Development of Spanish Broom and Flax Dressings with Glycyrrhetinic Acid-Loaded Films for Wound Healing: Characterization and Evaluation of Biological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Films on Cotton, Spanish Broom, and Flax Wound Dressings
2.3. Characterization of Cotton, Spanish Broom, and Flax Wound Dressings
2.4. Differential Scanning Calorimetry (DSC)
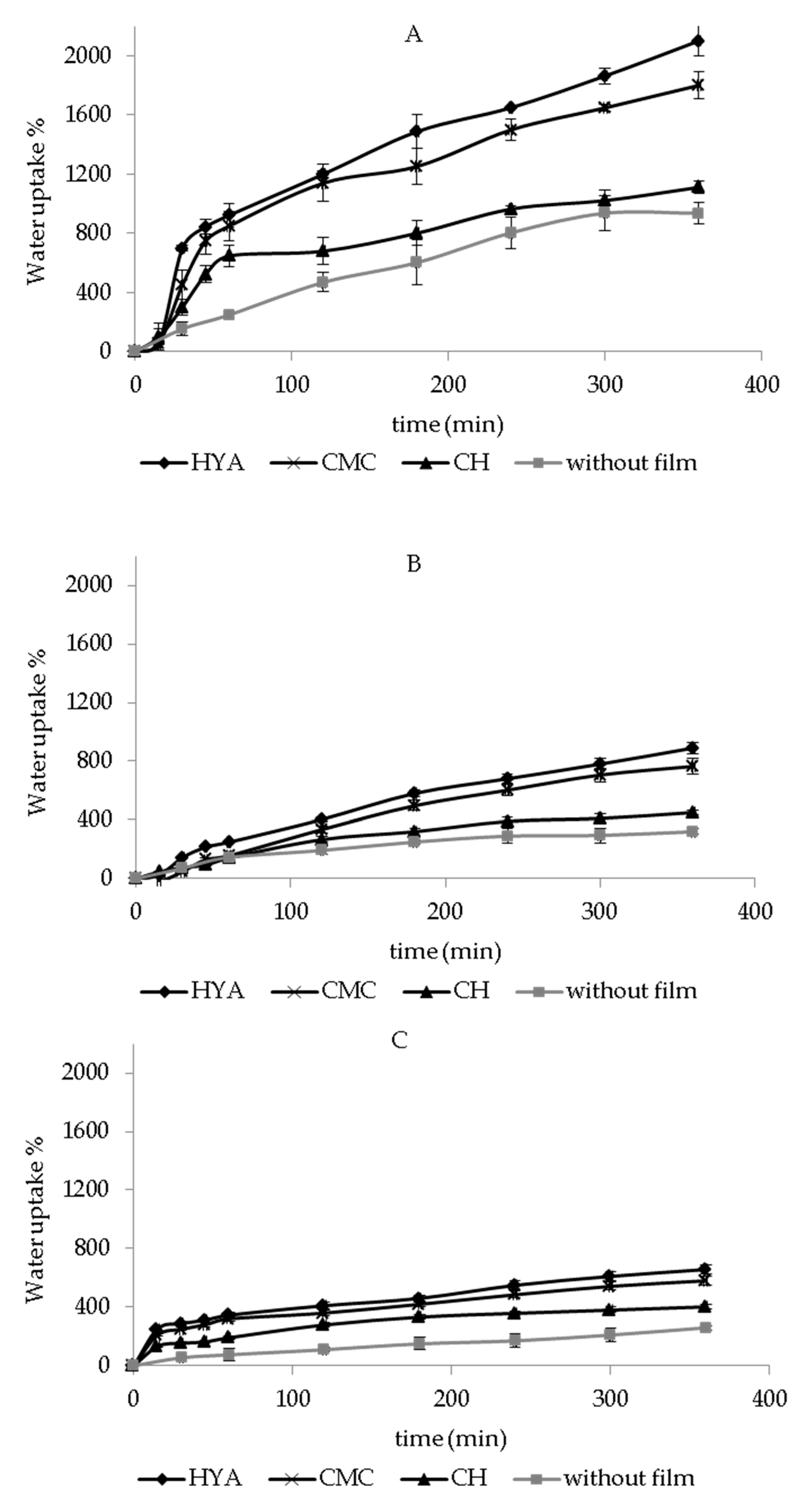
2.5. In Vitro Water-Uptake Ability
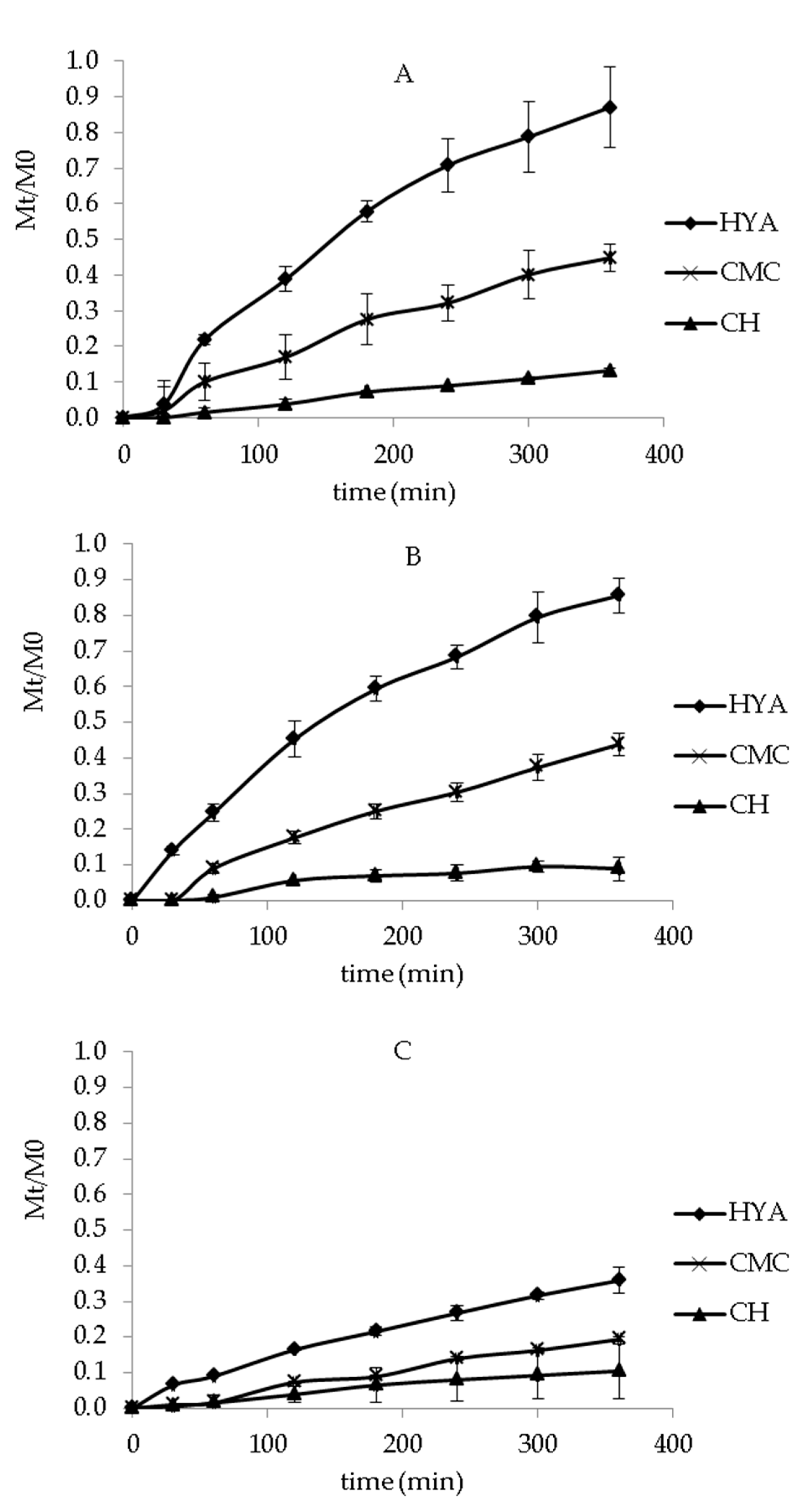
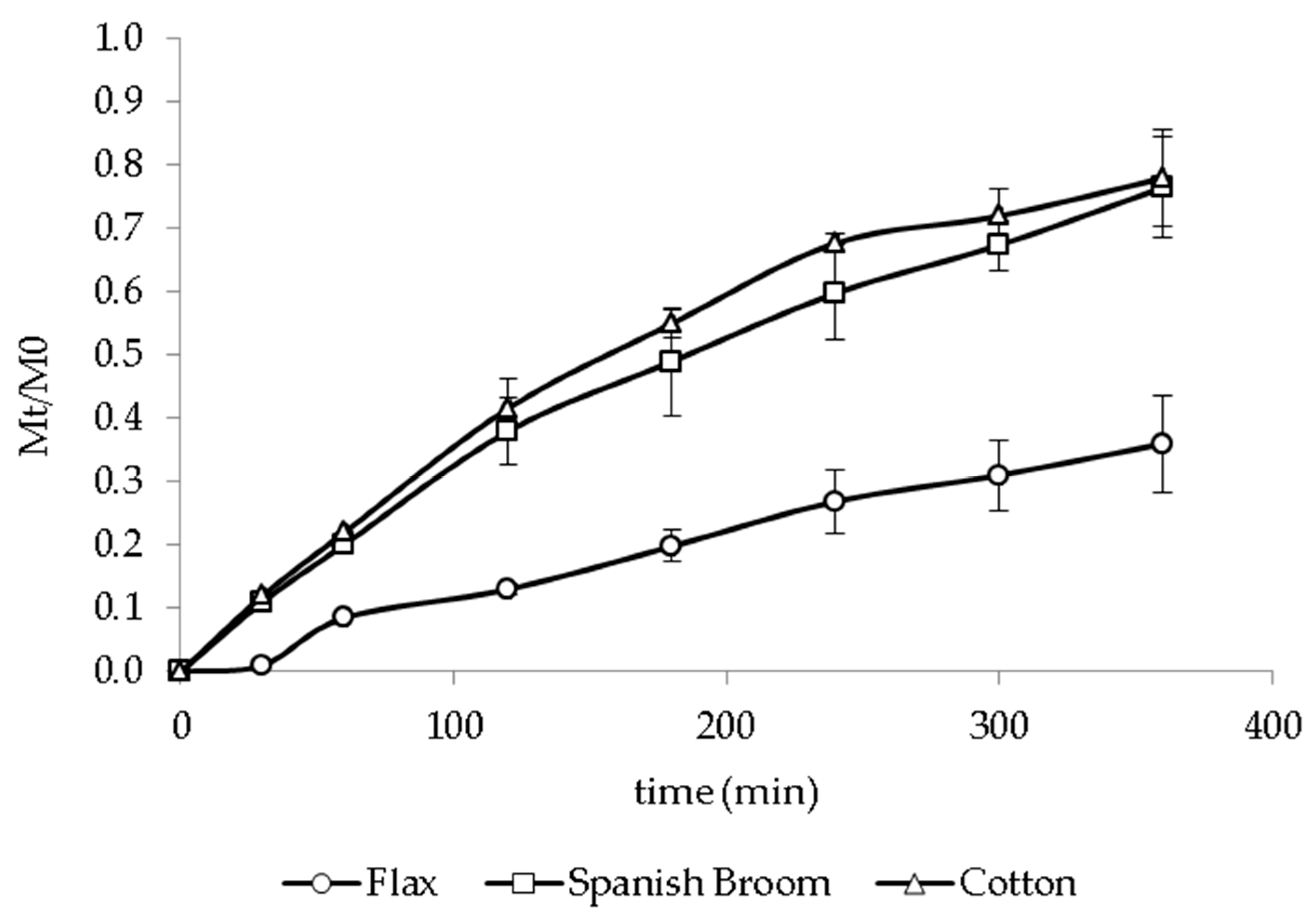
2.6. In Vitro Release Studies
2.7. Preparation of Extracts
2.8. Cell Culture and Treatment
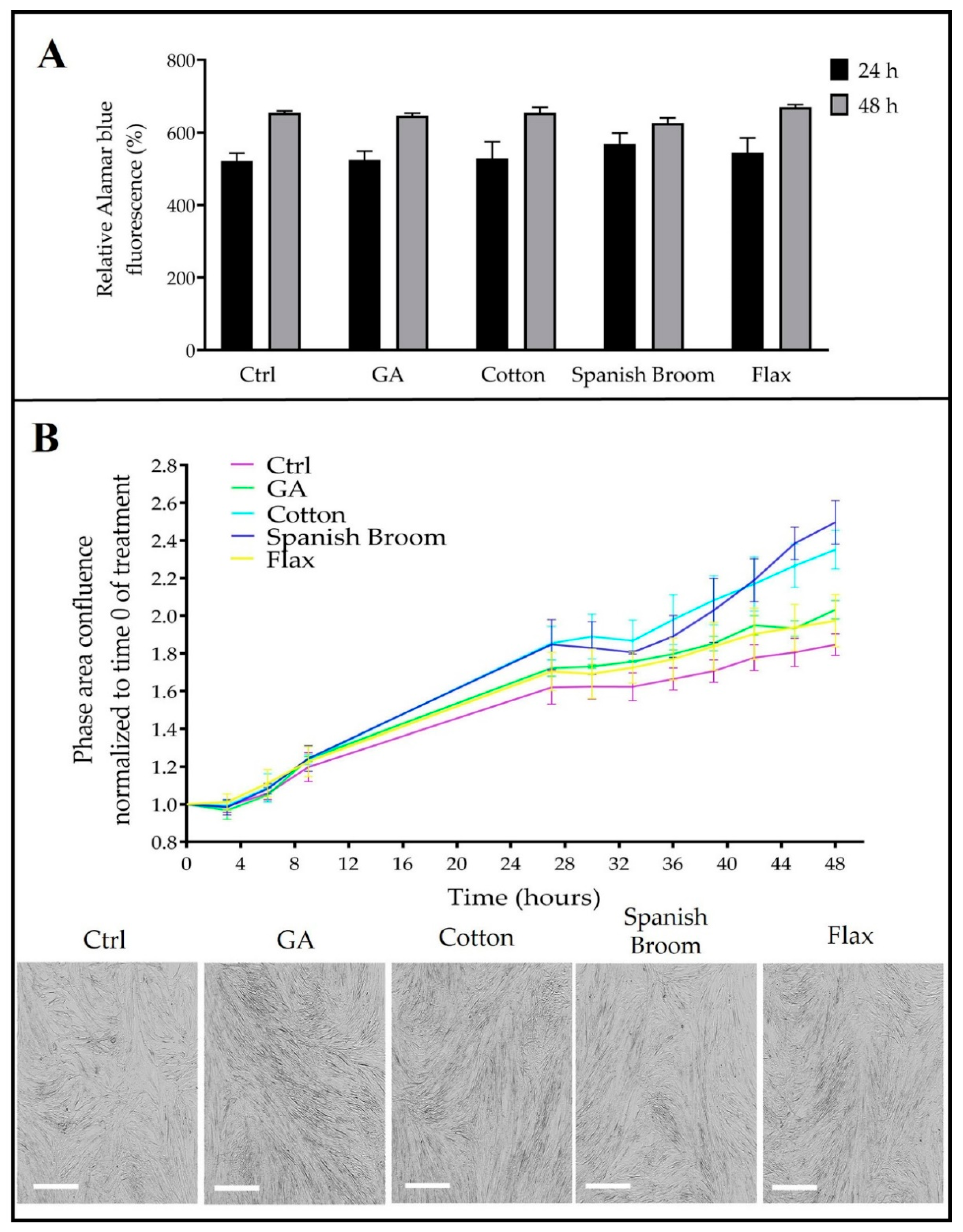
2.9. Cell-Viability Assay
2.10. Cell-Growth Analysis
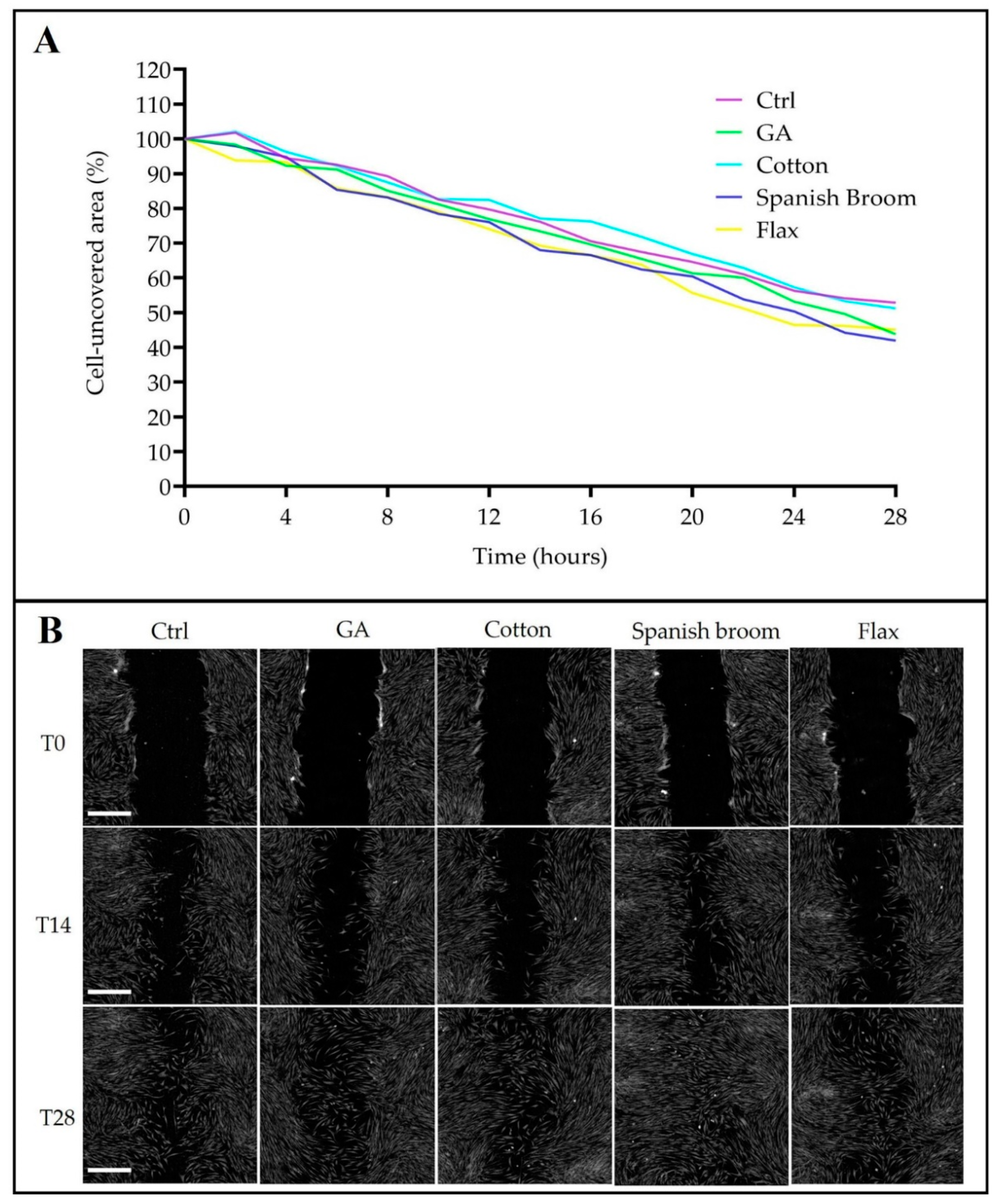
2.11. Wound-Healing Assay by Quantitative Phase Imaging (QPI) Microscopy
2.12. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Cotton, Spanish Broom, and Flax Wound Dressings
3.2. Differential Scanning Calorimetry (DSC)
3.3. In Vitro Water-Uptake Ability
3.4. In Vitro Release Studies
3.5. Biological Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, W.; Wang, Z.; Zha, Y.; Gu, X.; You, W.; Xiao, Y.; Wang, X.; Zhang, S.; Wang, J. High flexible and broad antibacterial nanodressing induces complete skin repair with angiogenic and follicle regeneration. Adv. Healthc. Mater. 2020, 9, 2000035. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, M.; He, B.; Gao, B. Intelligent patches for wound management: In Situ sensing and treatment. Anal. Chem. 2021, 93, 4687–4696. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, J.; Liu, Y.; Li, Y.; Zhang, C.; Qi, W.; Yeung, K.W.K.; Wong, T.M.; Zhao, X.; Pan, H. Electrospun chitosan/PVA/bioglass Nanofibrous membrane with spatially designed structure for accelerating chronic wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110083. [Google Scholar] [CrossRef]
