Iron Oxide Incorporated Conjugated Polymer Nanoparticles for Simultaneous Use in Magnetic Resonance and Fluorescent Imaging of Brain Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Particles
2.3. Steady State Absorption & Emission Techniques
2.4. Dynamic Light Scattering
2.5. TEM Analysis
2.6. Cell Culture
2.7. Cytotoxicity and In Vitro Uptake Analysis
2.8. PDT In Vitro Treatment
2.9. Magnetic Resonance Imaging
2.10. Fluorescence Imaging
2.11. Imaging Phantom Studies
2.12. Development of GBM In Vivo Models
2.13. Preclinical MRI Studies
- T2WI and T2 map acquisitions.
- Dynamic T2WI study in which 200 μL of IONP-doped CPN 1:6.4 solutions (50mg/L and corresponding to 320 mg/L of IONPs) flushed with 100 μL of saline, were injected intravenously after acquiring 4 basal images.
- T2WI and T2 map 30 min after the CPN injection.
2.13.1. IONP-Doped CPN MRI Evaluation of Orthotopic Tumors
2.13.2. MRI Evaluation of Heterotopic Tumors
2.13.3. Image Processing and Analysis
2.14. Statistical Analysis
2.15. Euthanasia and Organ Preservation for Fluorescence Imaging
2.16. Ex vivo Fluorescence Studies
3. Results
3.1. Preparation and Characterizations of IONP-Doped CPNs
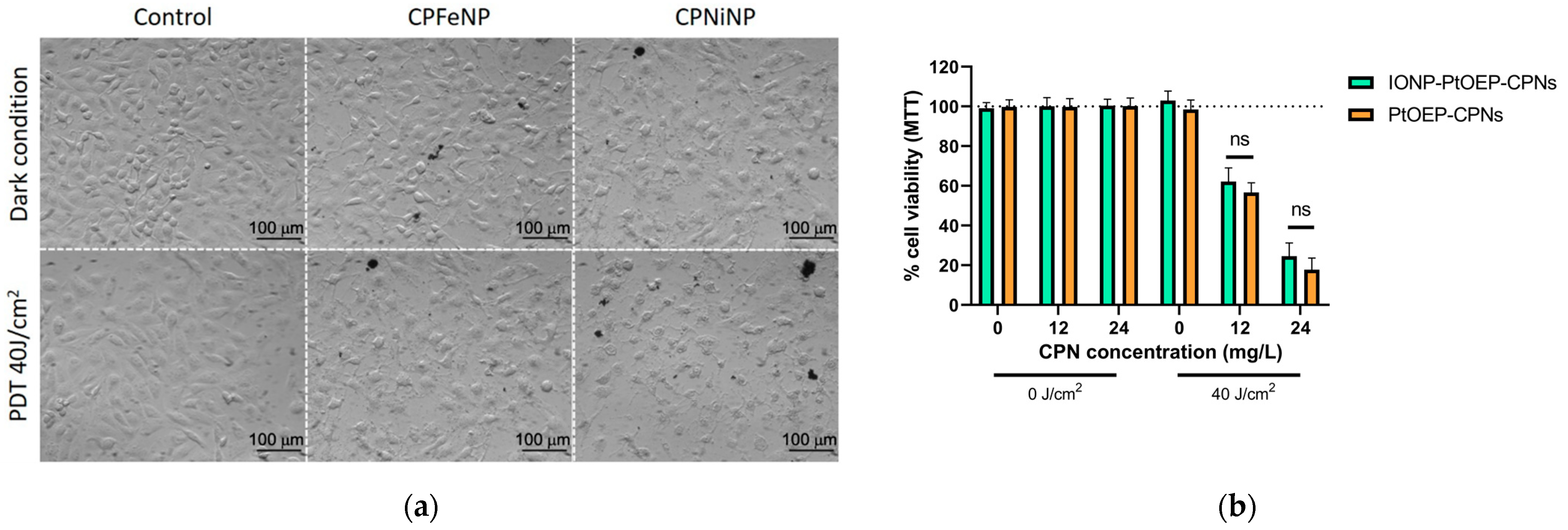
3.2. Biocompatibility and Nanoparticle Uptake in GBM Cells
3.3. PDT In Vitro Efficacy of IONP-Doped CPNs
3.4. In Vitro Imaging Phantom Studies
3.5. Preclinical MRI Studies Subsection
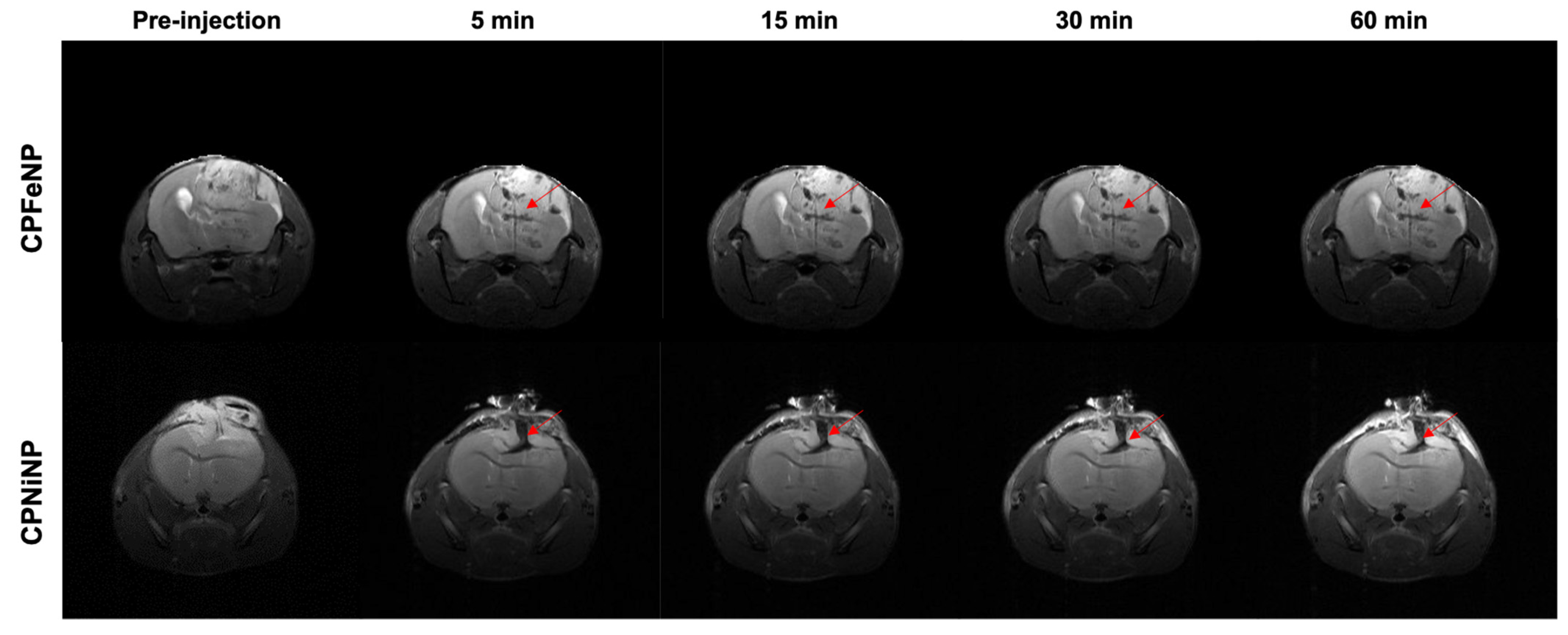
3.5.1. Biodistribution
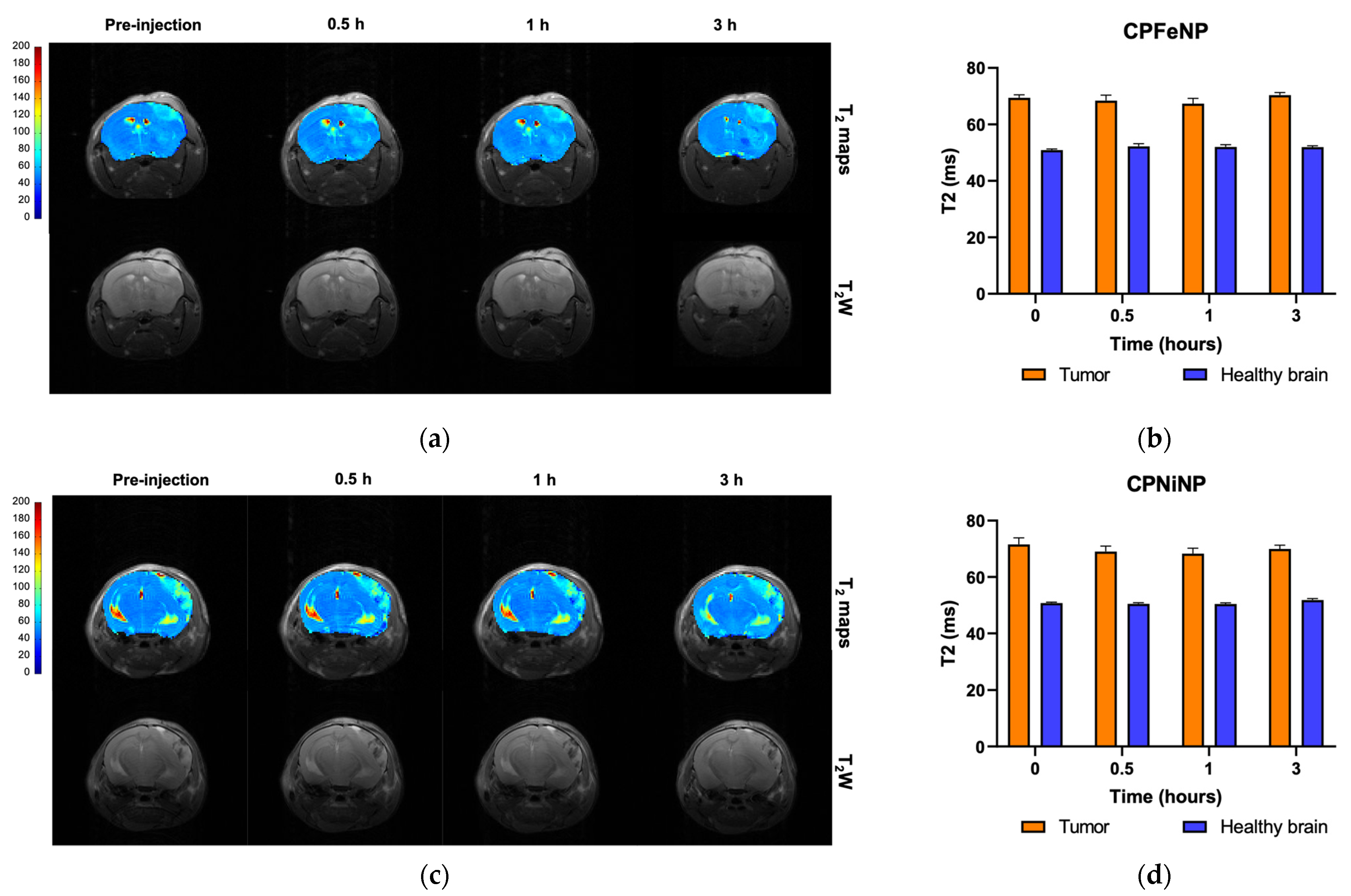
3.5.2. Evaluation of the Orthotopic Glioma Model
3.5.3. Evaluation of the Heterotopic Glioma Model
3.6. Fluorescence Ex Vivo Studies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alifieris, K.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef]
- Ibarra, L.E. Cellular Trojan horses for delivery of nanomedicines to brain tumors: Where do we stand and what is next? Nanomedicine 2021, 16, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Silantyev, A.S.; Falzone, L.; Libra, M.; Gurina, O.I.; Kardashova, K.S.; Nikolouzakis, T.K.; Nosyrev, A.E.; Sutton, C.W.; Mitsias, P.D.; Tsatsakis, A. Current and Future Trends on Diagnosis