxicity of chenodeoxycholic acid in the rhesus monkey. Gastroenterology 1975, 69, 333–337. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.H.; Lee, J.A.; Namkung, W.; Sun, A.; Ananthanarayanan, M.; Suchy, F.J.; Shin, D.M.; Muallem, S.; Lee, M.G. Transporter-mediated bile acid uptake causes Ca2+-dependent cell death in rat pancreatic acinar cells. Gastroenterology 2002, 122, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Picarella, D.E.; Kratz, A.; Li, C.; Ruddle, N.H.; Flavell, R. Transgenic tumor necrosis factor (TNF)-alpha production in pancreatic islets leads to insulitis, not diabetes. Distinct patterns of inflammation in TNF-alpha and TNF-beta transgenic mice. J. Immunol. 1993, 150, 4136–4150. [Google Scholar] [PubMed]
- Hollman, D.A.; Milona, A.; van Erpecum, K.J.; van Mil, S.W. Anti-inflammatory and metabolic actions of FXR: Insights into molecular mechanisms. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Shaik, F.B.; Panati, K.; Narasimha, V.R.; Narala, V.R. Chenodeoxycholic acid attenuates ovalbumin-induced airway inflamma-tion in murine model of asthma by inhibiting the TH2 cytokines. Biochem. Biophys. Res. Commun. 2015, 463, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Gadaleta, R.M.; Van Erpecum, K.J.; Oldenburg, B.; Willemsen, E.C.L.; Renooij, W.; Murzilli, S.; Klomp, L.W.J.; Siersema, P.D.; Schipper, M.E.I.; Danese, S.; et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011, 60, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Greve, J.W.; Gouma, D.J.; Buurman, W.A. Bile acids inhibit endotoxin-induced release of tumor necrosis factor by monocytes: Anin vitro study. Hepatology 1989, 10, 454–458. [Google Scholar] [CrossRef]
- Horikawa, T.; Oshima, T.; Li, M.; Kitayama, Y.; Eda, H.; Nakamura, K.; Tamura, A.; Ogawa, T.; Yamasaki, T.; Okugawa, T.; et al. Chenodeoxycholic acid releases proinflammatory cytokines from small intestinal epithelial cells through the farnesoid x receptor. Digestion 2019, 100, 286–294. [Google Scholar] [CrossRef]
- Chang, I.; Cho, N.; Kim, S.; Kim, J.Y.; Kim, E.; Woo, J.-E.; Nam, H.J.; Kim, S.J.; Lee, M.-S. Role of calcium in pancreatic islet cell death by IFN-γ/TNF-α. J. Immunol. 2004, 172, 7008–7014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeki, R.; Ogino, H.; Kaneko, S.; Unoura, M.; Kobayashi, K. Effects of chenodeoxycholic and ursodeoxycholic acids on inter-feron-γ production by peripheral blood mononuclear cells from patients with primary biliary cirrhosis. J. Gastroenterol. 1995, 30, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, T.; Yu, D.; Forman, B.M.; Huang, W. FXR Protects lung from lipopolysaccharide-induced acute injury. Mol. Endocrinol. 2012, 26, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Pirot, P.; Eizirik, D.L.; Cardozo, A.K. Interferon-γ potentiates endoplasmic reticulum stress-induced death by reducing pan-creatic beta cell defence mechanisms. Diabetologia 2006, 49, 1229–1236. [Google Scholar] [CrossRef] [Green Version]
- Oh, Y.S.; Lee, Y.-J.; Park, E.Y.; Jun, H.-S. Interleukin-6 treatment induces beta-cell apoptosis via STAT-3-mediated nitric oxide production. Diabetes Metab. Res. Rev. 2011, 27, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Calmus, Y.; Guechot, J.; Podevin, P.; Bonnefis, M.T.; Giboudeau, J.; Poupon, R. Differential effects of chenodeoxycholic and ur-sodeoxycholic acids on interleukin 1, interleukin 6 and tumor necrosis factor–α production by monocytes. Hepatology 1992, 16, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; Kohlmann, C.; Haselow, K.; Gehrmann, T.; Bode, J.G.; Häussinger, D. Bile acids inhibit interleukin-6 signaling via gp130 receptor-dependent and-independent pathways in rat liver. Hepatology 2006, 44, 1206–1217. [Google Scholar] [CrossRef]