- Fang, Y.; Zhu, X.; Wang, N.; Zhang, X.; Yang, D.; Nie, J.; Ma, G. Biodegradable core-shell electrospun nanofibers based on PLA and γ-PGA for wound healing. Eur. Polym. J. 2019, 116, 30. [Google Scholar] [CrossRef]
- Perumal, G.; Pappuru, S.; Chakraborty, D.; Nandkumar, A.M.; Chand, D.K.; Doble, M. Synthesis and characterization of curcumin loaded PLA—Hyperbranched polyglycerol electrospun blend for wound dressing applications. Mater. Sci. Eng. C 2017, 76, 1196–1204. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings–a review. Biomedicine 2015, 5, 24–48. [Google Scholar] [CrossRef]
- Martin, C.; Low, W.L.; Amin, M.C.I.M.; Radecka, I.; Raj, P.; Kenward, K. Current trends in the development of wound dressings, biomaterials and devices. Pharm. Pat. Analyst 2013, 2, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Cappadone, C.; Farruggia, G.; Luppi, B.; Bigucci, F.; Cerchiara, T. Glycyrrhetinic acid liposomes and hyalurosomes on spanish broom, flax, and hemp dressings to heal skin wounds. Molecules 2020, 25, 2558–2571. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Turillas, F.A.; de la Guardia, M. Environmental impact of Recover cotton in textile industry. Resour. Conserv. Recycl. 2017, 116, 107–115. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67–103. [Google Scholar] [CrossRef]
- Cerchiara, T.; Giordani, B.; Melgoza, L.M.; Prata, C.; Parolin, C.; Dalena, F.; Abruzzo, A.; Bigucci, F.; Luppi, B.; Vitali, B. New Spanish Broom dressings based on Vitamin E and Lactobacillus plantarum for superficial skin wounds. J. Drug Deliv. Sci. Technol. 2020, 56, 101499–101508. [Google Scholar] [CrossRef]