and Prognosis of Glioblastoma: From Molecular Biology to Proteomics. Cells 2019, 8, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibarra, L.E.; Vilchez, M.L.; Caverzán, M.D.; Sanabria, L.N.M. Understanding the glioblastoma tumor biology to optimize photodynamic therapy: From molecular to cellular events. J. Neurosci. Res. 2021, 99, 1024–1047. [Google Scholar] [CrossRef]
- Kirby, A.J.; Finnerty, G.T. New strategies for managing adult gliomas. J. Neurol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E. Nano-Therapies for Glioblastoma Treatment. Cancers 2020, 12, 242. [Google Scholar] [CrossRef] [Green Version]
- Cramer, S.W.; Chen, C.C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2020, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Ibarra, L.E.; Porcal, G.V.; Macor, L.P.; Ponzio, R.A.; Spada, R.M.; Lorente, C.; Chesta, C.A.; Rivarola, V.A.; Palacios, R.E. Metallated porphyrin-doped conjugated polymer nanoparticles for efficient photodynamic therapy of brain and colorectal tumor cells. Nanomedicine 2018, 13, 605–624. [Google Scholar] [CrossRef]
- Ibarra, L.E.; Beaugé, L.; Arias-Ramos, N.; A Rivarola, V.; A Chesta, C.; López-Larrubia, P.; E Palacios, R. Trojan horse monocyte-mediated delivery of conjugated polymer nanoparticles for improved photodynamic therapy of glioblastoma. Nanomedicine 2020, 15, 1687–1707. [Google Scholar] [CrossRef]
- Caverzán, M.D.; Beaugé, L.; Chesta, C.A.; Palacios, R.E.; Ibarra, L.E. Photodynamic therapy of Glioblastoma cells using doped conjugated polymer nanoparticles: An in vitro comparative study based on redox status. J. Photochem. Photobiol. B: Biol. 2020, 212, 112045. [Google Scholar] [CrossRef]
- Spada, R.M.; Macor, L.P.; Hernández, L.I.; Ponzio, R.A.; Ibarra, L.E.; Lorente, C.; Chesta, C.A.; Palacios, R.E. Amplified singlet oxygen generation in metallated-porphyrin doped conjugated polymer nanoparticles. Dye. Pigment. 2018, 149, 212–223. [Google Scholar] [CrossRef] [Green Version]
