Encapsulation of Active Pharmaceutical Ingredients in Lipid Micro/Nanoparticles for Oral Administration by Spray-Cooling
Abstract
1. Introduction
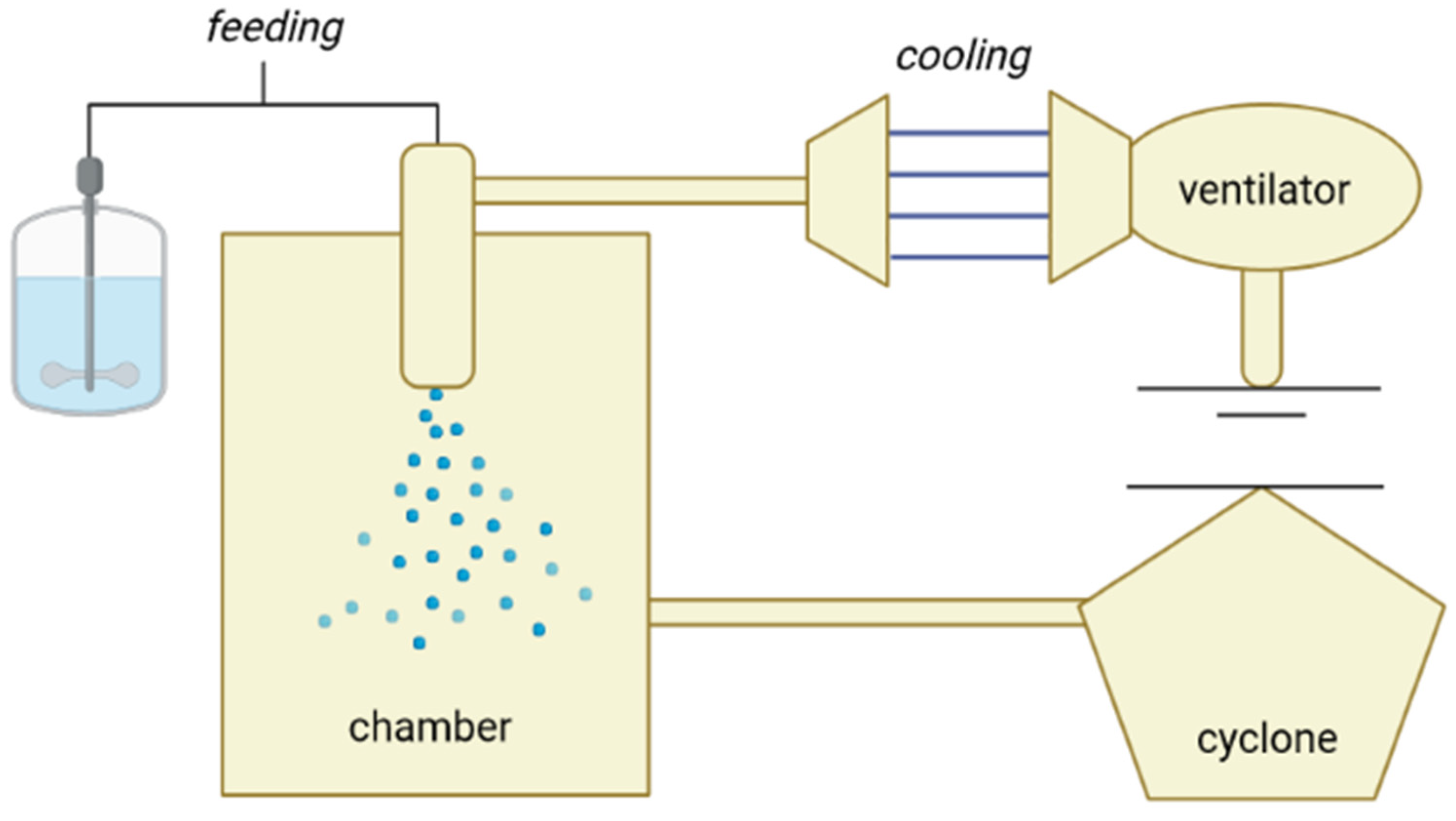
2. Encapsulation by Spray-Cooling
2.1. Process Variables
2.2. Morphological Characteristics of Particles
2.3. Encapsulating Agents
2.4. Release Mechanisms
2.5. Advantages of the Process
2.6. Disadvantages of the Process
3. Encapsulation of APIs by Spray Cooling
3.1. Improvement of the Dissolution Profile
3.2. Sustained-Release Systems
3.3. Increase of Stability
3.4. Flavour Changes
3.5. Encapsulation of Proteins and Peptides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bandaru, S.V.R.; Villanueva, W.; Thakre, S.; Bechta, S. Multi-nozzle spray cooling of a reactor pressure vessel steel plate for the application of ex-vessel cooling. Nucl. Eng. Des. 2021, 375, 111101. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Y.; Wei, W.; Hu, Z.; Li, P. Optimization Design of Spray Cooling Fan Based on CFD Simulation and Field Experiment for Horticultural Crops. Agriculture 2021, 11, 566. [Google Scholar] [CrossRef]
- Fini, A.; Cavallari, C.; Ospitali, F.; Gonzalez-Rodriguez, M.L. Theophylline-loaded compritol microspheres prepared by ultrasound-assisted atomization. J. Pharma Sci. 2011, 100, 743–757. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers—A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Doktorovová, S.; Kovačević, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef]
- Gouin, S. Microencapsulation: Industrial Appraisal of Existing Technologies and Trends. Trends Food Sci. Technol. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Thies, C. A Survey of Microencapsulation Processes. In Microencapsulation: Methods and Industrial Applications; Benita, S., Ed.; Marcel Dekker: New York, NY, USA, 1996; pp. 1–20. [Google Scholar]
- Maschke, A.; Becker, C.; Eyrich, D.; Kiermaier, J.; Blunk, T.; Göpferich, A. Development of a spray congealing process for the preparation of insulin-loaded lipid microparticles and characterization thereof. Eur. J. Pharm. Biopharm. 2007, 65, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Albertini, B.; Passerini, N.; Pattarino, F.; Rodriguez, L. New spray congealing atomizer for the microencapsulation of highly concentrated solid and liquid substances. Eur. J. Pharm. Biopharm. 2008, 69, 348–357. [Google Scholar] [CrossRef]
- Ilic, I.; Dreu, R.; Burjak, M.; Homar, M.; Kerc, J.; Srcic, S. Microparticle size control and glimepiride microencapsulation using spray congealing technology. Int. J. Pharm. 2009, 381, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.S.; Lee, K. Microencapsulation in the food industry. Food Sci. Technol. 1991, 24, 289–297. [Google Scholar]
- Risch, S.J. Encapsulation: Overview of Uses and Techniques. In Encapsulation and Controlled Release of Food Ingredients; Risch, S.J., Reineccius, G.A., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 2–7. [Google Scholar]