- Cerchiara, T.; Abruzzo, A.; Ñahui Palomino, R.A.; Vitali, B.; De Rose, R.; Chidichimo, G.; Ceseracciu, L.; Athanassiou, A.; Saladini, B.; Dalena, F.; et al. Spanish broom (Spartium junceum L.) fibers impregnated with vancomycin- loaded chitosan nanoparticles as new antibacterial wound dressing: Preparation, characterization and antibacterial activity. Eur. J. Pharm. Sci. 2017, 99, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Cerchiara, T.; Abruzzo, A.; Di Cagno, M.; Bigucci, F.; Bauer-Brandl, A.; Parolin, C.; Vitali, B.; Gallucci, M.C.; Luppi, B. Chitosan based micro- and nanoparticles for colontargeted delivery of vancomycin prepared by alternative processing methods. Eur. J. Pharm. Biopharm. 2015, 92, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Cerchiara, T.; Chidichimo, G.; Rondi, G.; Gallucci, M.C.; Gattuso, C.; Luppi, B.; Bigucci, F. Chemical composition, morphology and tensile properties of Spanish Broom (Spartium junceum L.) fibres in comparison with flax (Linum usitatissimum L.). Fibres Text. East. Eur. 2014, 22, 25–28. [Google Scholar]
- Hung, C.F.; Hsiao, C.Y.; Hsieh, W.H.; Li, H.J.; Tsai, Y.J.; Lin, C.N.; Chang, H.H.; Wu, N.L. 18β-glycyrrhetinic acid derivate promotes proliferation, migration and aquaporin-3 expression in human dermal fibroblasts. PLoS ONE 2017, 12, 1–14. [Google Scholar] [CrossRef]
- Graebin, C.S. The pharmacological activities of Glycyrrhizinic Acid (“Glycyrrhizin”) and Glycyrrhetinic Acid. In Sweeteners. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Berlin/Heidelberg, Germany; Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Kao, T.C.; Shyu, M.H.; Yen, G.C. Glycyrrhizic acid and 18beta-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/ GSK3beta signaling and glucocorticoid receptor activation. J. Agric. Food Chem. 2010, 58, 8623–8629. [Google Scholar] [CrossRef]
- Kalaiarasi, P.; Pugalendi, K.V. Protective effect of 18b-glycyrrhetinic acid on lipid peroxidation and antioxidant enzymes in experimental diabetes. J. Pharm. Res. 2011, 4, 107–111. [Google Scholar]