- Kuehne, A.J.C. Conjugated Polymer Nanoparticles toward In Vivo Theranostics—Focus on Targeting, Imaging, Therapy, and the Importance of Clearance. Adv. Biosyst. 2017, 1, 1700100. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.; Tang, Y.; Fang, X.; Yin, S.; Xu, H.; Wu, C. Incorporation of Porphyrin to π-Conjugated Backbone for Polymer-Dot-Sensitized Photodynamic Therapy. Biomacromolecules 2016, 17, 2128–2136. [Google Scholar] [CrossRef]
- Lopez-Larrubia, P.; Canadillas-Cardenas, E.; Metelo, M.A.; Arias, N.; Martinez-Maestro, M.; Salguero, A.; Cerda, S. Magnetic Resonance Imaging of Gliomas. In Advances in the Biology, Imaging and Therapies for Glioblastoma; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Borges, A.; López-Larrubia, P.; Marques, J.; Cerdan, S. MR Imaging Features of High-Grade Gliomas in Murine Models: How They Compare with Human Disease, Reflect Tumor Biology, and Play a Role in Preclinical Trials. Am. J. Neuroradiol. 2011, 33, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zheng, L.; Cai, H.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Facile One-Pot Synthesis of Fe3O4@Au Composite Nanoparticles for Dual-Mode MR/CT Imaging Applications. ACS Appl. Mater. Interfaces 2013, 5, 10357–10366. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.S.; Lee, N.; Kim, T.; Kim, H.; Yu, T.; Song, I.C.; Moon, W.K.; Hyeon, T. Multifunctional Uniform Nanoparticles Composed of a Magnetite Nanocrystal Core and a Mesoporous Silica Shell for Magnetic Resonance and Fluorescence Imaging and for Drug Delivery. Angew. Chem. 2008, 120, 8566–8569. [Google Scholar] [CrossRef]
- Li, Y.-H.; Song, T.; Liu, J.-Q.; Zhu, S.-J.; Chang, J. An efficient method for preparing high-performance multifunctional polymer beads simultaneously incorporated with magnetic nanoparticles and quantum dots. J. Mater. Chem. 2011, 21, 12520–12528. [Google Scholar] [CrossRef]
- Senders, J.T.; Muskens, I.S.; Schnoor, R.; Karhade, A.V.; Cote, D.J.; Smith, T.R.; Broekman, M.L.D. Agents for fluorescence-guided glioma surgery: A systematic review of preclinical and clinical results. Acta Neurochir. 2017, 159, 151–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maragkos, G.A.; Schüpper, A.J.; Lakomkin, N.; Sideras, P.; Price, G.; Baron, R.; Hamilton, T.; Haider, S.; Lee, I.Y.; Hadjipanayis, C.G.; et al. Fluorescence-Guided High-Grade Glioma Surgery More Than Four Hours After 5-Aminolevulinic Acid Administration. Front. Neurol. 2021, 12, 644804. [Google Scholar] [CrossRef]
