Achyrocline satureioides (Lam.) DC (Asteraceae) Extract-Loaded Nanoemulsions as a Promising Topical Wound Healing Delivery System: In Vitro Assessments in Human Keratinocytes (HaCaT) and HET-CAM Irritant Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of A. satureioides Extract (ASE)
2.3. Flavonoid Determination by Ultra-Fast Liquid Chromatography (UFLC)
2.4. Preparation of A. satureioides Extract-Loaded Nanoemulsions
2.5. Physicochemical Characterization of the Nanoemulsions
2.6. Keratinocyte Cell Culture and Treatment
2.6.1. Cell Viability (MTT) Assay
2.6.2. Propidium Iodide (PI) Assay
2.6.3. Cell Migration
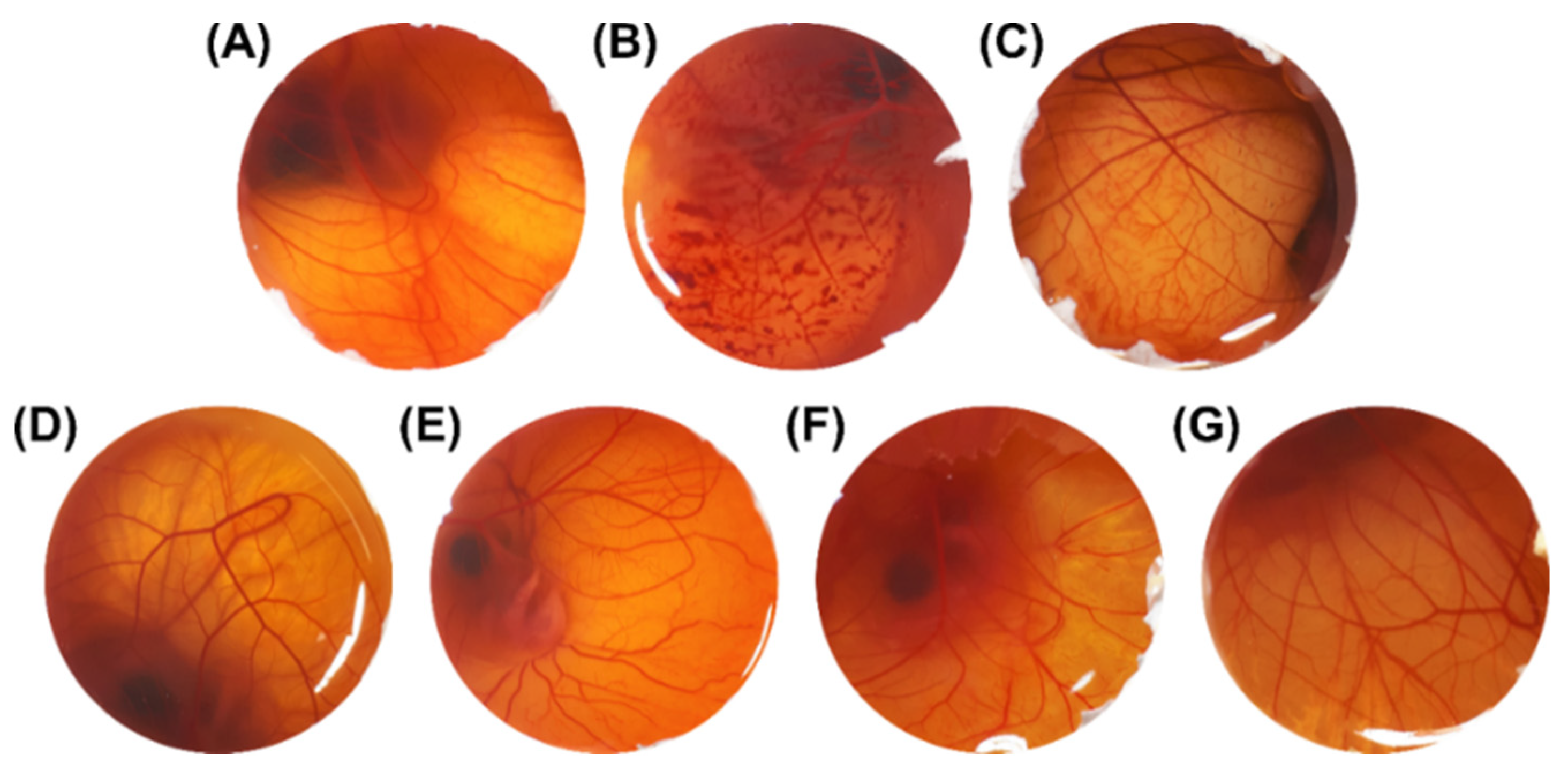
2.7. Hen’s Egg Chorioallantoic Membrane Test (HET-CAM)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of Nanoemulsions
3.2. In Vitro Cell Viability and Migration Evaluation in Keratinocytes
3.3. Hen’s Egg Chorioallantoic Membrane Test (HET-CAM)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Cienc. 2019, 91, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pazyar, N.; Yaghoobi, R.; Rafiee, E.; Mehrabian, A.; Feily, A. Skin wound healing and phytomedicine: A review. Skin Pharmacol. Physiol. 2014, 27, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Elkordy, A.A.; Haj-Ahmad, R.R.; Awaad, A.S.; Zaki, R.M. An overview on natural product drug formulations from conventional medicines to nanomedicines: Past, present and future. J. Drug Deliv. Sci. Technol. 2021, 63, 102459. [Google Scholar] [CrossRef]
- Sun, Y.; Qian, J. Botanical drug clinical trial: Common issues and future options. Acta Pharm. Sin. B 2021, 11, 300–303. [Google Scholar] [CrossRef]
- Gertsch, J. Botanical drugs, synergy, and network pharmacology: Forth and back to intelligent mixtures. Planta Med. 2011, 77, 1086–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retta, D.; Dellacassa, E.; Villamil, J.; Suárez, S.A.; Bandoni, A.L. Marcela, a promising medicinal and aromatic plant from Latin America: A review. Ind. Crops Prod. 2012, 38, 27–38. [Google Scholar] [CrossRef]
- Morquio, A.; Rivera-Megret, F.; Dajas, F. Photoprotection by topical application of Achyrocline satureioides (‘Marcela’). Phyther. Res. 2005, 19, 486–490. [Google Scholar] [CrossRef]
- Simões, C.M.O.; Schenkel, E.P.; Bauer, L.; Langeloh, A. Pharmacological investigations on Achyrocline satureioides (Lam.) DC., compositae. J. Ethnopharmacol. 1988, 22, 281–293. [Google Scholar] [CrossRef]
- De Souza, K.C.B.; Bassani, V.L.; Schapoval, E.E.S. Influence of excipients and technological process on anti-inflammatory activity of quercetin and Achyrocline satureioides (Lam.) D.C. extracts by oral route. Phytomedicine 2007, 14, 102–108. [Google Scholar] [CrossRef]
