The Pharmaceutical Technology Approach on Imaging Innovations from Italian Research
Abstract
1. Introduction
1.1. Imaging Techniques Most Frequently Used in the Clinical Practice
1.2. Limitations of the Imaging Tools
2. Main Technological Strategies and Examples of Imaging Agents Developed for Improving Diagnostic Efficiency
2.1. Chemical Modification, Prodrugs/Bio-Precursors
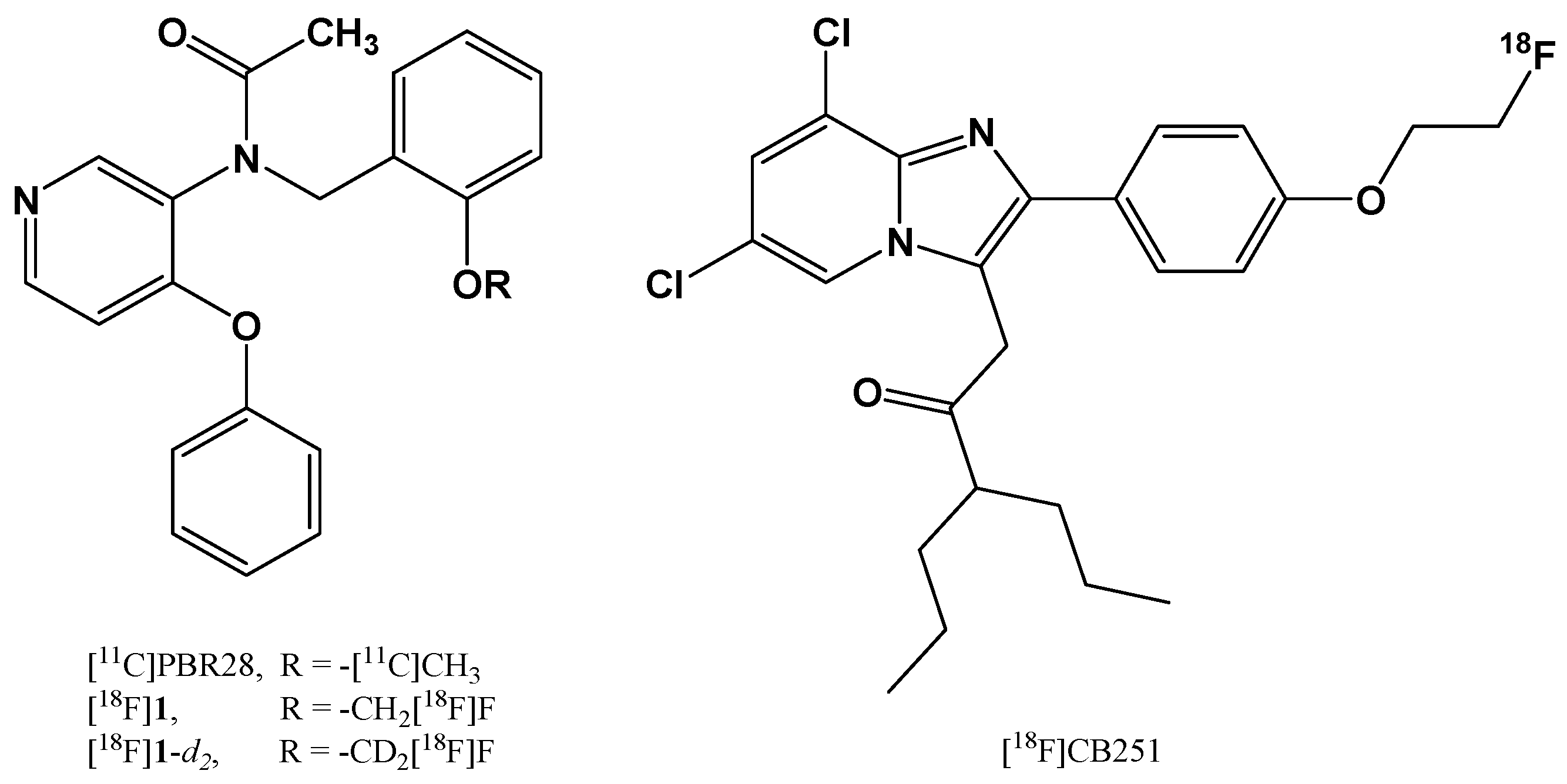
2.1.1. Chemical Modification of 18F-radiotracers to Increase their Metabolic Stability
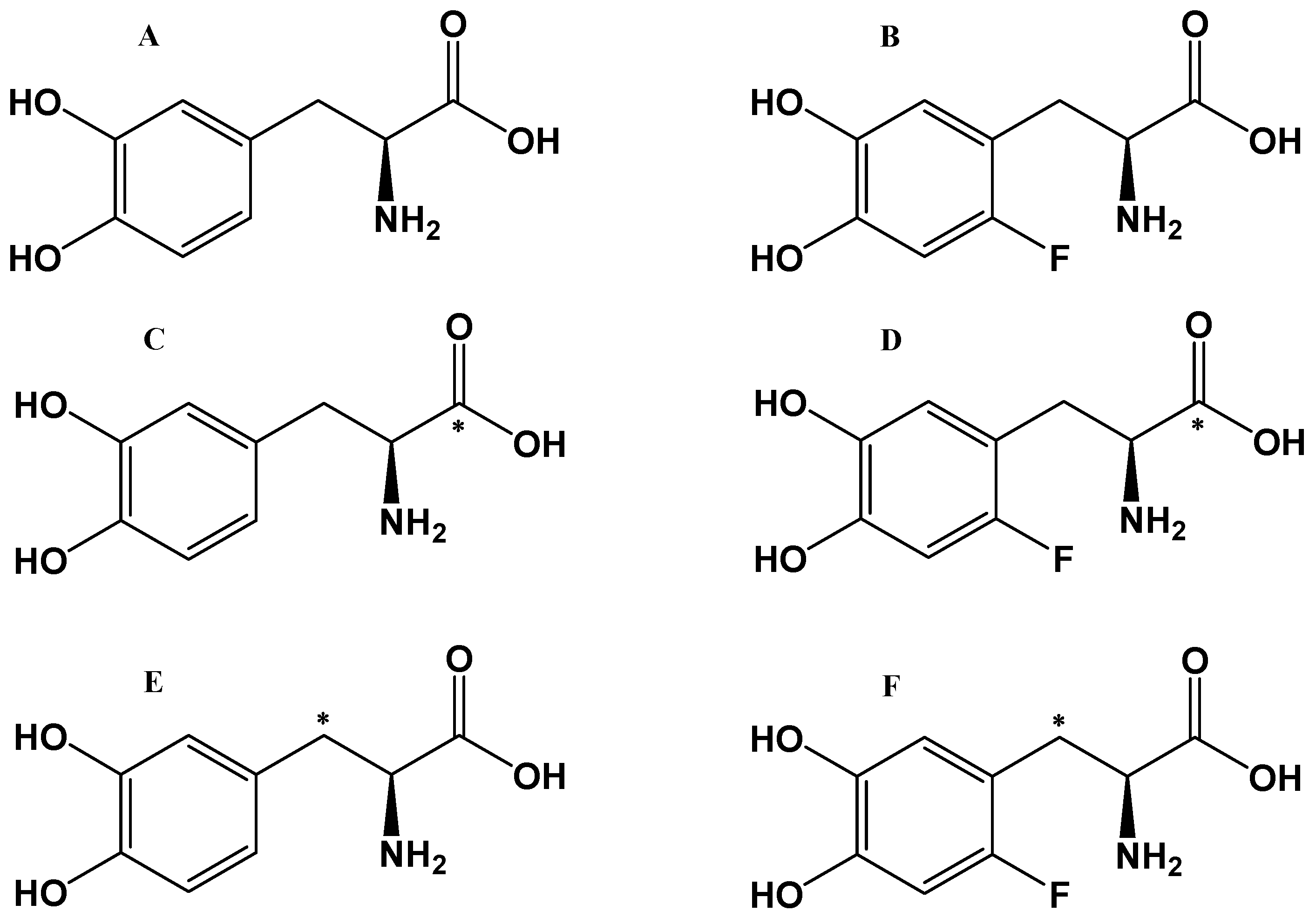
2.1.2. Labeled L-DOPA Bio-Precursors as Imaging Agents
2.2. Formulation Strategies: The Use of Buffers
2.2.1. Buffers for Gallium-68 Radionuclide Complexation
2.2.2. Buffers for DA Precursor Formulations
2.3. Conjugates for Active Targeting
2.3.1. Polysaccharide- and Albumin-Based Conjugates for Nuclear Imaging
2.3.2. Conjugates for PET Imaging
2.4. Nanoparticles (NPs) as Imaging Agents
2.4.1. Nanoparticles as MRI-Imaging Agents
2.4.2. Nanoparticles as PET Imaging Agents
2.4.3. Nanoparticles as Multimodal Imaging Agents
2.4.4. Nanoparticles as Optical, Near-Infrared (NIR), and PA-Imaging Agents
3. Translational Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasban, H.; Atomic, E.; Authority, E. A Comparative Study of Medical Imaging Techniques. Int. J. Latest Trends Eng. Technol. 2015, 4, 37–58. [Google Scholar]
- Ai, T.; Morelli, J.N.; Hu, X.; Hao, D.; Goerner, F.L.; Ager, B.; Runge, V.M. A historical overview of magnetic resonance imaging, focusing on technological innovations. Investig. Radiol. 2012, 47, 725–741. [Google Scholar] [CrossRef]
- Haris, M.; Yadav, S.K.; Rizwan, A.; Singh, A.; Wang, E.; Hariharan, H.; Reddy, R.; Marincola, F.M. Molecular magnetic resonance imaging in cancer. J. Transl. Med. 2015, 13, 313. [Google Scholar] [CrossRef] [PubMed]
- Saslow, D.; Boetes, C.; Burke, W.; Harms, S.; Leach, M.O.; Lehman, C.D.; Morris, E.; Pisano, E.; Schnall, M.; Sener, S.; et al. American cancer society guidelines for breast screening with MRI as an adjunct to mammography. Obstet. Gynecol. Surv. 2007, 62, 458–460. [Google Scholar] [CrossRef]
- Kirkham, A.P.S.; Emberton, M.; Allen, C. How Good is MRI at Detecting and Characterising Cancer within the Prostate? Eur. Urol. 2006, 50, 1163–1175. [Google Scholar] [CrossRef]
- Jack, C.; Bernstein, M.; Fox, N.C.; Thompson, P.M.; Alexander, G.; Harvey, D.; Borowski, B.; Britson, P.J.; Whitwell, J.L.; Ward, C.; et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J. Magn. Reson. Imaging 2008, 27, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Smieskova, R.; Kempton, M.J.; Ho, B.C.; Andreasen, N.C.; Borgwardt, S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci. Biobehav. Rev. 2013, 37, 1680–1691. [Google Scholar] [CrossRef]
- Brambilla, P.; Hardan, A.; Di Nemi, S.U.; Perez, J.; Soares, J.C.; Barale, F. Brain anatomy and development in autism: Review of structural MRI studies. Brain Res. Bull. 2003, 61, 557–569. [Google Scholar] [CrossRef]
- Bookheimer, S. Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu. Rev. Neurosci. 2002, 25, 151–188. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI contrast agents: Current challenges and new frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Hrvoje, L.; Greenstaff, M.W. X-Ray Computed Tomography Contrast Agents. Chem. Rev. 2014, 113, 1641–1666. [Google Scholar]
- The Essential Guide to Image Processing. J. Electron. Imaging 2007, 19, 029901. [CrossRef][Green Version]
- Olsen, O.; Gøtzsche, P.C. Screening for breast cancer with mammography. Cochrane Database Syst. Rev. 2001, CD001877. [Google Scholar] [CrossRef]
- Budoff, M.; Achenbach, S.; Hecht, H.N.J. Atlas of Cardiovascular Computed Tomography; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Jones, A.K. Fluoroscopic Imaging Systems; IAEA: Vienna, Austria, 2014; Chapter 8; ISBN 978-92-0-131010-1. [Google Scholar]
- Piccinonna, S.; Margiotta, N.; Denora, N.; Iacobazzi, R.M.; Pacifico, C.; Trapani, G.; Natile, G. A model radiopharmaceutical agent targeted to translocator protein 18 kDa (TSPO). Dalt. Trans. 2013, 42, 10112–10115. [Google Scholar] [CrossRef]
- Perrone, M.; Moon, B.S.; Park, H.S.; Laquintana, V.; Jung, J.H.; Cutrignelli, A.; Lopedota, A.; Franco, M.; Kim, S.E.; Lee, B.C.; et al. A Novel PET Imaging Probe for the Detection and Monitoring of Translocator Protein 18 kDa Expression in Pathological Disorders. Sci. Rep. 2016, 6, 20422. [Google Scholar] [CrossRef]
- Choi, J.Y.; Iacobazzi, R.M.; Perrone, M.; Margiotta, N.; Cutrignelli, A.; Jung, J.H.; Park, D.D.; Moon, B.S.; Denora, N.; Kim, S.E.; et al. Synthesis and evaluation of tricarbonyl 99mTc-Labeled 2-(4-Chloro)phenyl-imidazo[1,2-a] pyridine analogs as novel SPECT imaging radiotracer for TSPO-rich cancer. Int. J. Mol. Sci. 2016, 17, 1085. [Google Scholar] [CrossRef]