- Lee, C.; Kim, G.R.; Yoon, J.; Kim, S.E.; Yoo, J.S.; Piao, Y. In vivo delineation of glioblastoma by targeting tumor-associated macrophages with near-infrared fluorescent silica coated iron oxide nanoparticles in orthotopic xenografts for surgical guidance. Sci. Rep. 2018, 8, 11122. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, F.; Tang, F.; Ma, N.; Li, L. Preparation of hybrid fluorescent–magnetic nanoparticles for application to cellular imaging by self-assembly. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 103–109. [Google Scholar] [CrossRef]
- Howes, P.; Green, M.; Bowers, A.; Parker, D.; Varma, G.; Kallumadil, M.; Hughes, M.; Warley, A.; Brain, A.; Botnar, R. Magnetic Conjugated Polymer Nanoparticles as Bimodal Imaging Agents. J. Am. Chem. Soc. 2010, 132, 9833–9842. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.-G.; Chen, Y.-L.; Feng, P.; Xiao, X.; Dong, M.; Yu, J.-C.; Hu, Q.-Y.; Shen, Q.-D.; Gu, Z. Conjugated polymer nanomaterials for theranostics. Acta Pharmacol. Sin. 2017, 38, 764–781. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Feng, W.; Hu, C.; Lv, Z.; Wei, H.; Jiang, J.; Liu, S.; Zhao, Q. Manganese porphyrin-incorporated conjugated polymer nanoparticles for T1-enhanced magnetic resonance and fluorescent imaging. Inorg. Chim. Acta 2017, 466, 604–611. [Google Scholar] [CrossRef]
- Li, K.; Ding, D.; Huo, D.; Pu, K.-Y.; Thao, N.N.P.; Hu, Y.; Li, Z.; Liu, B. Conjugated Polymer Based Nanoparticles as Dual-Modal Probes for Targeted In Vivo Fluorescence and Magnetic Resonance Imaging. Adv. Funct. Mater. 2012, 22, 3107–3115. [Google Scholar] [CrossRef]
- Simonsen, J.B.; Kromann, E.B. Pitfalls and opportunities in quantitative fluorescence-based nanomedicine studies—A commentary. J. Control. Release 2021, 335, 660–667. [Google Scholar] [CrossRef]