- Bettega, J.M.R.; Teixeira, H.; Bassani, V.L.; Barardi, C.R.M.; Simões, C.M.O. Evaluation of the antiherpetic activity of standardized extracts of Achyrocline satureioides. Phyther. Res. 2004, 18, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Bidone, J.; Argenta, D.F.; Kratz, J.; Pettenuzzo, L.F.; Horn, A.P.; Koester, L.S.; Bassani, V.L.; Simões, C.M.O.; Teixeira, H.F. Antiherpes Activity and Skin/Mucosa Distribution of Flavonoids from Achyrocline satureioides Extract Incorporated into Topical Nanoemulsions. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alerico, G.C.; Beckenkamp, A.; Vignoli-Silva, M.; Buffon, A.; Von Poser, G.L. Proliferative effect of plants used for wound healing in Rio Grande do Sul state, Brazil. J. Ethnopharmacol. 2015, 176, 305–310. [Google Scholar] [CrossRef]
- Pereira, L.X.; Silva, H.K.C.; Longatti, T.R.; Silva, P.P.; Di Lorenzo Oliveira, C.; de Freitas Carneiro Proietti, A.B.; Thomé, R.G.; do Carmo Vieira, M.; Carollo, C.A.; Demarque, D.P.; et al. Achyrocline alata potentiates repair of skin full thickness excision in mice. J. Tissue Viability 2017, 26, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Doersch, K.M.; Newell-Rogers, M.K. The impact of quercetin on wound healing relates to changes in αV and β1 integrin expression. Exp. Biol. Med. 2017, 242, 1424–1431. [Google Scholar] [CrossRef] [Green Version]
- Yin, G.; Wang, Z.; Wang, Z.; Wang, X. Topical application of quercetin improves wound healing in pressure ulcer lesions. Exp. Dermatol. 2018, 27, 779–786. [Google Scholar] [CrossRef]
- Ozay, Y.; Guzel, S.; Erdogdu, I.H.; Yildirim, Z.; Pehlivanoglu, B.; Turk, B.A.; Darcan, S. Evaluation of the wound healing properties of luteolin ointments on excision and incision wound models in diabetic and non-diabetic rats. Rec. Nat. Prod. 2018, 12, 350–366. [Google Scholar] [CrossRef]
- Carvalho, E.L.S.; Zorzi, G.K.; Von Poser, G.L.; Teixeira, H.F.; Moreira, J.C.F.; Bassani, V.L. Nanoestrutura compreendendo extratos vegetais, processo deprodução de nanoestrutura compreendendo extratos vegetais e composições compreendendo as mesmas. Brazil Patent BR PI0805156A2 I, 20 November 2008. [Google Scholar]
- Bidone, J.; Zorzi, G.K.; Carvalho, E.L.S.; Simões, C.M.O.; Koester, L.S.; Bassani, V.L.; Teixeira, H.F. Incorporation of Achyrocline satureioides (Lam.) DC extracts into topical nanoemulsions obtained by means of spontaneous emulsification procedure. Ind. Crops Prod. 2014, 62, 421–429. [Google Scholar] [CrossRef]
- Balestrin, L.A.; Bidone, J.; Bortolin, R.C.; Moresco, K.; Moreira, J.C.; Teixeira, H.F. Protective effect of a hydrogel containing Achyrocline satureioides extract-loaded nanoemulsion against UV-induced skin damage. J. Photochem. Photobiol. B Biol. 2016, 163, 269–276. [Google Scholar] [CrossRef]
- Balestrin, L.A.; Fachel, F.N.S.; Koester, L.S.; Bassani, V.L.; Teixeira, H.F. A stability-indicating ultra-fast liquid chromatography method for the assay of the main flavonoids of Achyrocline satureioides (Marcela) in porcine skin layers and nanoemulsions. Phytochem. Anal. 2020, 1–10. [Google Scholar] [CrossRef]