- Schöder, H.; Erdi, Y.E.; Larson, S.M.; Yeung, H.W.D. PET/CT: A new imaging technology in nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1419–1437. [Google Scholar] [CrossRef] [PubMed]
- Fass, L. Imaging and cancer: A review. Mol. Oncol. 2008, 2, 115–152. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.S.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef]
- Torresan, V.; Forrer, D.; Guadagnini, A.; Badocco, D.; Pastore, P.; Casarin, M.; Selloni, A.; Coral, D.; Ceolin, M.; Fernández van Raap, M.B.; et al. 4D Multimodal Nanomedicines Made of Nonequilibrium Au–Fe Alloy Nanoparticles. ACS Nano 2020, 14, 12840–12853. [Google Scholar] [CrossRef]
- Huang, W.Y.; Davis, J.J. Multimodality and nanoparticles in medical imaging. Dalt. Trans. 2011, 40, 6087–6103. [Google Scholar] [CrossRef]
- Kim, G.; Paeng, J.; Jung, J.; Moon, B.; Lopalco, A.; Denora, N.; Lee, B.; Kim, S. Assessment of TSPO in a Rat Experimental Autoimmune Myocarditis Model: A Comparison Study between [18F]Fluoromethyl-PBR28 and [18F]CB251. Int. J. Mol. Sci. 2018, 19, 276. [Google Scholar] [CrossRef]
- Moon, B.S.; Jung, J.H.; Park, H.S.; Contino, M.; Denora, N.; Lee, B.C.; Kim, S.E. Preclinical comparison study between [18F]fluoromethyl-PBR28 and its deuterated analog in a rat model of neuroinflammation. Bioorg. Med. Chem. Lett. 2018, 28, 2925–2929. [Google Scholar] [CrossRef]
- Scilimati, A.; Denora, N.; Tricarico, D.D.M. Stable F-Dopa Formulations and Uses Thereof. International Application No. PCT/IB2017/057720, 7 December 2017. [Google Scholar]
- Boschi, A.; Pasquali, M.; Trapella, C.; Massi, A.; Martini, P.; Duatti, A.; Guerrini, R.; Zanirato, V.; Fantinati, A.; Marzola, E.; et al. Design and Synthesis of (TcN)-Tc-99m-Labeled Dextran-Mannose Derivatives for Sentinel Lymph Node Detection. Pharmaceuticals 2018, 11, 70. [Google Scholar] [CrossRef]
- Caviglioli, G.; Chinol, M.; Baldassari, S.; Garaboldi, L.; Zuccari, G.; Petretto, A.; Drava, G.; Sinico, C.; Paganelli, G. A new microdispersed albumin derivative potentially useful for radio-guided surgery of occult breast cancer lesions. Sci. Rep. 2019, 9, 5623. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, S.; Baldassari, S.; Ailuno, G.; Zuccari, G.; Drava, G.; Petretto, A.; Cossu, V.; Marini, C.; Alfei, S.; Florio, T.; et al. Two Novel PET Radiopharmaceuticals for Endothelial Vascular Cell Adhesion Molecule-1 (VCAM-1) Targeting. Pharmaceutics 2021, 13, 1025. [Google Scholar] [CrossRef]
- Caviglioli, G.; Baldassari, S.; Zuccari, G.; Pastorino, S.; Florio, T.; Sambuceti, M.; Ailuno, G. Compounds and Methods for Detecting Early Atherosclerotic Lesions in Blood. Vessels. Patent WO2019175019A1, 19 September 2019. [Google Scholar]
- Orteca, G.; Pisaneschi, F.; Rubagotti, S.; Liu, T.W.; Biagiotti, G.; Piwnica-Worms, D.; Iori, M.; Capponi, P.C.; Ferrari, E.; Asti, M. Development of a potential gallium-68-labelled radiotracer based on DOTA-curcumin for colon-rectal carcinoma: From synthesis to in vivo studies. Molecules 2019, 24, 644. [Google Scholar] [CrossRef]
- Iacobazzi, R.M.; Porcelli, L.; Lopedota, A.A.; Laquintana, V.; Lopalco, A.; Cutrignelli, A.; Altamura, E.; Di Fonte, R.; Azzariti, A.; Franco, M.; et al. Targeting human liver cancer cells with lactobionic acid-G(4)-PAMAM-FITC sorafenib loaded dendrimers. Int. J. Pharm. 2017, 528, 485–497. [Google Scholar] [CrossRef]
- Arduino, I.; Depalo, N.; Re, F.; Dal Magro, R.; Panniello, A.; Margiotta, N.; Fanizza, E.; Lopalco, A.; Laquintana, V.; Cutrignelli, A.; et al. PEGylated solid lipid nanoparticles for brain delivery of lipophilic kiteplatin Pt(IV) prodrugs: An in vitro study. Int. J. Pharm. 2020, 583, 119351. [Google Scholar] [CrossRef]
- Laquintana, V.; Denora, N.; Lopalco, A.; Lopedota, A.; Cutrignelli, A.; Lasorsa, F.M.; Agostino, G.; Franco, M. Translocator Protein Ligand–PLGA Conjugated Nanoparticles for 5-Fluorouracil Delivery to Glioma Cancer Cells. Mol. Pharm. 2014, 11, 859–871. [Google Scholar] [CrossRef]
- Lopalco, A.; Cutrignelli, A.; Denora, N.; Perrone, M.; Iacobazzi, R.M.; Fanizza, E.; Lopedota, A.; Depalo, N.; De Candia, M.; Franco, M.; et al. Delivery of proapoptotic agents in glioma cell lines by TSPO ligand–dextran nanogels. Int. J. Mol. Sci. 2018, 19, 1155. [Google Scholar] [CrossRef]
- Denora, N.; Laquintana, V.; Lopalco, A.; Iacobazzi, R.M.; Lopedota, A.; Cutrignelli, A.; Iacobellis, G.; Annese, C.; Cascione, M.; Leporatti, S.; et al. In vitro targeting and imaging the translocator protein TSPO 18-kDa through G(4)-PAMAM-FITC labeled dendrimer. J. Control. Release 2013, 172, 1111–1125. [Google Scholar] [CrossRef]