- Castangia, I.; Caddeo, C.; Manca, M.L.; Casu, L.; Latorre, A.C.; Díez-Sales, O.; Ruiz-Saurí, A.; Bacchetta, G.; Fadda, A.M.; Manconi, M. Delivery of liquorice extract by liposomes and hyalurosomes to protect the skin against oxidative stress injuries. Carbohydr. Polym. 2015, 134, 657–663. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Q.; Zhong, Y.; Cui, X.; Jiang, Z.; Wu, P.; Zheng, X.; Zhang, K.; Zhao, S. Synthesis, anti-microbial and anti-inflammatory activities of 18-β-glycyrrhetinic acid derivatives. Bioorganic. Chem. 2020, 101, 103985–103995. [Google Scholar] [CrossRef]
- Pachuau, L. Recent developments in novel drug delivery systems for wound healing. Expert Opin. Drug Deliv. 2015, 12, 1895–1909. [Google Scholar] [CrossRef]
- Rezvanian, M.; Mohd Amin, M.C.I.; Ng, S.F. Development and physicochemical characterization of alginate composite film loaded with simvastatin as a potential wound dressing. Carbohydr. Polym. 2016, 137, 295–304. [Google Scholar] [CrossRef]
- Jin, S.G.; Yousaf, A.M.; Kim, K.S.; Kim, D.W.; Kim, D.S.; Kim, J.K.; Yong, C.S.; Youn, Y.S.; Kim, J.O.; Choi, H.-G. Influence of hydrophilic polymers on functional properties and wound healing efficacy of hydrocolloid based wound dressings. Int. J. Pharm. 2016, 501, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Catanzano, O.; D’Esposito, V.; Acierno, S.; Ambrosio, M.R.; De Caro, C.; Avagliano, C.; Russo, P.; Russo, R.; Miro, A.; Ungaro, F.; et al. Alginate-hyaluronan composite hydrogels accelerate wound healing process. Carbohydr. Polym. 2015, 131, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Vivcharenko, V.; Przekora, A. Modifications of Wound Dressings with Bioactive Agents to Achieve Improved Pro-Healing Properties. Appl. Sci. 2021, 11, 4114. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmogahzy, Y. Engeenering textiles. Integrating the Design and Manufacture of Textile Products, 2nd ed.; Woodhead Publishing: Duxford, UK, 2019; p. 407. [Google Scholar]