- Fachel, F.N.S.; Medeiros-Neves, B.; Dal Prá, M.; Schuh, R.S.; Veras, K.S.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. Box-Behnken design optimization of mucoadhesive chitosan-coated nanoemulsions for rosmarinic acid nasal delivery—In vitro studies. Carbohydr. Polym. 2018, 199, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Reichling, J.; Kehm, R.; Sharaf, M.M.; Zentgraf, H.; Schneele, J.; Schnitzler, P. Efficacy of anise oil, dwarf-pine oil and chamomile oil against thymidine-kinase-positive and thymidine-kinase-negative herpesviruses. J. Pharm. Pharmacol. 2008, 60, 1545–1550. [Google Scholar] [CrossRef]
- Luepke, N.P. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]
- ICCVAM Recommended Test Method Protocol: Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Test Method. NIH Publication No. 10-7553. 2010. Available online: https://ntp.niehs.nih.gov/iccvam/docs/protocols/ivocular-hetcam.pdf (accessed on 13 May 2021).
- Pereira, R.L.; Leites, F.I.; Paese, K.; Sponchiado, R.M.; Michalowski, C.B.; Guterres, S.S.; Schapoval, E.E.S. Hydrogel containing adapalene- and dapsone-loaded lipid-core nanocapsules for cutaneous application: Development, characterization, in vitro irritation and permeation studies. Drug Dev. Ind. Pharm. 2016, 42, 2001–2008. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Lmmunological Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Chassot, J.M.; Ribas, D.; Silveira, E.F.; Grünspan, L.D.; Pires, C.C.; Farago, P.V.; Braganhol, E.; Tasso, L.; Cruz, L. Beclomethasone Dipropionate-Loaded Polymeric Nanocapsules: Development, In Vitro Cytotoxicity, and In Vivo Evaluation of Acute Lung Injury. J. Nanosci. Nanotechnol. 2015, 15, 855–864. [Google Scholar] [CrossRef]
- Dengler, W.A.; Schulte, J.; Berger, D.P.; Mertelsmann, R.; Fiebig, H.H. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anticancer. Drugs 1995, 6, 522–532. [Google Scholar] [CrossRef]
- Gottrup, F.; Ågren, M.S.; Karlsmark, T. Models for use in wound healing research: A survey focusing on in vitro and in vivo adult soft tissue. Wound Repair Regen. 2000, 8, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Erikss, J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef] [Green Version]
- Shanmugapriya, K.; Kim, H.; Saravana, P.S.; Chun, B.S.; Kang, H.W. Astaxanthin-alpha tocopherol nanoemulsion formulation by emulsification methods: Investigation on anticancer, wound healing, and antibacterial effects. Colloids Surf. B Biointerfaces 2018, 172, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Luepke, N.P.; Kemper, F.H. The HET-CAM test: An alternative to the draize eye test. Food Chem. Toxicol. 1986, 24, 495–496. [Google Scholar] [CrossRef]
- Reis Mansur, M.C.P.P.; Leitão, S.G.; Cerqueira-Coutinho, C.; Vermelho, A.B.; Silva, R.S.; Presgrave, O.A.F.; Leitão, Á.A.C.; Leitão, G.G.; Ricci-Júnior, E.; Santos, E.P. In vitro and in vivo evaluation of efficacy and safety of photoprotective formulations containing antioxidant extracts. Rev. Bras. Farmacogn. 2016, 26, 251–258. [Google Scholar] [CrossRef] [Green Version]