- Desai, K.G.H.; Park, H.J. Preparation of cross-linked chitosan microspheres by spray drying: Effect of cross-linking agent on the properties of spray dried microspheres. J. Microencapsul. 2005, 22, 377–395. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Shimoni, E. Overview of Microencapsulates for use in Food Products or Processes and Methods to Make Them. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Zuidam, N.J., Nedović, V.A., Eds.; Springer: La Vergne, TN, USA, 2010; pp. 10–11. [Google Scholar]
- Samad, A.; Tariq, M.; Alam, M.I.; Akhter, M.S. Microsphere: A Novel Drug Delivery System. In Colloids in Drug Delivery; Fanun, M., Ed.; CRC Press: New York, NY, USA, 2010. [Google Scholar]
- Martins, S.; Silva, A.C.; Ferreira, D.C.; Souto, E.B. Improving oral absorption of Salmon calcitonin by trimyristin lipid nanoparticles. J. Biomed. Nanotechnol. 2009, 5, 76–83. [Google Scholar] [CrossRef]
- Almeida, A.J.; Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 2007, 59, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Killen, M.J. Process of spray drying and spray congealing. Pharm. Eng. 1993, 56, 58–62. [Google Scholar]
- Chambi, H.N.M.; Alvim, I.D.; Barrera-Arellano, D.; Grosso, C.R.F. Solid lipid microparticles containing water-soluble compounds of different molecular mass: Production, characterization and release profiles. Food Res. Int. 2008, 41, 229–236. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Kermasha, S.; Alli, I.; Mulligan, C.N. Encapsulation in the food industry: A review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar] [PubMed]
- Akiyama, Y.; Yoshioka, A.; Oribe, H.; Hirai, S.; Kitamori, N.; Toguchi, H. Novel oral controlled-release microspheres using polyglycerol esters of fatty acids. J. Control. Release 1993, 26, 1–10. [Google Scholar] [CrossRef]
- Yajima, T.; Umeki, N.; Itai, S. Optimum spray congealing conditions for masking the bitter taste of clarithromycin in wax matrix. Chem. Pharm. Bull. 1999, 47, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Baldim, I.; Rosa, D.M.; Souza, C.R.F.; Ana, R.d.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Oliveira, W.P. Factors Affecting the Retention Efficiency and Physicochemical Properties of Spray Dried Lipid Nanoparticles Loaded with Lippia sidoides Essential Oil. Biomolecules 2020, 10, 693. [Google Scholar] [CrossRef]
- Baldim, I.; Souza, C.R.F.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Oliveira, W.P. Spray-Dried Structured Lipid Carriers for the Loading of Rosmarinus officinalis: New Nutraceutical and Food Preservative. Foods 2020, 9, 1110. [Google Scholar] [CrossRef]
- Yajima, T.; Itai, S.; Takeuchi, H.; Kawashima, Y. Optimum heat treatment conditions for masking the bitterness of the clarithromycin wax matrix. Chem. Pharm. Bull. 2003, 51, 1223–1226. [Google Scholar] [CrossRef] [PubMed]
- Passerini, N.; Perissutti, B.; Moneghini, M.; Voinovich, D.; Albertini, B.; Cavallari, C.; Rodriguez, L. Characterization of carbamazepine-Gelucire 50/13 microparticles prepared by a spray-congealing process using ultrasounds. J. Pharm. Sci. 2002, 91, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Cánovas, G.V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Drying. In Food Powders. Physical Properties, Processing, and Functionality; Barbosa-Cánovas, G.V., Ortega-Rivas, E., Eds.; Springer-Verlag: New York, NY, USA, 2005. [Google Scholar]