- Fanizza, E.; Iacobazzi, R.M.; Laquintana, V.; Valente, G.; Caliandro, G.; Striccoli, M.; Agostiano, A.; Cutrignelli, A.; Lopedota, A.; Curri, M.L.; et al. Highly selective luminescent nanostructures for mitochondrial imaging and targeting. Nanoscale 2016, 8, 3350–3361. [Google Scholar] [CrossRef]
- Denora, N.; Lee, C.; Iacobazzi, R.M.; Choi, J.Y.; Song, I.H.; Yoo, J.S.; Piao, Y.; Lopalco, A.; Leonetti, F.; Lee, B.C.; et al. TSPO-targeted NIR-fluorescent ultra-small iron oxide nanoparticles for glioblastoma imaging. Eur. J. Pharm. Sci. 2019, 139, 105047. [Google Scholar] [CrossRef]
- Armanetti, P.; Chillà, A.; Margheri, F.; Biagioni, A.; Menichetti, L.; Margheri, G.; Ratto, F.; Centi, S.; Bianchini, F.; Severi, M.; et al. Enhanced Antitumoral Activity and Photoacoustic Imaging Properties of AuNP-Enriched Endothelial Colony Forming Cells on Melanoma. Adv. Sci. 2021, 8, 2001175. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Argenziano, M.; Vigna, E.; Giustetto, P.; Torres, E.; Aime, S.; Terreno, E. Preparation and in vitro characterization of chitosan nanobubbles as theranostic agents. Colloids Surf. B Biointerfaces 2015, 129, 39–46. [Google Scholar] [CrossRef]
- Torresan, V.; Guadagnini, A.; Badocco, D.; Pastore, P.; Muñoz Medina, G.A.; Fernàndez van Raap, M.B.; Postuma, I.; Bortolussi, S.; Bekić, M.; Čolić, M.; et al. Biocompatible Iron–Boron Nanoparticles Designed for Neutron Capture Therapy Guided by Magnetic Resonance Imaging. Adv. Healthc. Mater. 2021, 10, 2001632. [Google Scholar] [CrossRef]
- Adamiano, A.; Iafisco, M.; Sandri, M.; Basini, M.; Arosio, P.; Canu, T.; Sitia, G.; Esposito, A.; Iannotti, V.; Ausanio, G.; et al. On the use of superparamagnetic hydroxyapatite nanoparticles as an agent for magnetic and nuclear in vivo imaging. Acta Biomater. 2018, 73, 458–469. [Google Scholar] [CrossRef]
- Truffi, M.; Sevieri, M.; Morelli, L.; Monieri, M.; Mazzucchelli, S.; Sorrentino, L.; Allevi, R.; Bonizzi, A.; Zerbi, P.; Marchini, B.; et al. Anti-madcam-1-conjugated nanocarriers delivering quantum dots enable specific imaging of inflammatory bowel disease. Int. J. Nanomed. 2020, 15, 8537–8552. [Google Scholar] [CrossRef]
- Piras, A.M.; Fabiano, A.; Sartini, S.; Zambito, Y.; Braccini, S.; Chiellini, F.; Erba, P.A. pH-Responsive Carboxymethylcellulose Nanoparticles for 68Ga-WBC Labeling in PET Imaging. Polymers 2019, 11, 1615. [Google Scholar] [CrossRef]
- Rainone, P.; Riva, B.; Belloli, S.; Sudati, F.; Ripamonti, M.; Verderio, P.; Colombo, M.; Colzani, B.; Gilardi, M.C.; Moresco, R.M.; et al. Development of99mtc-radiolabeled nanosilica for targeted detection of HER2-positive breast cancer. Int. J. Nanomed. 2017, 12, 3447–3461. [Google Scholar] [CrossRef]
- Rainone, P.; De Palma, A.; Sudati, F.; Roffia, V.; Rigamonti, V.; Salvioni, L.; Colombo, M.; Ripamonti, M.; Spinelli, A.E.; Mazza, D.; et al. 99mTc-radiolabeled silica nanocarriers for targeted detection and treatment of HER2-positive breast cancer. Int. J. Nanomed. 2021, 16, 1943–1960. [Google Scholar] [CrossRef]
- Piccionello, A.P.; Menichetti, L.; Armanetti, P.; Flori, A.; Monaco, I.; Maturi, M.; Pace, A.; Locatelli, E. Photoluminescent decoration of iron oxide magnetic nanoparticles for dual-imaging applications. J. Nanopart. Res. 2018, 20, 259. [Google Scholar] [CrossRef]
- Armanetti, P.; Pocoví-Martínez, S.; Flori, A.; Avigo, C.; Cassano, D.; Menichetti, L.; Voliani, V. Dual photoacoustic/ultrasound multi-parametric imaging from passion fruit-like nano-architectures. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1787–1795. [Google Scholar] [CrossRef]
- Kuchar, M.; Mamat, C. Methods to increase the metabolic stability of 18F-radiotracers. Molecules 2015, 20, 16186–16220. [Google Scholar] [CrossRef]
- Gant, T.G. Using Deuterium in Drug Discovery: Leaving the Label in the Drug. J. Med. Chem. 2014, 57, 3595–3611. [Google Scholar] [CrossRef]
- Nag, S.; Lehmann, L.; Kettschau, G.; Toth, M.; Heinrich, T.; Thiele, A.; Varrone, A.; Halldin, C. Development of a novel fluorine-18 labeled deuterated fluororasagiline ([18F]fluororasagiline-D2) radioligand for PET studies of monoamino oxidase B (MAO-B). Bioorg. Med. Chem. 2013, 21, 6634–6641. [Google Scholar] [CrossRef]
- Lee, I.; Choe, Y.S.; Choi, J.Y.; Lee, K.-H.; Kim, B.-T. Synthesis and Evaluation of 18 F-Labeled Styryltriazole and Resveratrol Derivatives for β-Amyloid Plaque Imaging. J. Med. Chem. 2012, 55, 883–892. [Google Scholar] [CrossRef]
- Rosenthal, M.S.; Bosch, A.L.; Nickles, R.J.; Gatley, S.J. Synthesis and some characteristics of no-carrier added [18F]fluorotrimethylsilane. Int. J. Appl. Radiat. Isot. 1985, 36, 318–319. [Google Scholar] [CrossRef]
