In Vitro Evaluation of Poly(lactide-co-glycolide) In Situ Forming Gels for Bedaquiline Fumarate Salt and Pharmacokinetics Following Subcutaneous Injection in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation Preparations
2.3. In Vitro Evaluations
2.3.1. In Vitro Release Study
2.3.2. Analytical Method for the In Vitro Release Study
2.3.3. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
2.4. In Vivo Pharmacokinetic Study in Rats
2.4.1. Animals
2.4.2. Pharmacokinetics
2.4.3. Bioanalytical Method for Pharmacokinetics
2.4.4. Clinical Observations
3. Results
3.1. In Vitro Evaluations
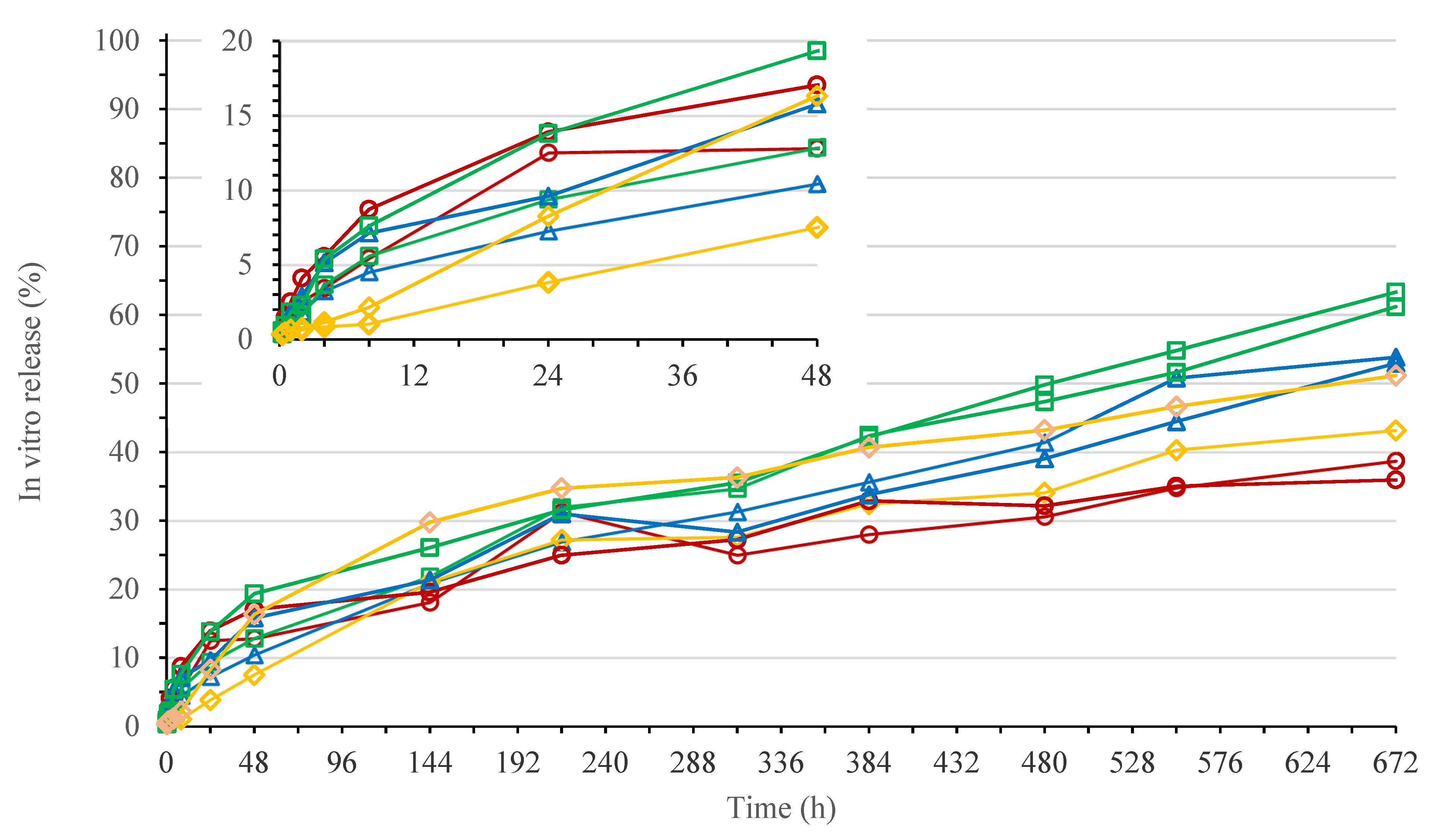
3.1.1. In Vitro Release Study for 200 mg eq./g Bedaquiline Fumarate Salt in Polymer/NMP 20/80% (w/w)
3.1.2. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
3.2. In Vivo Pharmacokinetic Study in Rats
Pharmacokinetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2020. Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 12 July 2021).