- Liu, X.D.; Atarashi, T.; Furuta, T.; Yoshii, H.; Aishima, S.; Ohkawara, M. Microencapsulation of emulsified hydrophobic flavours by spray drying. Drying Technol. 2001, 19, 1361–1374. [Google Scholar] [CrossRef]
- Lakkis, J.M. Encapsulation and Controlled Release in Bakery Applications. In Encapsulation and Controlled Release Technologies in Food Systems; Lakkis, J.M., Ed.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 116–177. [Google Scholar]
- Lakkis, J.M. Marketing Perspective of Encapsulation Technologies. In Encapsulation and Controlled Release Technologies in Food Systems; Lakkis, J.M., Ed.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 217–218. [Google Scholar]
- Chen, D.; Batycky, R.P.; Johnston, L.; Mintzes, J. Control of Process Humidity to Produce Large, Porous Particles. U.S. Patent 7,469,488, 30 December 2008. [Google Scholar]
- Eldem, T.; Speiser, P.; Altorfer, H. Polymorphic behavior of sprayed lipid micropellets and its evaluation by differential scanning calorimetry and scanning electron microscopy. Pharm. Res. 1991, 8, 178–184. [Google Scholar] [CrossRef]
- Campos, J.R.; Fernandes, A.R.; Severino, P.; Gracia, M.L.; Souto, S.B.; Souto, E.B. Phase Behavior of Polymorphic Fats in Drug Delivery Systems—A Review of the State of Art. Curr. Pharm. Des. 2018, 24, 2508–2512. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Rodriguez, L.; Cavallari, C.; Albertini, B.; Passerini, N. Ultrasound compacted and spray-congealed indomethacin/polyethylene glycol systems. Int. J. Pharm. 2002, 247, 11–22. [Google Scholar] [CrossRef]
- Rodriguez, L.; Passerini, N.; Cavallari, C.; Cini, M.; Sancin, P.; Fini, A. Description and preliminary evaluation of a new ultrasonic atomiser for spray congealing processes. Int. J. Pharm. 1999, 183, 133–143. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Nedovic, V.A. Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer: New York, NY, USA, 2010. [Google Scholar]
- Leonel, A.J. Production and characterization of lipid microparticles produced by spray cooling encapsulating a low molar mass hydrophilic compound. Ciênc. Tecnol. Aliment. 2010, 30, 276–281. [Google Scholar] [CrossRef][Green Version]
- Severino, P.; Silveira, E.F.; Loureiro, K.; Chaud, M.V.; Antonini, D.; Lancellotti, M.; Sarmento, V.H.; da Silva, C.F.; Santana, M.H.A.; Souto, E.B. Antimicrobial activity of polymyxin-loaded solid lipid nanoparticles (PLX-SLN): Characterization of physicochemical properties and in vitro efficacy. Eur. J. Pharm. Sci. 2017, 106, 177–184. [Google Scholar] [CrossRef]
- Achanta, A.S.; Adusumilli, P.S.; James, K.W.; Rhodes, C.T. Development of hot melt coating methods. Drug Dev. Ind. Pharm. 1997, 23, 441–449. [Google Scholar] [CrossRef]
- Heng, P.W.S.; Wong, T.W. Melt Processes for Oral Solid Dosage Forms. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J., Ed.; Informa Healthcare: New York, NY, USA, 2007; Volume 4, pp. 2257–2261. [Google Scholar]