- Holleman, A.; Wiberg, N.; Holleman, A.; Wiberg, N.; Wiberg, E. Lehrbuch der Anorganischen Chemie, 121st ed.; Lehrbuch der Anorganischen Chemie, 102nd; Holleman, A., Wiberg, N., Wiberg, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Li, Z.; Lin, T.P.; Liu, S.; Huang, C.W.; Hudnall, T.W.; Gabbaï, F.P.; Conti, P.S. Rapid aqueous [18F]-labeling of a bodipy dye for positron emission tomography/fluorescence dual modality imaging. Chem. Commun. 2011, 47, 9324–9326. [Google Scholar] [CrossRef]
- Hendricks, J.A.; Keliher, E.J.; Wan, D.; Hilderbrand, S.A.; Weissleder, R.; Mazitschek, R. Synthesis of [18 F]BODIPY: Bifunctional Reporter for Hybrid Optical/Positron Emission Tomography Imaging. Angew. Chem. Int. Ed. 2012, 51, 4603–4606. [Google Scholar] [CrossRef]
- Velikyan, I.; Beyer, G.J.; Långström, B. Microwave-Supported Preparation of 68 Ga Bioconjugates with High Specific Radioactivity. Bioconjug. Chem. 2004, 15, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, A.; Denora, N. Nanoformulations for Drug Delivery: Safety, Toxicity, and Efficacy; Humana Press: New York, NY, USA, 2018; Volume 1800. [Google Scholar]
- Di Stefano, A.; Sozio, P.; Iannitelli, A.; Cerasa, L.S. New drug delivery strategies for improved Parkinson’s disease therapy. Expert Opin. Drug Deliv. 2009, 6, 389–404. [Google Scholar] [CrossRef]
- Lopalco, A.; Cutrignelli, A.; Denora, N.; Lopedota, A.; Franco, M.; Laquintana, V. Transferrin Functionalized Liposomes Loading Dopamine HCl: Development and Permeability Studies across an In Vitro Model of Human Blood–Brain Barrier. Nanomaterials 2018, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Cassano, T.; Lopalco, A.; De Candia, M.; Laquintana, V.; Lopedota, A.; Cutrignelli, A.; Perrone, M.; Iacobazzi, R.M.; Bedse, G.; Franco, M.; et al. Oxazepam-Dopamine Conjugates Increase Dopamine Delivery into Striatum of Intact Rats. Mol. Pharm. 2017, 14, 3178–3187. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, R.M.; Lopalco, A.; Cutrignelli, A.; Laquintana, V.; Lopedota, A.; Franco, M.; Denora, N. Bridging Pharmaceutical Chemistry with Drug and Nanoparticle Targeting to Investigate the Role of the 18-kDa Translocator Protein TSPO. ChemMedChem 2017, 12, 1263–1274. [Google Scholar] [CrossRef]
- Denora, N.; Cassano, T.; Laquintana, V.; Lopalco, A.; Trapani, A.; Cimmino, C.S.; Laconca, L.; Giuffrida, A.; Trapani, G. Novel codrugs with GABAergic activity for dopamine delivery in the brain. Int. J. Pharm. 2012, 437, 221–231. [Google Scholar] [CrossRef]
- Denora, N.; Lopedota, A.; de Candia, M.; Cellamare, S.; Degennaro, L.; Luisi, R.; Mele, A.; Tricarico, D.; Cutrignelli, A.; Laquintana, V.; et al. Pharmaceutical development of novel lactate-based 6-fluoro-l-DOPA formulations. Eur. J. Pharm. Sci. 2017, 99, 361–368. [Google Scholar] [CrossRef]
- Cumming, P.; Deep, P.; Rousset, O.; Evans, A.; Gjedde, A. On the Rate of Decarboxylation of Dopa to Dopamine in Living Mammalian Brain. Ann. N. Y. Acad. Sci. 1997, 835, 274–308. [Google Scholar] [CrossRef]
- Nakamura, T.; Dhawan, V.; Chaly, T.; Fukuda, M.; Ma, Y.; Breeze, R.; Greene, P.; Fahn, S.; Freed, C.; Eidelberg, D. Blinded positron emission tomography study of dopamine cell implantation for Parkinson’s disease. Ann. Neurol. 2001, 50, 181–187. [Google Scholar] [CrossRef]
- DeJesus, O.T. Positron-labeled DOPA analogs to image dopamine terminals. Drug Dev. Res. 2003, 59, 249–260. [Google Scholar] [CrossRef]
- Hartvig, P.; Reibring, L.; Tedroff, J.; Bjurling, P.; Kihlberg, T.; Långström, B. Brain kinetics of L-[?-11 C]DOPA in humans studied by positron emission tomography. J. Neural Transm. 1991, 86, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Reiffers, S.; der Molen, H.D.B.; Vaalburg, W.; Ten Hoeve, W.; Paans, A.M.J.; Korf, J.; Woldring, M.G.; Wynberg, H. Rapid synthesis and purification of carbon-11 labelled DOPA: A potential agent for brain studies. Int. J. Appl. Radiat. Isot. 1977, 28, 955–958. [Google Scholar] [CrossRef]
- Korf, J.; Reiffers, S.; Beerling-Van Der Molen, H.D.; Lakke, J.P.W.F.; Paans, A.M.J.; Vaalburg, W.; Woldring, M.G. Rapid decarboxylation of carbon-11 labelled dl-dopa in the brain: A potential approach for external detection of nervous structures. Brain Res. 1978, 145, 59–67. [Google Scholar] [CrossRef]
- Bjurling, P.; Antoni, G.; Malmborg, P.; Watanabe, Y.; Långström, B. Multi-enzymatic syntheses of L-tyrosine and L-DOPA. 11C labeling in two positions. Acta Radiol. Suppl. 1991, 376, 107–108. [Google Scholar] [PubMed]
- Torstenson, R.; Tedroff, J.; Hartvig, P.; Fasth, K.-J.; Lågström, B. A Comparison of 11 C-Labeled l -DOPA and l -Fluorodopa as Positron Emission Tomography Tracers for the Presynaptic Dopaminergic System. J. Cereb. Blood Flow Metab. 1999, 19, 1142–1149. [Google Scholar] [CrossRef]