- Foutsizoglou, S. A practical guide to the most commonly used dressings in wound care. PMFA J. 2018, 6, 1. [Google Scholar]
- Seaman, S. Dressing selection in chronic wound management. J. Am. Podiatr. Med. Assoc. 2002, 92, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zuo, Z.; Cheung, C.K.C.; Leung, S.S.Y. Updates on thermosensitive hydrogel for nasal, ocular and cutaneous delivery. Int. J. Pharm. 2019, 559, 86–101. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced therapeutic dressings for effective wound healing—A review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [Green Version]
- Luppi, B.; Bigucci, F.; Baldini, M.; Abruzzo, A.; Cerchiara, T.; Corace, G.; Zecchi, V. Hydroxypropyl methyl cellulose films for prolonged delivery of the antipsychotic drug chlorpromazine. J. Pharm. Pharmacol. 2010, 62, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhu, W.; Han, C.; Sui, X.; Liu, C.; Ma, X.; Dong, Y. Preparation of glycyrrhetinic acid liposomes using lyophilization monophase solution method: Preformulation, optimization, and in vitro evaluation. Nanoscale Res. Lett. 2018, 13, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Qiu, Y.; Zhang, S.; Gao, Y. Enhanced transdermal delivery of 18β-glycyrrhetic acid via elastic vesicles: In vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2012, 38, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Bertram, U.; Bodmeier, R. Effect of polymer molecular weight and of polymer blends on the properties of rapidly gelling nasal inserts. Drug Dev. Ind. Pharm. 2012, 38, 659–669. [Google Scholar] [CrossRef]