- Chahine, E.B.; Karaoui, L.R.; Mansour, H. Bedaquiline: A novel diarylquinoline for multidrug-resistant tuberculosis. Ann. Pharmacother. 2014, 48, 107–115. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Consolidated Guidelines on Tuberculosis, Module 4: Treatment—Drug-Resistant Tuberculosis Treatment. Available online: https://www.who.int/publications/i/item/9789240007048 (accessed on 12 July 2021).
- U.S. National Library of Medicine, DailyMed Label: SIRTURO—Bedaquiline Fumarate Tablet. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1534c9ae-4948-4cf4-9f66-222a99db6d0e (accessed on 12 July 2021).
- Swindells, S.; Siccardi, M.; Barrett, S.E.; Olsen, D.B.; Grobler, J.A.; Podany, A.T.; Nuermberger, E.; Kim, P.; Barry, C.E.; Owen, A.; et al. Long-acting formulations for the treatment of latent tuberculous infection: Opportunities and challenges. Int. J. Tuberc. Lung Dis. 2018, 22, 125–132. [Google Scholar] [CrossRef]
- Kaushik, A.; Ammerman, N.C.; Tyagi, S.; Saini, V.; Vervoort, I.; Lachau-Durand, S.; Nuermberger, E.; Andries, K. Activity of a long-acting injectable bedaquiline formulation in a paucibacillary mouse model of latent tuberculosis infection. Antimicrob. Agents Chemother. 2019, 63, e00007-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.; Bevernage, J.; Darville, N.; Tistaert, C.; Van Bocxlaer, J.; Rossenu, S.; Vermeulen, A. Linking in vitro intrinsic dissolution rate and thermodynamic solubility with pharmacokinetic profiles of bedaquiline long-acting aqueous microsuspensions in rats. Mol. Pharm. 2021, 18, 952–965. [Google Scholar] [CrossRef]
- Kempe, S.; Mäder, K. In situ forming implants—an attractive formulation principle for parenteral depot formulations. J. Control. Release 2012, 161, 668–679. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine, DailyMed Label: ELIGARD—Leuprolide Acetate Kit. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b78d1919-9dee-44fa-90f9-e0a26d32481d (accessed on 12 July 2021).
- U.S. National Library of Medicine, DailyMed Label: ATRIDOX—Doxycycline Hyclate Kit. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2550dbc0-8bdd-4bca-81d5-eec77ee6fbd5 (accessed on 12 July 2021).
- U.S. National Library of Medicine, DailyMed Label: SUBLOCADE—Buprenorphine Solution. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6189fb21-9432-45f8-8481-0bfaf3ccde95 (accessed on 12 July 2021).