- Velikyan, I. 68Ga-Based Radiopharmaceuticals: Production and Application Relationship. Molecules 2015, 20, 12913–12943. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, M.; Chekol, R.; Vanbilloen, H.; Bormans, G.; Verbruggen, A. Optimal buffer choice of the radiosynthesis of 68Ga–Dotatoc for clinical application. Nucl. Med. Commun. 2010, 31, 753–758. [Google Scholar] [CrossRef]
- Le Roux, J.; Kleynhans, J.; Rubow, S. The use of HEPES-buffer in the production of gallium-68 radiopharmaceuticals—Time to reconsider strict pharmacopoeial limits? EJNMMI Radiopharm. Chem. 2021, 6, 15. [Google Scholar] [CrossRef]
- Eppard, E.; Wuttke, M.; Nicodemus, P.L.; Rösch, F. Ethanol-Based Post-processing of Generator-Derived 68 Ga Toward Kit-Type Preparation of 68 Ga-Radiopharmaceuticals. J. Nucl. Med. 2014, 55, 1023–1028. [Google Scholar] [CrossRef]
- Pfaff, S.; Nehring, T.; Pichler, V.; Cardinale, J.; Mitterhauser, M.; Hacker, M.; Wadsak, W. Development and evaluation of a rapid analysis for HEPES determination in 68Ga-radiotracers. EJNMMI Res. 2018, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Eu, P.; Jackson, P.; Hong, E.; Binns, D.; Iravani, A.; Murphy, D.; Mitchell, C.; Siva, S.; Hicks, R.J.; et al. Cold Kit for Prostate-Specific Membrane Antigen (PSMA) PET Imaging: Phase 1 Study of 68 Ga-Tris(Hydroxypyridinone)-PSMA PET/CT in Patients with Prostate Cancer. J. Nucl. Med. 2018, 59, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Calderoni, L.; Farolfi, A.; Pianori, D.; Maietti, E.; Cabitza, V.; Lambertini, A.; Ricci, G.; Telo, S.; Lodi, F.; Castellucci, P.; et al. Evaluation of an Automated Module Synthesis and a Sterile Cold Kit–Based Preparation of 68 Ga-PSMA-11 in Patients with Prostate Cancer. J. Nucl. Med. 2020, 61, 716–722. [Google Scholar] [CrossRef] [PubMed]
- TLX591-CDX PROSTATE CANCER, Imaging (68Ga-PSMA-11 Use in Prostate Cancer Imaging). Available online: https://telixpharma.com/pipeline/tlx591-cdx-illumet/ (accessed on 1 August 2021).
- Fugazza, L.; Filannino, A.; Mariani, M.F. Process for the Preparation of Complexes of 68Ga. European Patent 3718991A1, 7 October 2020. [Google Scholar]
- Urbanová, K.; Seifert, D.; Vinšová, H.; Vlk, M.; Lebeda, O. Simple new method for labelling of PSMA-11 with 68Ga in NaHCO3. Appl. Radiat. Isot. 2021, 172, 109692. [Google Scholar] [CrossRef]
- Lopalco, A.; Dalwadi, G.; Niu, S.; Schowen, R.L.; Douglas, J.; Stella, V.J. Mechanism of Decarboxylation of Pyruvic Acid in the Presence of Hydrogen Peroxide. J. Pharm. Sci. 2016, 105, 705–713. [Google Scholar] [CrossRef]
- Lopalco, A.; Douglas, J.; Denora, N.; Stella, V.J. Determination of pKa and Hydration Constants for a Series of α-Keto-Carboxylic Acids Using Nuclear Magnetic Resonance Spectrometry. J. Pharm. Sci. 2016, 105, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, A.; Stella, V.J. Effect of Molecular Structure on the Relative Hydrogen Peroxide Scavenging Ability of Some α-Keto Carboxylic Acids. J. Pharm. Sci. 2016, 105, 2879–2885. [Google Scholar] [CrossRef]
- Lopalco, A.; Marinaro, W.A.; Day, V.W.; Stella, V.J. Isolation, Solubility, and Characterization of D-Mannitol Esters of 4-Methoxybenzeneboronic Acid. J. Pharm. Sci. 2017, 106, 601–610. [Google Scholar] [CrossRef]
- Lopalco, A.; Stella, V.J.; Thompson, W.H. Origins, and formulation implications, of the pK difference between boronic acids and their esters: A density functional theory study. Eur. J. Pharm. Sci. 2018, 124, 10–16. [Google Scholar] [CrossRef]
- Lopalco, A.; Deeken, R.; Douglas, J.; Denora, N.; Stella, V.J. Some Preformulation Studies of Pyruvic Acid and Other α-Keto Carboxylic Acids in Aqueous Solution: Pharmaceutical Formulation Implications for These Peroxide Scavengers. J. Pharm. Sci. 2019, 108, 3281–3288. [Google Scholar] [CrossRef]
- Lopalco, A.; Lopedota, A.A.; Laquintana, V.; Denora, N.; Stella, V.J. Boric Acid, a Lewis Acid with Unique and Unusual Properties: Formulation Implications. J. Pharm. Sci. 2020, 109, 2375–2386. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, A.; Iacobazzi, R.M.; Denora, N.; Stella, V.J. Bortezomib Aqueous Solubility in the Presence and Absence of D-Mannitol: A Clarification With Formulation Implications. J. Pharm. Sci. 2021, 110, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Wahl, L.; Nahmias, C. Modeling of fluorine-18-6-fluoro-L-Dopa in humans. J. Nucl. Med. 1996, 37, 432–437. [Google Scholar]
- Rubello, D.; Nanni, C.; Fanti, S. 18F-DOPA PET and PET/CT. J. Nucl. Med. 2007, 48, 1577–1579. [Google Scholar] [CrossRef]
- Package Leaflet: Information for the Patient Iasodopa 0. 3 GBq/mL, Concentrate for Solution for Injection. Available online: https://www.synektik.com.pl/assets/Uploads/Synektik-ulotka-dla-pacjenta-IASOdopa-ENG.pdf (accessed on 1 August 2021).