- Rampersad, N. Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Thu, H.; Ng, S. Gelatine enhances drug dispersion in alginate bilayer film via the formation of crystalline microaggregates. Int. J. Pharm. 2013, 454, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Mai, Y.; Liu, Q.; Zhang, W.; Yang, J. Mechanism and improved dissolution of glycyrrhetinic acid solid dispersion by alkalizers. Pharmaceutics 2020, 12, 82–106. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Qin, Q.; Liao, X.L.; Yang, B. Host-guest inclusion system of glycyrrhetinic acid with polyamine-β-cyclodextrin: Preparation, characterization, and anticancer activity. J. Mol. Struct. 2017, 1149, 155–161. [Google Scholar] [CrossRef]
- Rumondor, A.C.F.; Dhareshwar, S.S.; Kesisoglou, F. Amorphous solid dispersions or prodrugs: Complementary strategies to increase drug absorption. J. Pharm. Sci. 2016, 105, 2498–2508. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Sharma, S.; Dhiman, A. Design of antibiotic containing hydrogel wound dressings: Biomedical properties and histological study of wound healing. Int. J. Pharm. 2013, 457, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Giordani, B.; Abruzzo, A.; Prata, C.; Nicoletta, F.P.; Dalena, F.; Cerchiara, T.; Luppi, B.; Bigucci, F. Ondansetron buccal administration for paediatric use: A comparison between films and wafers. Int. J. Pharm. 2020, 580, 119228. [Google Scholar] [CrossRef]
- Camponeschi, F.; Atrei, A.; Rocchigiani, G.; Mencuccini, L.; Uva, M.; Barbucci, R. New formulations of polysaccharide-based hydrogels for drug release and tissue engineering. Gels 2015, 1, 3–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kononova, S.V.; Kruchinina, E.V.; Petrova, V.A.; Baklagina, Y.G.; Klechkovskaya, V.V.; Orekhov, A.S.; Vlasova, E.N.; Popova, E.N.; Gubanova, G.N.; Skorik, Y.A. Pervaporation membranes of a simplex type with polyelectrolyte layers of chitosan and sodium hyaluronate. Carbohydr. Polym. 2019, 209, 10–19. [Google Scholar] [CrossRef]