- Yokel, A.R. Nanoparticle brain delivery: A guide to verification methods. Nanomedicine 2020, 15, 409–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busquets, M.A.; Estelrich, J.; Sánchez-Martín, M.J. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef] [Green Version]
- Champagne, P.-O.; Westwick, H.; Bouthillier, A.; Sawan, M. Colloidal stability of superparamagnetic iron oxide nanoparticles in the central nervous system: A review. Nanomedicine 2018, 13, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Bomati-Miguel, O.; de Alejo, R.P.; Ruiz-Cabello, J.; Veintemillas-Verdaguer, S.; O’Grady, K. Contrast agents for MRI based on iron oxide nanoparticles prepared by laser pyrolysis. J. Magn. Magn. Mater. 2003, 266, 102–109. [Google Scholar] [CrossRef]
- Yuan, Y.; Hou, W.; Sun, Z.; Liu, J.; Ma, N.; Li, X.; Yin, S.; Qin, W.; Wu, C. Measuring Cellular Uptake of Polymer Dots for Quantitative Imaging and Photodynamic Therapy. Anal. Chem. 2021, 93, 7071–7078. [Google Scholar] [CrossRef]
- Ahmad, T.; Rhee, I.; Hong, S.; Chang, Y.; Lee, J. Ni-Fe2O4 Nanoparticles as Contrast Agents for Magnetic Resonance Imaging. J. Nanosci. Nanotechnol. 2011, 11, 5645–5650. [Google Scholar] [CrossRef]
- Fernández-Barahona, I.; Muñoz-Hernando, M.; Ruiz-Cabello, J.; Herranz, F.; Pellico, J. Iron Oxide Nanoparticles: An Alternative for Positive Contrast in Magnetic Resonance Imaging. Inorganics 2020, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Sona, M.M.; Viswanadh, M.K.; Singh, S.; Agrawal, P.; Mehata, A.K.; Pawde, D.M.; Narendra; Sonkar, R.; Muthu, M.S. Nanotheranostics: Emerging Strategies for Early Diagnosis and Therapy of Brain Cancer. Nanotheranostics 2018, 2, 70–86. [Google Scholar] [CrossRef]
- D’Angelo, M.; Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Dominguez-Benot, R.; Pitari, G.; Ippoliti, R.; Cimini, A. Theranostic Nanomedicine for Malignant Gliomas. Front. Bioeng. Biotechnol. 2019, 7, 325. [Google Scholar] [CrossRef] [PubMed]
- Giakoumettis, D.; Kritis, A.; Foroglou, N. C6 cell line: The gold standard in glioma research. Hippokratia 2018, 22, 105–112. [Google Scholar] [PubMed]