- Gbq, I. Summary of product characteristics. Pharmaceut. Med. 2014, 87–89. [Google Scholar] [CrossRef]
- Nataf, V.; Balard, M.; de Beco, V.; Kerrou, K.; Gutman, F.; Grahek, D.; Montravers, F.; Talbot, J.-N. Safety of 18F-DOPA injection for PET of carcinoid tumor. J. Nucl. Med. 2006, 47, 1732. [Google Scholar]
- Verhoeven, M.; Seimbille, Y.; Dalm, S.U. Therapeutic applications of pretargeting. Pharmaceutics 2019, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Warram, J.M.; de Boer, E.; Sorace, A.G.; Chung, T.K.; Kim, H.; Pleijhuis, R.G.; van Dam, G.M.; Rosenthal, E.L. Antibody-based imaging strategies for cancer. Cancer Metastasis Rev. 2014, 33, 809–822. [Google Scholar] [CrossRef]
- Dammes, N.; Peer, D. Monoclonal antibody-based molecular imaging strategies and theranostic opportunities. Theranostics 2020, 10, 938–955. [Google Scholar] [CrossRef]
- Malviya, G.D.; Alessandria, C.; Lanzolla, T.; Lenza, A.; Conti, F.V.G. 99mTechnetium labelled anti-TNF-α antibodies for the therapy-decision making and follow-up of patients with rheumatoid arthritis. Q. J. Nucl. Med. Mol. Imaging 2008, 52, 13. [Google Scholar]
- Ceccarelli, F.; Perricone, C.; Galli, F.; Valesini, G.; Conti, F. Use of 99mTc-labelled Anti-TNF Monoclonal Antibodies to Assess Patients Affected by Inflammatory Arthropathies. Int. J. Radiol. Med. Imaging 2015, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kurdziel, K.A.; Mena, E.; McKinney, Y.; Wong, K.; Adler, S.; Sissung, T.; Lee, J.; Lipkowitz, S.; Lindenberg, L.; Turkbey, B.; et al. First-in-human phase 0 study of 111In-CHX-A”-DTPA trastuzumab for HER2 tumor imaging. J. Transl. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Aghevlian, S.; Lu, Y.; Winnik, M.A.; Hedley, D.W.; Reilly, R.M. Panitumumab Modified with Metal-Chelating Polymers (MCP) Complexed to 111 In and 177 Lu—An EGFR-Targeted Theranostic for Pancreatic Cancer. Mol. Pharm. 2018, 15, 1150–1159. [Google Scholar] [CrossRef]
- Lohrke, J.; Siebeneicher, H.; Berger, M.; Reinhardt, M.; Berndt, M.; Mueller, A.; Zerna, M.; Koglin, N.; Oden, F.; Bauser, M.; et al. 18F-GP1, a novel PET tracer designed for high-sensitivity, low-background detection of thrombi. J. Nucl. Med. 2017, 58, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.J.; Bu, J.; Kubiatowicz, L.J.; Chen, S.S.; Kim, Y.S.; Hong, S. Peptide–nanoparticle conjugates: A next generation of diagnostic and therapeutic platforms? Nano Converg. 2018, 5, 38. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef]
- Koudrina, A.; Derosa, M.C. Advances in medical imaging: Aptamer- And peptide-targeted MRI and CT contrast agents. ACS Omega 2020, 5, 22691–22701. [Google Scholar] [CrossRef]
- Yin, J.; Yao, D.; Yin, G.; Huang, Z.; Pu, X. Peptide-Decorated Ultrasmall Superparamagnetic Nanoparticles as Active Targeting MRI Contrast Agents for Ovarian Tumors. ACS Appl. Mater. Interfaces 2019, 11, 41038–41050. [Google Scholar] [CrossRef]
- Boschi, A.; Massi, A.; Uccelli, L.; Pasquali, M.; Duatti, A. PEGylated N-methyl-S-methyl dithiocarbazate as a new reagent for the high-yield preparation of nitrido Tc-99m and Re-188 radiopharmaceuticals. Nucl. Med. Biol. 2010, 37, 927–934. [Google Scholar] [CrossRef]
- Bolzati, C.; Dolmella, A. Nitrido technetium-99m core in radiopharmaceutical applications: Four decades of research. Inorganics 2020, 8, 3. [Google Scholar] [CrossRef]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery—New applications on the horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef]
- Chinol, M.; Caviglioli, G.; Baldassari, S.; Russo, E.; Parodi, B.; Carollo, A.; Paganelli, G. A new conjugate of human albumin and p-SCN-Bn-DOTA for radioguided occult lesion localization in breast cancer (ROLL). J. Nucl. Med. 2014, 55, 613. [Google Scholar]
- Ailuno, G.; Baldassari, S.; Zuccari, G.; Schlich, M.; Caviglioli, G. Peptide-based nanosystems for vascular cell adhesion molecule-1 targeting: A real opportunity for therapeutic and diagnostic agents in inflammation associated disorders. J. Drug Deliv. Sci. Technol. 2020, 55, 101461. [Google Scholar] [CrossRef]
- Ailuno, G.; Zuccari, G.; Baldassari, S.; Lai, F.; Caviglioli, G. Anti-Vascular Cell Adhesion Molecule-1 Nanosystems: A Promising Strategy against Inflammatory Based Diseases. J. Nanosci. Nanotechnol. 2021, 21, 2793–2807. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.A.; Nahrendorf, M.; Yu, A.M.; Reynolds, F.; Weissleder, R. In vivo phage display selection yields atherosclerotic plaque targeted peptides for imaging. Mol. Imaging Biol. 2006, 8, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Asti, M.; Ferrari, E.; Croci, S.; Atti, G.; Rubagotti, S.; Iori, M.; Capponi, P.C.; Zerbini, A.; Saladini, M.; Versari, A. Synthesis and characterization of 68Ga-labeled curcumin and curcuminoid complexes as potential radiotracers for imaging of cancer and alzheimers disease. Inorg. Chem. 2014, 53, 4922–4933. [Google Scholar] [CrossRef]