- Parent, M.; Nouvel, C.; Koerber, M.; Sapin, A.; Maincent, P.; Boudier, A. PLGA in situ implants formed by phase inversion: Critical physicochemical parameters to modulate drug release. J. Control. Release 2013, 172, 292–304. [Google Scholar] [CrossRef]
- Brodbeck, K.J.; DesNoyer, J.R.; McHugh, A.J. Phase inversion dynamics of PLGA solutions related to drug delivery. Part II. The role of solution thermodynamics and bath-side mass transfer. J. Control. Release 1999, 62, 333–344. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Ahmed, T. Review: Approaches to develop PLGA based in situ gelling system with low initial burst. Pak. J. Pharm. Sci. 2015, 28, 657–665. [Google Scholar]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Park, T.G. Degradation of poly(d,l-lactic acid) microspheres: Effect of molecular weight. J. Control. Release 1994, 30, 161–173. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Ibrahim, H.M.; Ibrahim, F.; Samy, A.M.; Kaseem, A.; Nutan, M.T.; Hussain, M.D. Development of biodegradable in situ implant and microparticle injectable formulations for sustained delivery of haloperidol. J. Pharm. Sci. 2012, 101, 3753–3762. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Ibrahim, H.M.; Samy, A.M.; Kaseem, A.; Nutan, M.T.; Hussain, M.D. Biodegradable injectable in situ implants and microparticles for sustained release of montelukast: In vitro release, pharmacokinetics, and stability. AAPS PharmSciTech 2014, 15, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.A.; Alharby, Y.A.; El-Helw, A.R.; Hosny, K.M.; El-Say, K.M. Depot injectable atorvastatin biodegradable in situ gel: Development, optimization, in vitro, and in vivo evaluation. Drug Des. Dev. Ther. 2016, 10, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Lin, X.; Zheng, X.; Feng, Y.; Shen, L. Injectable long-acting systems for Radix Ophiopogonis polysaccharide based on mono-PEGylation and in situ formation of a PLGA depot. Int. J. Nanomed. 2014, 9, 5555–5563. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, H.M.; Ahmed, T.A.; Hussain, M.D.; Rahman, Z.; Samy, A.M.; Kaseem, A.A.; Nutan, M.T. Development of meloxicam in situ implant formulation by quality by design principle. Drug Dev. Ind. Pharm. 2014, 40, 66–73. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A.; Zhao, X.; Liu, X.; Wang, D.; Sun, F.; Li, Y. Design of a long-term antipsychotic in situ forming implant and its release control method and mechanism. Int. J. Pharm. 2012, 427, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, R.; Mobedi, H.; Najafi, J.; Enayati, M. In-vitro/in-vivo comparison of leuprolide acetate release from an in-situ forming PLGA system. DARU J. Pharm. Sci. 2013, 21, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avachat, A.M.; Kapure, S.S. Asenapine maleate in situ forming biodegradable implant: An approach to enhance bioavailability. Int. J. Pharm. 2014, 477, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Lihong, W.; Qiuyuan, L.; Hongzhuo, L. Injectable long-term control-released in situ gels of hydrochloric thiothixene for the treatment of schizophrenia: Preparation, in vitro and in vivo evaluation. Int. J. Pharm. 