- Passerini, N.; Perissutti, B.; Albertini, B.; Voinovich, D.; Moneghini, M.; Rodriguez, L. Controlled release of verapamil hydrochloride from waxy microparticles prepared by spray congealing. J. Control. Release 2003, 88, 263–275. [Google Scholar] [CrossRef]
- Albertini, B.; Passerini, N.; González-Rodríguez, M.L.; Perissutti, B.; Rodriguez, L. Effect of Aerosil® on the properties of lipid controlled release microparticles. J. Control. Release 2004, 100, 233–246. [Google Scholar] [CrossRef]
- Cavallari, C.; Rodriguez, L.; Albertini, B.; Passerini, N.; Rosetti, F.; Fini, A. Thermal and fractal analysis of diclofenac/Gelucire 50/13 microparticles obtained by ultrasound-assisted atomization. J. Pharm. Sci. 2005, 94, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Passerini, N.; Albertini, B.; Perissutti, B.; Rodriguez, L. Evaluation of melt granulation and ultrasonic spray congealing as techniques to enhance the dissolution of praziquantel. Int. J. Pharm. 2006, 318, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Yajima, T.; Nogata, A.; Demachi, M.; Umeki, N.; Itai, S. Particle design for taste-masking using a spray-congealing technique. Chem. Pharm. Bull. 1996, 44, 187–191. [Google Scholar] [CrossRef]
- Wanasundara, U.N.; Shahidi, F. Storage stability of microencapsulated seal blubber oil. J. Food Lipids 1995, 2, 73–86. [Google Scholar] [CrossRef]
- Whorton, C. Factors Influencing Volatile Release from Encapsulation Matrices. In Encapsulation and Controlled Release of Food Ingredients; Rish, S.J., Reineccius, G.A., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 134–142. [Google Scholar]
- Depypere, F.; Dewettinck, K.; Ronsse, F.; Pieters, J.G. Food powder microencapsulation: Principles, problems and opportunities. Appl. Biotechnol. Food Sci. Pol. 2003, 1, 75–94. [Google Scholar]
- Brazel, C.S. Microencapsulation: Offering solutions for the food industry. Cereal Foods World 1999, 44, 388–390. [Google Scholar]
- Fávaro-Trindade, C.S.; Grosso, C.R.F. Microencapsulation of L. acidophilus and B. lactis and evaluation of their survival at the pHs values of the stomach and in bile. J. Microencapsul. 2002, 19, 485–494. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Kim, D.B.; Naa, K.; Choi, H.K. Preparation and characterization of solid lipid nanoparticles (SLN) made of cacao butter and curdlan. Eur. J. Pharm. Sci. 2005, 24, 199–205. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Schubert, W.; Bonnekoh, B.; Pommer, A.J.; Philipsen, L.; Bockelmann, R.; Malykh, Y.; Gollnick, H.; Friedenberger, M.; Bode, M.; Dress, A.W. Analyzing proteome topology and function by automated multidimensional fluorescence microscopy. Nat. Biotechnol. 2006, 24, 1270–1278. [Google Scholar] [CrossRef]
- Zaky, A.; Elbakry, A.; Ehmer, A.; Breunig, M.; Goepferich, A. The mechanism of protein release from triglyceride microspheres. J. Control. Release 2010, 147, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Albertini, B.; Passerini, N.; Di Sabatino, M.; Monti, D.; Burgalassi, S.; Chetoni, P.; Rodriguez, L. Poloxâmero 407 microspheres for orotransmucosal drug delivery. Part I: Formulation, manufacturing and characterization. Int. J. Pharm. 2010, 399, 71–79. [Google Scholar] [CrossRef]