- Jena, L.; McErlean, E.; McCarthy, H. Delivery across the blood-brain barrier: Nanomedicine for glioblastoma multiforme. Drug Deliv. Transl. Res. 2019, 10, 304–318. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.; Tatiparti, K.; Sau, S.; Iyer, A.K. Recent advances in nano delivery systems for blood-brain barrier (BBB) penetration and targeting of brain tumors. Drug Discov. Today 2021, S1359644621001951. [Google Scholar] [CrossRef]
- Kang, J.H.; Cho, J.; Ko, Y.T. Investigation on the effect of nanoparticle size on the blood–brain tumour barrier permeability by in situ perfusion via internal carotid artery in mice. J. Drug Target. 2019, 27, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Saric, A.; Boucher, M.; Slomianny, C.; Geffroy, F.; Mériaux, S.; Lalatonne, Y.; Petit, P.X.; Motte, L. Antioxidative Theranostic Iron Oxide Nanoparticles toward Brain Tumors Imaging and ROS Production. ACS Chem. Biol. 2016, 11, 2812–2819. [Google Scholar] [CrossRef]
- Suarez-Garcia, S.; Arias-Ramos, N.; Frias, C.; Candiota, A.P.; Arús, C.; Lorenzo, J.; Ruiz-Molina, D.; Novio, F. Dual T1/T2 Nanoscale Coordination Polymers as Novel Contrast Agents for MRI: A Preclinical Study for Brain Tumor. ACS Appl. Mater. Interfaces 2018, 10, 38819–38832. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Joh, D.Y.; Al-Zaki, A.; Stangl, M.; Murty, S.; Davis, J.J.; Baumann, B.; Alonso-Basanta, M.; Kao, G.D.; Tsourkas, A.; et al. Theranostic Application of Mixed Gold and Superparamagnetic Iron Oxide Nanoparticle Micelles in Glioblastoma Multiforme. J. Biomed. Nanotechnol. 2016, 12, 347–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes, M.; Sousa, J.J.; Pais, A.; Vitorino, C. Targeted Theranostic Nanoparticles for Brain Tumor Treatment. Pharmaceutics 2018, 10, 181. [Google Scholar] [CrossRef] [Green Version]
- Nam, L.; Coll, C.; Erthal, L.C.S.; De La Torre, C.; Serrano, D.; Martínez-Máñez, R.; Santos-Martínez, M.J.; Ruiz-Hernández, E. Drug Delivery Nanosystems for the Localized Treatment of Glioblastoma Multiforme. Materials 2018, 11, 779. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, S.M.; Schneider, M.; E Türeli, A.; Türeli, N.G. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef]