- Trastullo, R.; Abruzzo, A.; Saladini, B.; Gallucci, M.C.; Cerchiara, T.; Luppi, B.; Bigucci, F. Design and evaluation of buccal films as paediatric dosage form for transmucosal delivery of ondansetron. Eur. J. Pharm. Biopharm. 2016, 105, 115–121. [Google Scholar] [CrossRef]
- Cheng, M.; Gao, X.; Wang, Y.; Chen, H.; He, B.; Xu, H.; Li, Y.; Han, J.; Zhang, Z. Synthesis of glycyrrhetinic acid-mod ified chitosan 5-fluorouracil nanoparticles and its inhibition of liver cancer characteristics in vitro and in vivo. Mar. Drugs 2013, 11, 3517–3536. [Google Scholar] [CrossRef]
- Mali, K.K.; Dhawale, S.C.; Dias, R.J.; Ghorpade, V.S.; Dhane, N.S. Development of vancomycin-loaded polysaccharide-based hydrogel wound dressings: In vitro and in vivo evaluation. Asian J. Pharm. 2018, 12, 94–105. [Google Scholar]
- Boateng, J.S.; Matthews, K.H.; Auffret, A.D.; Humphrey, M.J.; Eccleston, G.M.; Stevens, H.N. Comparison of the in vitro release characteristics of mucosal freeze-dried wafers and solvent-cast films containing an insoluble drug. Drug Dev. Ind. Pharm. 2012, 38, 47–54. [Google Scholar] [CrossRef]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biologicalfunctions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [Green Version]
- Frenkel, J.S. The role of hyaluronan in wound healing. Int. Wound J. 2012, 11, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Aljghami, M.E.; Saboor, S.; Amini-Nik, S. Emerging innovative wound dressings. Ann. Biomed. Eng. 2019, 47, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Components of Polymeric Solutions | HYA | CMC | HPMC | CH |
|---|---|---|---|---|
| Polymer | 1 | 1 | 1 | 1 |
| GA | 0.05 | 0.05 | 0.05 | 0.05 |
| Ethanol | 10 | 10 | 10 | 10 |
| Propylene glycol | 13.65 | 13.65 | 13.65 | 13.65 |
| Lactic acid | 0 | 0 | 0 | 0.9 |
| Water | 75.3 | 75.3 | 75.3 | 74.4 |