2014, 469, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kamali, H.; Khodaverdia, E.; Hadizadeh, F.; Yazdian-Robatic, R.; Haghbine, A.; Zohurif, G. An in-situ forming implant formulation of naltrexone with minimum initial additive: In-vitro, ex-vivo, and in-vivo release evaluation. J. Drug Deliv. Sci. Technol. 2018, 47, 95–105. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; Eissa, R.G.; El-Megrab, N.A.; El-Nahas, H.M. Morphological characterization of optimized risperidone-loaded in-situ gel forming implants with pharmacokinetic and behavioral assessments in rats. J. Drug Deliv. Sci. Technol. 2021, 61, 102195. [Google Scholar] [CrossRef]
- Patel, R.B.; Carlson, A.N.; Solorio, L.; Exner, A.A. Characterization of formulation parameters affecting low molecular weight drug release from in situ forming drug delivery systems. J. Biomed. Mater. Res. A 2010, 94, 476–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S. Lipophilic and hydrophilic drug loaded PLA/PLGA in situ implants: Studies on thermal behavior of drug & polymer and observation of parameters influencing drug burst release with corresponding effects on loading efficiency & morphology of implants. Int. J. Pharm. Pharm. Sci. 2011, 3, 181–188. [Google Scholar]
- Koocheki, S.; Madaeni, S.S.; Niroomandi, P. Development of an enhanced formulation for delivering sustained release of buprenorphine hydrochloride. Saudi Pharm. J. 2011, 19, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Kilicarslan, M.; Koerber, M.; Bodmeier, R. In situ forming implants for the delivery of metronidazole to periodontal pockets: Formulation and drug release studies. Drug Dev. Ind. Pharm. 2014, 40, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.A. Simvastatin in-situ forming implants: Preparation and characterization. Int. J. Pharm. Pharm. Res. 2016, 7, 44–57. [Google Scholar]
- Moore, J.W.; Flanner, H.H. Mathematical comparison of dissolution profiles. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]
- Center for Drug Evaluation and Research, Application Number: 204384Orig1S000, Pharmacology Review(s). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/204384Orig1s000PharmR.pdf (accessed on 12 July 2021).
- Madhu, M.; Shaila, L.; Anwar, B.J. Biodegradable injectable implant systems for sustained delivery using poly (lactide-co-glycolide) copolymers. Int. J. Pharm. Pharm. Sci. 2009, 1, 103–107. [Google Scholar]
- Solorio, L.; Exner, A.A. Effect of the subcutaneous environment on phase-sensitive in situ-forming implant drug release, degradation, and microstructure. J. Pharm. Sci. 2015, 104, 4322–4328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passerini, N.; Craig, D.Q. An investigation into the effects of residual water on the glass transition temperature of polylactide microspheres using modulated temperature DSC. J. Control. Release 2001, 73, 111–115. [Google Scholar] [CrossRef]






| Formulation Number (Type) | Polymer Grade | Solvent | Bedaquiline Fumarate Salt Concentration | Polymer/Solvent Ratio | Assessments |
|---|---|---|---|---|---|
| 1 (ISG) | PLGA5050A | NMP | 200 mg eq./g | 20/80% (w/w) | In vitro and in vivo drug release, SEM, EDX |
| 2 (ISG) | PLGA7525A | NMP | 200 mg eq./g | 20/80% (w/w) | |
| 3 (ISG) | PLGA7525E | NMP | 200 mg eq./g | 20/80% (w/w) | In vitro and in vivo drug release |
| 4 (ISG) | PDLLA | NMP | 200 mg eq./g | 20/80% (w/w) | |