- Rubagotti, S.; Croci, S.; Ferrari, E.; Orteca, G.; Iori, M.; Capponi, P.C.; Versari, A.; Asti, M. Uptake of Ga-curcumin derivatives in different cancer cell lines: Toward the development of new potential 68Ga-labelled curcuminoids-based radiotracers for tumour imaging. J. Inorg. Biochem. 2017, 173, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, N.; Hyeon, T. Recent development of nanoparticles for molecular imaging. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20170022. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; Grodzinski, P.; Cai, W.; Liu, C.H. Big Potential from Small Agents: Nanoparticles for Imaging-Based Companion Diagnostics. ACS Nano 2018, 12, 2106–2121. [Google Scholar] [CrossRef]
- Mauri, M.; Collico, V.; Morelli, L.; Das, P.; García, I.; Penaranda Avila, J.; Bellini, M.; Rotem, R.; Truffi, M.; Corsi, F.; et al. MnO Nanoparticles Embedded in Functional Polymers as T 1 Contrast Agents for Magnetic Resonance Imaging. ACS Appl. Nano Mater. 2020, 3, 3787–3797. [Google Scholar] [CrossRef]
- Rossi, L.M.; Shi, L.; Quina, F.H.; Rosenzweig, Z. Stöber Synthesis of Monodispersed Luminescent Silica Nanoparticles for Bioanalytical Assays. Langmuir 2005, 21, 4277–4280. [Google Scholar] [CrossRef]
- Riva, B.; Bellini, M.; Corvi, E.; Verderio, P.; Rozek, E.; Colzani, B.; Avvakumova, S.; Radeghieri, A.; Rizzuto, M.A.; Morasso, C.; et al. Impact of the strategy adopted for drug loading in nonporous silica nanoparticles on the drug release and cytotoxic activity. J. Colloid Interface Sci. 2018, 519, 18–26. [Google Scholar] [CrossRef]
- Cassano, D.; David, J.; Luin, S.; Voliani, V. Passion fruit-like nanoarchitectures: A general synthesis route. Sci. Rep. 2017, 7, 43795. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M.; Bakr, O.M.; Riello, P.; Polizzi, S.; Anjum, D.H.; Fiameni, S.; Arosio, P.; Orlando, T.; de Julian Fernandez, C.; et al. Coexistence of plasmonic and magnetic properties in Au89Fe11 nanoalloys. Nanoscale 2013, 5, 5611. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, L.; Zhao, M.J.; Zhang, T.; Liu, Y.; Jiang, F.L. Mitochondria-Targeted BODIPY Nanoparticles for Enhanced Photothermal and Photoacoustic Imaging in Vivo. ACS Appl. Bio Mater. 2021, 4, 1760–1770. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, W.; Kuang, X.; Hou, S.; Liu, H. Nanopreparations for mitochondria targeting drug delivery system: Current strategies and future prospective. Asian J. Pharm. Sci. 2017, 12, 498–508. [Google Scholar] [CrossRef]
- Pathak, R.; Kolishetti, N.; Dhar, S. Targeted nanoparticles in mitochondrial medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, L.E.; Dukes, M.J.; Hunt, C.R.; Casey, J.D.; Bornhop, D.J. TSPO Targeted Dendrimer Imaging Agent: Synthesis, Characterization, and Cellular Internalization. Bioconjug. Chem. 2009, 20, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Iacobazzi, R.M.; Natile, G.; Margiotta, N. Metal complexes targeting the Translocator Protein 18 kDa (TSPO). Coord. Chem. Rev. 2017, 341, 1–18. [Google Scholar] [CrossRef]
- De Maria Marchiano, R.; Di Sante, G.; Piro, G.; Carbone, C.; Tortora, G.; Boldrini, L.; Pietragalla, A.; Daniele, G.; Tredicine, M.; Cesario, A.; et al. Translational research in the era of precision medicine: Where we are and where we will go. J. Pers. Med. 2021, 11, 216. [Google Scholar] [CrossRef]
- Laghi, L.; Ricciardiello, L. The changing approach for identifying hereditary colorectal cancer syndromes. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 593–594. [Google Scholar] [CrossRef] [PubMed]
- Dama, E.; Melocchi, V.; Dezi, F.; Pirroni, S.; Carletti, R.M.; Brambilla, D.; Bertalot, G.; Casiraghi, M.; Maisonneuve, P.; Barberis, M.; et al. An Aggressive Subtype of Stage I Lung Adenocarcinoma with Molecular and Prognostic Characteristics Typical of Advanced Lung Cancers. Clin. Cancer Res. 2017, 23, 62–72. [Google Scholar] [CrossRef] [PubMed]




| Formulation Strategy | Formulation Innovation | Imaging Probe | Imaging Technique | Application | Tests | Ref. |
|---|---|---|---|---|---|---|
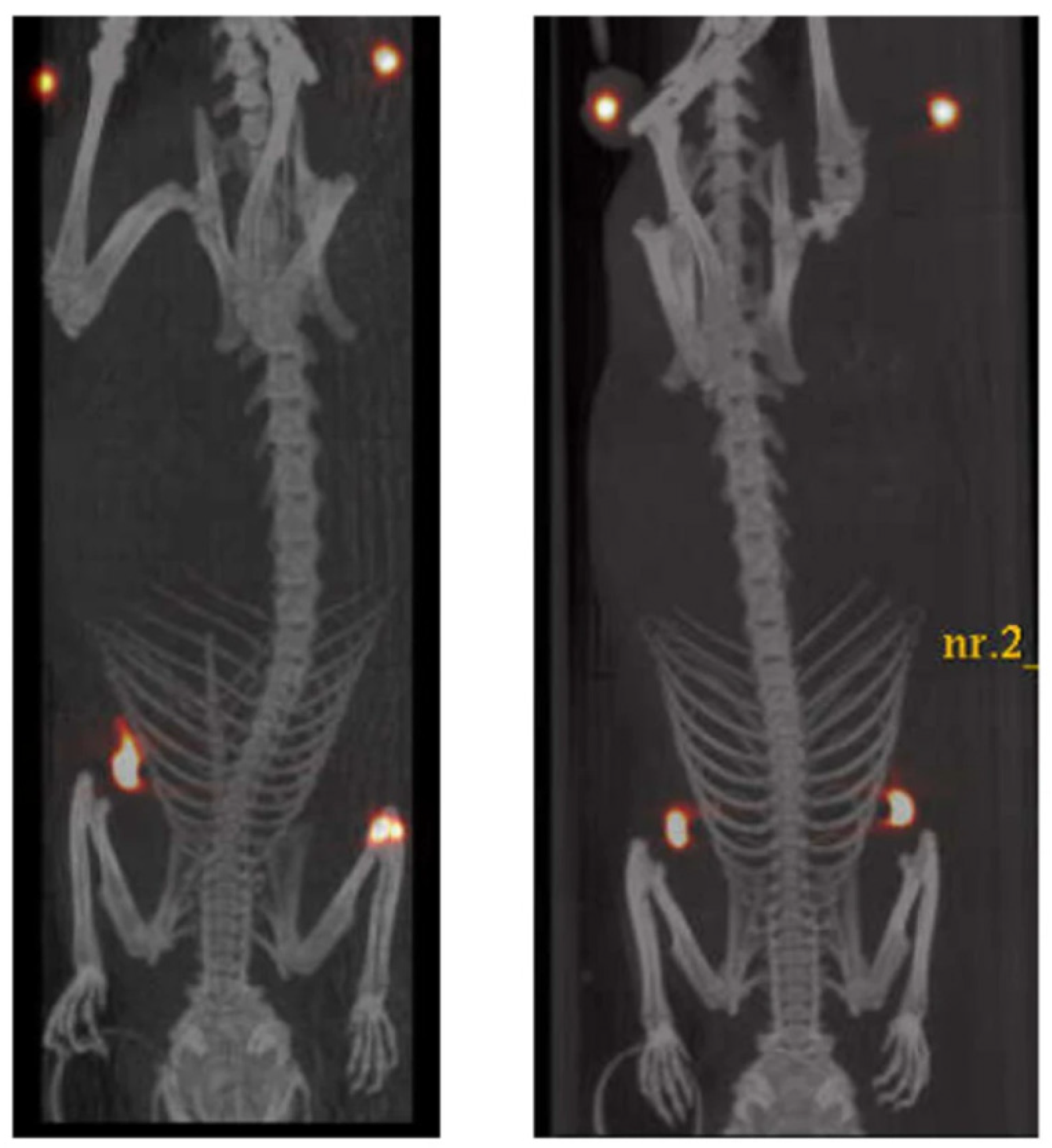
| Chemical modification, prodrugs and bio-precursors | Insertion of a deuterium atom in [18F]fluoromethyl-PBR28 | 18F | PET | Detection of inflammation associated to TSPO overexpression; diagnosis of neuroinflammation, neurodegeneration, and tumor progression | In vitro: human leukocyte membranes and rat cerebrocortical samples; Ex vivo: ICR mice In vivo: rat models of LPS-induced inflammation and of experimental autoimmune myocarditis | [24,25] |
| Fluorine-substituted TSPO ligand | 18F | PET | Detection of neuroinflammation, neurodegeneration, and tumor progression | Ex vivo: ICR mice In vivo: U87-MG xenografted Balb/c nu/nu mice | [17] | |
| Use of buffers | Use of lactate buffer for [18F]F-DOPA dissolution | 18F | PET | Detection of loss of functional dopaminergic neuron terminals in the striatum | In vitro: mouse skeletal muscle fibers In vivo: Wistar rats and C57BL/6J mice | [26] |
| Conjugates for active targeting | Synthesis of dextran-based multifunctional ligands | 99mTc | Lymphoscintigraphy | Sentinel lymph node detection for diagnosis of different types of cancers | In vitro: stability monitored in saline or serum | [27] |