- Emås, M.; Nyqvist, H. Methods of studying aging and stabilization of spray congealed solid dispersions with carnauba wax. 1. Microcalorimetric investigation. Int. J. Pharm. 2000, 197, 117–127. [Google Scholar] [CrossRef]
- Li, L.C.; Zhu, L.; Song, J.F.; Deng, J.S.; Bandopadhyay, R.; Wurster, D.E. Effect of solid state transition on the physical stability of suspensions containing bupivacaine lipid microparticles. Pharm. Dev. Technol. 2005, 10, 309–318. [Google Scholar] [CrossRef]
- Turton, R.; Cheng, X.X. Cooling Processes and Congealing. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J., Ed.; Informa Healthcare: New York, NY, USA, 2007; Volume 2, pp. 761–773. [Google Scholar]
- Souto, E.B.; Doktorovova, S.; Zielinska, A.; Silva, A.M. Key production parameters for the development of solid lipid nanoparticles by high shear homogenization. Pharm. Dev. Technol. 2019, 24, 1181–1185. [Google Scholar] [CrossRef]
- Qi, S.; Marchaud, D.; Craig, D. An investigation into the mechanism of dissolution rate enhancement of poorly water-soluble drugs from spray chilled gelucire 50/13 microspheres. J. Pharm. Sci. 2010, 99, 262–274. [Google Scholar] [CrossRef]
- Dixit, M.; Kini, A.; Kulkarni, P. Preparation and characterization of microparticles of piroxicam by spray drying and spray chilling methods. Res. Pharm. Sci. 2010, 5, 89–97. [Google Scholar]
- Martins, R. Desenvolvimento de Dispersões Sólidas Microparticuladas Contando Carbamazepina por Spray Congealing; Universidade de São Paulo: Ribeirão Preto, Brazil, 2010. [Google Scholar]
- Savolainen, M.; Herder, J.; Khoo, C.; Lövqvist, K.; Dahlqvist, C.; Glad, H.; Juppo, A.M. Evaluation of polar lipid-hydrophilic polymer microparticles. Int. J. Pharm. 2003, 262, 47–62. [Google Scholar] [CrossRef]
- Pedroso, D.L.; Dogenski, M.; Thomazini, M.; Heinemann, R.J.B.; Favaro-Trindade, C.S. Microencapsulation of Bifidobacterium animalis subsp. lactis and Lactobacillus acidophilus in cocoa butter using spray chilling technology. Braz. J. Microbiol. 2014, 44, 777–783. [Google Scholar] [CrossRef]
- Karaman, R. Prodrugs for masking bitter taste of antibacterial drugs—A computational approach. J. Mol. Model. 2013, 19, 2399–2412. [Google Scholar] [CrossRef]
- Bilati, U.; Allémann, E.; Doelker, E. Strategic approaches for overcoming peptide and protein instability within biodegradable nano- and microparticles. Eur. J. Pharm. Biopharm. 2005, 59, 375–388. [Google Scholar] [CrossRef]
- Dashevsky, A. Protein loss by the microencapsulation of an enzyme (lactase) in alginate beads. Int. J. Pharma 1998, 161, 1–5. [Google Scholar] [CrossRef]
- Reithmeier, H.; Herrmann, J.; Göpferich, A. Development and characterization of lipid microparticles as a drug carrier for somatostatina. Int. J. Pharm. 2001, 218, 133–143. [Google Scholar] [CrossRef]