| 5 (Solution) | PEG400 | Water | 5 mg eq./mL | 50/50% (v/v) | In vivo drug release |
| Group | N | Formulation Number (Type) | Dosing Route | Dose (mg eq./kg) | Dosing Volume (mL/kg) | Assessments |
|---|---|---|---|---|---|---|
| 1 | 3 | 1 (ISG) | SC (Day 1) | 80 | 0.4 | Pharmacokinetics:0–4032 h (168 days) |
| 2 | 3 | 2 (ISG) | SC (Day 1) | 80 | 0.4 | |
| 3 | 3 | 3 (ISG) | SC (Day 1) | 80 | 0.4 | |
| 4 | 3 | 4 (ISG) | SC (Day 1) | 80 | 0.4 | |
| 5 | 3 | 5 (Solution) | SC (Day 1) | 4 | 0.8 |
| Analyte | Bedaquiline | |||||
|---|---|---|---|---|---|---|
| Species/Sex | Sprague Dawley Rat/Male | |||||
| Dosing Route | Subcutaneous | |||||
| Formulation Number (Type) | 1 (ISG) | 2 (ISG) | 3 (ISG) | 4 (ISG) | 5 (Solution) | |
| Dose (mg eq./kg) | 80 | 80 | 80 | 80 | 4 | |
| PK parameter: | ||||||
| n | 3 | 3 | 3 | 3 | 3 | |
| Cmax a (ng/mL) | Mean (SD) | 152 (109) | 100 (44) | 71.6 (26.2) | 78 (59) | 56.5 (20.4) |
| tmax a (h) | Min-Max | 4.0–7.0 | 7.0 | 4.0–7.0 | 4.0 | 4.0–7.0 |
| tlast (h) | Min-Max | 4032 | 4032 | 4032 | 4032 | 2688 |
| AUC72h (ng·h/mL) | Mean (SD) | 5511 (3958) | 3639 (1152) | 2572 (1199) | 4115 (2325) | 2186 (807) |
| AUClast (ng·h/mL) | Mean (SD) | 101,258 (44,708) | 63,060 (22,519) | 52,698 (26,072) | 77,457 (42,363) | 10,697 (5121) |
| AUC∞ b,c (ng·h/mL) | Mean (SD) | 123,231 (54,055) | 80,651 (26,971) | 64,712 (37,540) | 88,128 (49,186) | 11,344 (4853) |
| t1/2 b,d (h) | Mean (SD) | 1695 (275) | 1980 (594) | 1606 (210) | 1411 (122) | 854 (264) |
| Cmax/Dose (10−6/mL) | Mean (SD) | 1.90(1.36) | 1.25 (0.54) | 0.895 (0.328) | 0.974 (0.379) | 14.1 (5.1) |
| AUC72h/Dose (10−6·h/mL) | Mean (SD) | 68.9 (49.5) | 45.5 (14.4) | 32.1 (15.0) | 51.4 (29.1) | 547 (202) |
| AUClast/Dose (10−6·h/mL) | Mean (SD) | 1266 (559) | 788 (281) | 659 (326) | 968 (530) | 2674 (1280) |
| AUC∞/Dose (10−6·h/mL) | Mean (SD) | 1540 (676) | 1008 (337) | 809 (469) | 1102 (615) | 2836 (1213) |
| AUClast/Dose vs. F5 | Ratio of means | 0.47 | 0.29 | 0.25 | 0.36 | - |
| Cmax/Dose vs. F5 | Ratio of means | 0.13 | 0.09 | 0.06 | 0.07 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Hemelryck, S.; Wens, R.; van Poppel, H.; Luijks, M.; Shahidi, K.; Marczak, M.; Kahnt, A.; Holm, R.; Mannaert, E.; Langguth, P. In Vitro Evaluation of Poly(lactide-co-glycolide) In Situ Forming Gels for Bedaquiline Fumarate Salt and Pharmacokinetics Following Subcutaneous Injection in Rats. Pharmaceutics 2021, 13, 1231. https://doi.org/10.3390/pharmaceutics13081231
Van Hemelryck S, Wens R, van Poppel H, Luijks M, Shahidi K, Marczak M, Kahnt A, Holm R, Mannaert E, Langguth P. In Vitro Evaluation of Poly(lactide-co-glycolide) In Situ Forming Gels for Bedaquiline Fumarate Salt and Pharmacokinetics Following Subcutaneous Injection in Rats. Pharmaceutics. 2021; 13(8):1231. https://doi.org/10.3390/pharmaceutics13081231
Chicago/Turabian StyleVan Hemelryck, Sandy, Rani Wens, Hannelore van Poppel, Milou Luijks, Koosha Shahidi, Marcin Marczak, Ariane Kahnt, René Holm, Erik Mannaert, and Peter Langguth. 2021. "In Vitro Evaluation of Poly(lactide-co-glycolide) In Situ Forming Gels for Bedaquiline Fumarate Salt and Pharmacokinetics Following Subcutaneous Injection in Rats" Pharmaceutics 13, no. 8: 1231. https://doi.org/10.3390/pharmaceutics13081231