| Conjugation of an albumin derivative with a DOTA ring | 177Lu, 111In, 64Cu | PET | Radio-guided occult lesion localization | In vivo: female adult rats | [28] | |
| Conjugation of a VCAM-1-binding peptide with a DOTA ring; development of a biotin/avidin three-step pretargeting system based on the same VCAM-1-binding peptide | 68Ga | PET | Early diagnosis of atherosclerosis | In vitro: TNF-α activated HUVEC | [29,30] | |
| Conjugation of curcumin with a DOTA ring | 68Ga | PET | Detection of colorectal cancer | In vitro: HT29 colorectal cancer cells In vivo: HT29 tumor-bearing mice | [31] | |
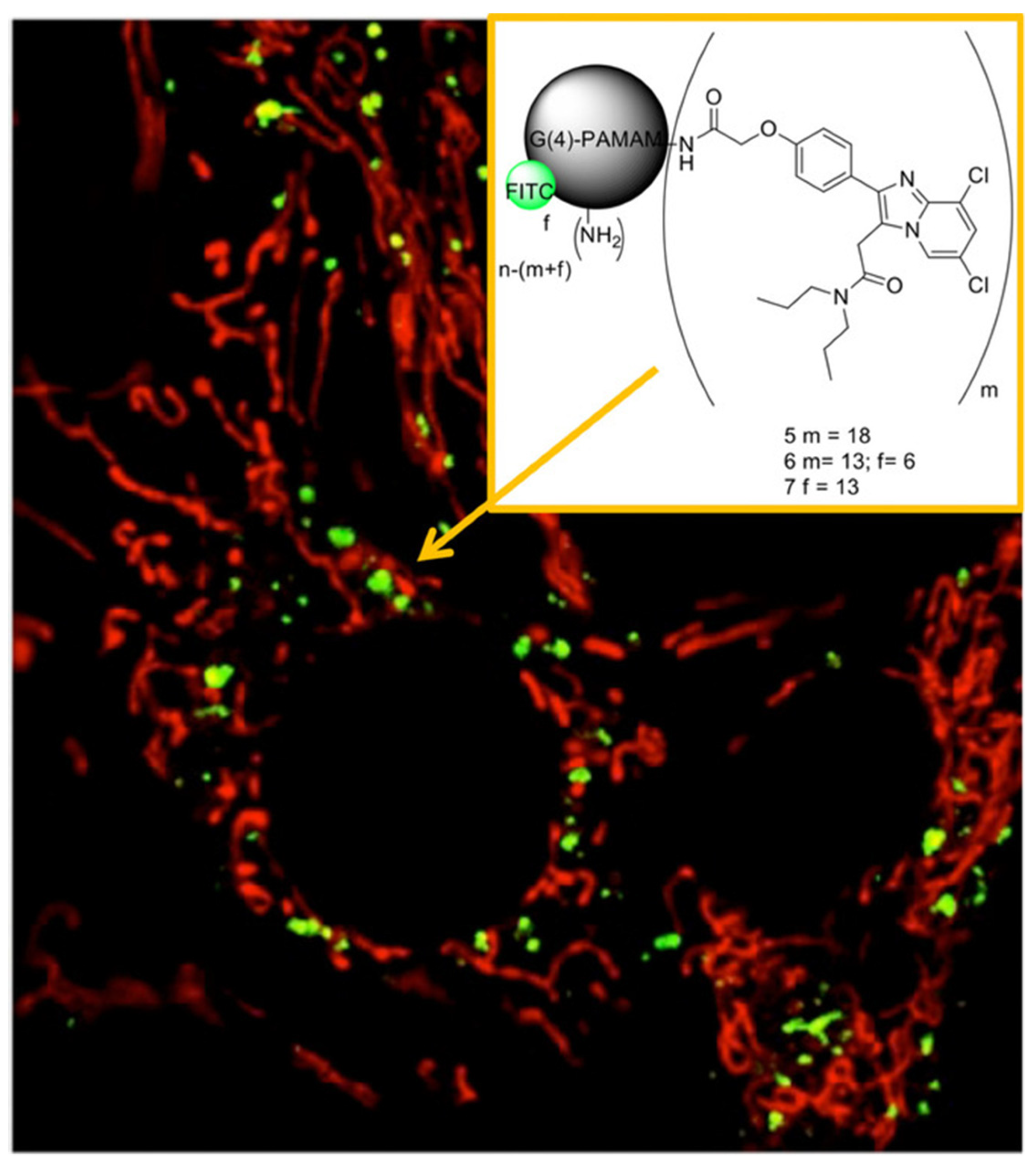
| Nanoparticles | PAMAM dendrimer encapsulating sorafenib | FITC | Fluorescence optical imaging | Detection and treatment of liver cancer overexpressing ASGPR2 | In vitro: HepG-2 human liver cancer cells | [32] |
| PEG-decorated SLNs encapsulating Pt(IV)-prodrugs | Carbon dots | Fluorescence optical imaging | Assessment of BBB permeability | In vitro: hCMEC/D3 cells and polarized hCMEC/D3 endothelial cells seeded on a porous membrane (BBB model) | [33] | |
| PLGA-TSPO NPs | FITC | Fluorescence optical imaging | Subcellular targeting and imaging of TSPO-overexpressing cells | In vitro: rat C6 glioma cells | [34] | |
| TSPO-dextran nanogel | FITC | Fluorescence optical imaging | Subcellular targeting and imaging of TSPO-overexpressing cells | In vitro: rat C6 glioma cells | [35] | |
| PAMAM dendrimer | FITC | Fluorescence optical imaging | Detection of tumors or neurodegenerative diseases associated to TSPO overexpression | In vitro: C6 rat glioma cells | [36] | |
| Silica shell functionalized QDs | QDs | Fluorescence optical imaging | Detection of tumors or neurodegenerative diseases associated to TSPO overexpression | In vitro: C6 rat glioma cells | [37] | |
| USPIONs | Cyanine 5.5 fluorescent dye | NIR optical imaging | Detection of glioblastoma exploiting TSPO targeting | In vitro: U87-MG glioblastoma cells and PC3 prostate cancer cells In vivo: Balb/c athymic mice | [38] | |
| ECFC cells encapsulating AuNPs | AuNPs | PA imaging | Detection and treatment of cancer | In vitro: stack of chicken breast muscle Ex vivo: mouse melanoma, liver and spleen In vivo: CD1 immunodeficient mice | [39] | |
| Chitosan nanobubbles | Gd(III)-DOTP complex | MRI | Detection and treatment of cancer | In vitro: preliminary evaluation of US properties in agar gel suspension | [40] | |
| Fe-B NPs enveloped by polyvinylpyrrolidone | Iron | MRI | Optimization of NCT procedures for cancer treatment | In vitro: L929 fibroblasts, 4T1 mammary carcinoma cells and B16 melanoma cells In vivo: Balb/c mice | [41] | |
| Iron-doped hydroxyapatite NPs | Iron | MRI coupled to SPECT and/or PET | - | In vivo: C57BL/6 mice | [42] | |
| PLGA-PEG micelles encapsulating QDs | QDs | MRI | Detection of inflammatory bowel disease by MAdCAM-1 targeting | In vitro: SKBR3 cells In vivo: C57BL/6 mice | [43] | |
| Carboxymethylcellulose NPs | 68Ga | PET | White blood cells imaging | In vitro: human leukocytes | [44] | |
| SiNPs | 99mTc | SPECT | Detection of HER2-positive breast cancer | In vitro: SK-BR-3 cells Ex vivo, in vivo: SK-BR-3 tumor-bearing mice | [45,46] | |
| Fe3O4 NPs | Fe3O4 and 7-nitrobenzofurazan fluorescent dye | Optical imaging/MRI | Detection and treatment of cancer | In vitro: preliminary evaluation of fluorescence and MRI properties | [47] | |
| SiNPs embedding dye-modified Au NPs | IRDye 800CW and AuNPs | US/PA imaging | Detection and treatment of cancer | Ex vivo: chicken breast samples | [48] | |
| Au-Fe nanoalloys | Au and Fe | X-ray CT/MRI | - | In vivo: Balb/c mice | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailuno, G.; Iacobazzi, R.M.; Lopalco, A.; Baldassari, S.; Arduino, I.; Azzariti, A.; Pastorino, S.; Caviglioli, G.; Denora, N. The Pharmaceutical Technology Approach on Imaging Innovations from Italian Research. Pharmaceutics 2021, 13, 1214. https://doi.org/10.3390/pharmaceutics13081214
Ailuno G, Iacobazzi RM, Lopalco A, Baldassari S, Arduino I, Azzariti A, Pastorino S, Caviglioli G, Denora N. The Pharmaceutical Technology Approach on Imaging Innovations from Italian Research. Pharmaceutics. 2021; 13(8):1214. https://doi.org/10.3390/pharmaceutics13081214
Chicago/Turabian StyleAiluno, Giorgia, Rosa Maria Iacobazzi, Antonio Lopalco, Sara Baldassari, Ilaria Arduino, Amalia Azzariti, Sara Pastorino, Gabriele Caviglioli, and Nunzio Denora. 2021. "The Pharmaceutical Technology Approach on Imaging Innovations from Italian Research" Pharmaceutics 13, no. 8: 1214. https://doi.org/10.3390/pharmaceutics13081214
APA StyleAiluno, G., Iacobazzi, R. M., Lopalco, A., Baldassari, S., Arduino, I., Azzariti, A., Pastorino, S., Caviglioli, G., & Denora, N. (2021). The Pharmaceutical Technology Approach on Imaging Innovations from Italian Research. Pharmaceutics, 13(8), 1214. https://doi.org/10.3390/pharmaceutics13081214