- Mookerjee, S.A.; Goncalves, R.L.; Gerencser, A.A.; Nicholls, D.G.; Brand, M. The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1847, 171–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katona, M.; Hegyi, P.; Kui, B.; Balla, Z.; Rakonczay, Z., Jr.; Rázga, Z.; Tiszlavicz, L. A novel, protective role of ursodeoxycholate in bile-induced pancreatic ductal injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G193–G204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teodoro, J.; Rolo, A.; Jarak, I.; Palmeira, C.M.; Carvalho, R. The bile acid chenodeoxycholic acid directly modulates metabolic pathways in white adipose tissue in vitro: Insight into how bile acids decrease obesity. NMR Biomed. 2016, 29, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. AG protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broeders, E.P.; Nascimento, E.; Havekes, B.; Brans, B.; Roumans, K.H.; Tailleux, A.; Schaart, G.; Kouach, M.; Charton, J.; Deprez, B.; et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab. 2015, 22, 418–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comeglio, P.; Cellai, I.; Mello, T.; Filippi, S.; Maneschi, E.; Corcetto, F.; Corno, C.; Sarchielli, E.; Morelli, A.; Rapizzi, E.; et al. INT-767 prevents NASH and promotes visceral fat brown adipogenesis and mitochondrial function. J. Endocrinol. 2018, 238, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, M.H.; Burgstahler, A.D.; Masyuk, A.; Larusso, N.F. Stimulation of ATP secretion in the liver by therapeutic bile acids. Biochem. J. 2001, 358, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Heidari, R.; Abdoli, N.; Ommati, M.M.; Jamshidzadeh, A.; Niknahad, H. Mitochondrial impairment induced by chenodeoxycholic acid: The protective effect of taurine and carnosine supplementation. Trends Pharm. Sci. 2018, 4, 2. [Google Scholar]
- Ferreira, M.; Coxito, P.M.; Sardão, V.A.; Palmeira, C.M.; Oliveira, P.J. Bile Acids Are Toxic for Isolated Cardiac Mitochondria: A Possible Cause for Hepatic-Derived Cardiomyopathies? Cardiovasc. Toxicol. 2005, 5, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Maechler, P. Mitochondrial function and insulin secretion. Mol. Cell. Endocrinol. 2013, 379, 12–18. [Google Scholar] [CrossRef]
- Knupp, J.; Arvan, P.; Chang, A. Increased mitochondrial respiration promotes survival from endoplasmic reticulum stress. Cell Death Differ. 2018, 26, 487–501. [Google Scholar] [CrossRef] [Green Version]
- Tekin, Z.; Garfinkel, M.R.; Chon, W.J.; Schenck, L.; Golab, K.; Savari, O.; Thistlethwaite, J.R.; Philipson, L.H.; Majewski, C.; Pannain, S.; et al. Outcomes of pancreatic islet allotransplantation using the edmonton protocol at the university of chicago. Transplant. Direct 2016, 2, e105. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mooranian, A.; Foster, T.; Ionescu, C.M.; Walker, D.; Jones, M.; Wagle, S.R.; Kovacevic, B.; Chester, J.; Johnston, E.; Wong, E.; et al. Enhanced Bilosomal Properties Resulted in Optimum Pharmacological Effects by Increased Acidification Pathways. Pharmaceutics 2021, 13, 1184. https://doi.org/10.3390/pharmaceutics13081184
Mooranian A, Foster T, Ionescu CM, Walker D, Jones M, Wagle SR, Kovacevic B, Chester J, Johnston E, Wong E, et al. Enhanced Bilosomal Properties Resulted in Optimum Pharmacological Effects by Increased Acidification Pathways. Pharmaceutics. 2021; 13(8):1184. https://doi.org/10.3390/pharmaceutics13081184
Chicago/Turabian StyleMooranian, Armin, Thomas Foster, Corina M. Ionescu, Daniel Walker, Melissa Jones, Susbin Raj Wagle, Bozica Kovacevic, Jacqueline Chester, Edan Johnston, Elaine Wong, and et al. 2021. "Enhanced Bilosomal Properties Resulted in Optimum Pharmacological Effects by Increased Acidification Pathways" Pharmaceutics 13, no. 8: 1184. https://doi.org/10.3390/pharmaceutics13081184
APA StyleMooranian, A., Foster, T., Ionescu, C. M., Walker, D., Jones, M., Wagle, S. R., Kovacevic, B., Chester, J., Johnston, E., Wong, E., Atlas, M. D., Mikov, M., & Al-Salami, H. (2021). Enhanced Bilosomal Properties Resulted in Optimum Pharmacological Effects by Increased Acidification Pathways. Pharmaceutics, 13(8), 1184. https://doi.org/10.3390/pharmaceutics13081184