| NEB | NEASE | |
|---|---|---|
| Size (nm) | 200 ± 20 | 307 ± 11.0 |
| Polydispersity index | 0.06 ± 0.01 | 0.12 ± 0.01 |
| ζ-potential (mV) | −21.17 ± 3.21 | −43.90 ± 3.92 |
| Viscosity (cP) | 1.66 ± 5.09 | 2.32 ± 2.9 |
| Flavonoids content (μg/mL) | - | 1130.3 ± 7.5 * |
| Treatments | Concentrations (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Control | 0.625 | 1.25 | 2.5 | 5 | 10 | ||
| ASE | M | No | No | No | Yes | Yes | Yes |
| PI | No | No | No | Yes | Yes | Yes | |
| NEASE | M | No | No | No | No | No | No |
| PI | No | No | No | No | No | No | |
| Substance | IS (RSD %) | Classification |
|---|---|---|
| NaCl 0.9% w/v | 0 (0) | Non-irritant |
| NaOH 0.1 M | 13.26 (1.41) * | Extreme irritant |
| Sodium lauryl sulfate 1% w/v | 10.51 (2.29) * | Extreme irritant |
| Olive oil | 0 (0) | Non-irritant |
| ASE:Olive oil (1:100) w/v | 0 (0) | Non-irritant |
| NEB | 0 (0) | Non-irritant |
| NEASE | 0 (0) | Non-irritant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestrin, L.A.; Kreutz, T.; Fachel, F.N.S.; Bidone, J.; Gelsleichter, N.E.; Koester, L.S.; Bassani, V.L.; Braganhol, E.; Dora, C.L.; Teixeira, H.F. Achyrocline satureioides (Lam.) DC (Asteraceae) Extract-Loaded Nanoemulsions as a Promising Topical Wound Healing Delivery System: In Vitro Assessments in Human Keratinocytes (HaCaT) and HET-CAM Irritant Potential. Pharmaceutics 2021, 13, 1241. https://doi.org/10.3390/pharmaceutics13081241
Balestrin LA, Kreutz T, Fachel FNS, Bidone J, Gelsleichter NE, Koester LS, Bassani VL, Braganhol E, Dora CL, Teixeira HF. Achyrocline satureioides (Lam.) DC (Asteraceae) Extract-Loaded Nanoemulsions as a Promising Topical Wound Healing Delivery System: In Vitro Assessments in Human Keratinocytes (HaCaT) and HET-CAM Irritant Potential. Pharmaceutics. 2021; 13(8):1241. https://doi.org/10.3390/pharmaceutics13081241
Chicago/Turabian StyleBalestrin, Lucélia Albarello, Tainá Kreutz, Flávia Nathiely Silveira Fachel, Juliana Bidone, Nicolly Espindola Gelsleichter, Letícia Scherer Koester, Valquiria Linck Bassani, Elizandra Braganhol, Cristiana Lima Dora, and Helder Ferreira Teixeira. 2021. "Achyrocline satureioides (Lam.) DC (Asteraceae) Extract-Loaded Nanoemulsions as a Promising Topical Wound Healing Delivery System: In Vitro Assessments in Human Keratinocytes (HaCaT) and HET-CAM Irritant Potential" Pharmaceutics 13, no. 8: 1241. https://doi.org/10.3390/pharmaceutics13081241
APA StyleBalestrin, L. A., Kreutz, T., Fachel, F. N. S., Bidone, J., Gelsleichter, N. E., Koester, L. S., Bassani, V. L., Braganhol, E., Dora, C. L., & Teixeira, H. F. (2021). Achyrocline satureioides (Lam.) DC (Asteraceae) Extract-Loaded Nanoemulsions as a Promising Topical Wound Healing Delivery System: In Vitro Assessments in Human Keratinocytes (HaCaT) and HET-CAM Irritant Potential. Pharmaceutics, 13(8), 1241. https://doi.org/10.3390/pharmaceutics13081241