| Cotton | Spanish Broom | Flax | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Weight (mg/cm2) | Thickness (mm) | Drug Content (µg/cm2) | Weight (mg/cm2) | Thickness (mm) | Drug Content (µg/cm2) | Weight (mg/cm2) | Thickness (mm) | Drug Content (µg/cm2) | |
| HYA | 7.2 ± 0.4 | 0.39 ± 0.03 | 244.8 ± 10.0 | 18.7 ± 0.9 | 0.46 ± 0.02 | 232.4 ± 16.1 | 39.1 ± 1.8 | 0.54 ± 0.02 | 257.7 ± 11.2 |
| CMC | 7.6 ± 0.3 | 0.34 ± 0.03 | 246.0 ± 11.8 | 19.7 ± 0.5 | 0.47 ± 0.02 | 263.9 ± 18.8 | 42.0 ± 1.9 | 0.54 ± 0.03 | 282.2 ± 13.5 |
| HPMC | 5.0 ± 0.2 | 0.39 ± 0.02 | 143.1 ± 85.7 | 19.2 ± 0.5 | 0.44 ± 0.02 | 147.5 ± 50.0 | 41.3 ± 1.2 | 0.57 ± 0.02 | 158.9 ± 56.9 |
| CH | 11.7 ± 0.4 | 0.38 ± 0.03 | 266.2 ± 18.8 | 25.4 ± 1.5 | 0.45 ± 0.03 | 237.9 ± 17.3 | 46.9 ± 1.2 | 0.53 ± 0.03 | 263.9 ± 16.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abruzzo, A.; Cappadone, C.; Sallustio, V.; Picone, G.; Rossi, M.; Nicoletta, F.P.; Luppi, B.; Bigucci, F.; Cerchiara, T. Development of Spanish Broom and Flax Dressings with Glycyrrhetinic Acid-Loaded Films for Wound Healing: Characterization and Evaluation of Biological Properties. Pharmaceutics 2021, 13, 1192. https://doi.org/10.3390/pharmaceutics13081192
Abruzzo A, Cappadone C, Sallustio V, Picone G, Rossi M, Nicoletta FP, Luppi B, Bigucci F, Cerchiara T. Development of Spanish Broom and Flax Dressings with Glycyrrhetinic Acid-Loaded Films for Wound Healing: Characterization and Evaluation of Biological Properties. Pharmaceutics. 2021; 13(8):1192. https://doi.org/10.3390/pharmaceutics13081192
Chicago/Turabian StyleAbruzzo, Angela, Concettina Cappadone, Valentina Sallustio, Giovanna Picone, Martina Rossi, Fiore Pasquale Nicoletta, Barbara Luppi, Federica Bigucci, and Teresa Cerchiara. 2021. "Development of Spanish Broom and Flax Dressings with Glycyrrhetinic Acid-Loaded Films for Wound Healing: Characterization and Evaluation of Biological Properties" Pharmaceutics 13, no. 8: 1192. https://doi.org/10.3390/pharmaceutics13081192