- Kwak, H.H.; Shim, W.S.; Choi, M.K.; Son, M.K.; Kim, H.J.; Yang, H.C.; Kim, T.H.; Lee, G.I.; Kim, B.H.; Kang, S.H.; et al. Development of a sustained-release recombinant human growth hormone formulation. J. Control. Release 2009, 137, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Sah, H. Stabilization of proteins against methylene chloride/water interface-induced denaturation and aggregation. J. Control. Release 1999, 58, 143–151. [Google Scholar] [CrossRef]
- Albertini, B.; Di Sabatino, M.; Calonghi, N.; Rodriguez, L.; Passerini, N. Novel multifunctional platforms for potential treatment of cutaneous wounds: Development and in vitro characterization. Int. J. Pharm. 2013, 440, 238–249. [Google Scholar] [CrossRef]


| Parameters | Spray Drying | Spray Chilling |
|---|---|---|
| Energy flux | Energy applied to the droplets, forcing evaporation of the medium | Energy removed to the droplets, forcing the medium to solidify |
| Equipment | Feed tubes without heating | Heated feed tubes (to prevent solidification) |
| Flow in the equipment chamber | Hot air | Cold air or liquid nitrogen |
| Average particle size | 5–150 µm | 20–200 µm |
| Release mechanism | Dissolution | Difussion, heating |
| Morphology of particle | Particle with irregular geometry and porous surface due to solvent evaporation | Dense, spherical and smooth surface (absence of the evaporation effects of the solvent) |
| Coating | Water-soluble polymers | Waxes, fatty acids, water-soluble and water-insoluble polymers, monomers |
| Food ingredients | Vitamins, flavors, starter cultures, carotenoids, oils and fats, enzymes, acidulants | Ferrous sulphate, vitamins, minerals, acidulants |
| Steps in the process | (1) Disperse or dissolve the asset in the aqueous coating solution (2) Atomization (3) Dehydration | (1) Disperse or dissolve active in the melted lipid mixture (2) Atomization (3) Cooling |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favaro-Trindade, C.S.; de Matos Junior, F.E.; Okuro, P.K.; Dias-Ferreira, J.; Cano, A.; Severino, P.; Zielińska, A.; Souto, E.B. Encapsulation of Active Pharmaceutical Ingredients in Lipid Micro/Nanoparticles for Oral Administration by Spray-Cooling. Pharmaceutics 2021, 13, 1186. https://doi.org/10.3390/pharmaceutics13081186
Favaro-Trindade CS, de Matos Junior FE, Okuro PK, Dias-Ferreira J, Cano A, Severino P, Zielińska A, Souto EB. Encapsulation of Active Pharmaceutical Ingredients in Lipid Micro/Nanoparticles for Oral Administration by Spray-Cooling. Pharmaceutics. 2021; 13(8):1186. https://doi.org/10.3390/pharmaceutics13081186
Chicago/Turabian StyleFavaro-Trindade, Carmen S., Fernando E. de Matos Junior, Paula K. Okuro, João Dias-Ferreira, Amanda Cano, Patricia Severino, Aleksandra Zielińska, and Eliana B. Souto. 2021. "Encapsulation of Active Pharmaceutical Ingredients in Lipid Micro/Nanoparticles for Oral Administration by Spray-Cooling" Pharmaceutics 13, no. 8: 1186. https://doi.org/10.3390/pharmaceutics13081186
APA StyleFavaro-Trindade, C. S., de Matos Junior, F. E., Okuro, P. K., Dias-Ferreira, J., Cano, A., Severino, P., Zielińska, A., & Souto, E. B. (2021). Encapsulation of Active Pharmaceutical Ingredients in Lipid Micro/Nanoparticles for Oral Administration by Spray-Cooling. Pharmaceutics, 13(8), 1186. https://doi.org/10.3390/pharmaceutics13081186