- Gregory, J.V.; Kadiyala, P.; Doherty, R.; Cadena, M.; Habeel, S.; Ruoslahti, E.; Lowenstein, P.R.; Castro, M.G.; Lahann, J. Systemic brain tumor delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef] [PubMed]
- Mapanao, A.K.; Giannone, G.; Summa, M.; Ermini, M.L.; Zamborlin, A.; Santi, M.; Cassano, D.; Bertorelli, R.; Voliani, V. Biokinetics and clearance of inhaled gold ultrasmall-in-nano architectures. Nanoscale Adv. 2020, 2, 3815–3820. [Google Scholar] [CrossRef]
- Gholam, P.; Bosselmann, I.; Enk, A.; Fink, C. Impact of red versus blue light on tolerability and efficacy of PDT: A randomized controlled trial. J. Dtsch. Dermatol. Ges. 2018, 16, 711–717. [Google Scholar] [CrossRef]
- Akasov, R.A.; Sholina, N.V.; Khochenkov, D.A.; Alova, A.; Gorelkin, P.V.; Erofeev, A.S.; Generalova, A.N.; Khaydukov, E.V. Photodynamic therapy of melanoma by blue-light photoactivation of flavin mononucleotide. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]






| Object | Type | TR 1 (ms) | TE 2 (ms) | Av 3 | FOV 4 (mm2) | Acq 5 | Resolution (μm3) | Additional Parameters |
|---|---|---|---|---|---|---|---|---|
| Phantom 6 | T2WI | 3000 | 23 | 3 | 40 × 40 | 3 min 36 s | 156 × 156 × 1000 | RARE 8 = 8 |
| T2 maps | 5000 | 12 | 1 | 40 × 40 | 10 min 40 s | 312 × 312 × 1000 | NE 9 = 50, RARE = 8 | |
| Mouse-body 6 | T2WI 11,12 | 1000 | 17 | 3 | 40 × 40 | 2 min 27 s | 156 × 156 × 1000 | RARE = 4 |
| T2 maps 11 | 3000 | 7 | 1 | 40 × 40 | 6 min 42 s | 312 × 312 × 1000 | NE = 32, RARE = 8 | |
| Dynamic 11 | 1000 | 16 | 1 | 40 × 40 | 32 min | 312 × 312 × 1000 | NR 10 = 60, RARE = 4 | |
| Mouse-head 7 | T2WI 12 | 2500 | 26 | 4 | 23 × 23 | 4 min | 90 × 90 × 1000 | RARE = 8 |
| T2 maps 12 | 3000 | 7 | 1 | 23 × 23 | 6 min 42 s | 180 × 180 × 1000 | NE = 32, RARE = 8 | |
| Dynamic 12 | 1000 | 16 | 1 | 23 × 23 | 32 min | 180 × 180 × 1000 | NR 10 = 60, RARE = 4 |
| Compound | Concentration (mg/L) 1 | T2 (ms) |
|---|---|---|
| CPFeNP | 50 | 37.2 ± 0.8 |
| 25 | 84.3 ± 0.5 | |
| 5 | 237 ± 2 | |
| CPNiNP | 50 | 33.1 ± 0.5 |
| 25 | 106 ± 2 | |
| 5 | 200 ± 2 | |
| CPN/no-met 2 | 20 | 488 ± 7 |
| PBS | - | 508 ± 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Ramos, N.; Ibarra, L.E.; Serrano-Torres, M.; Yagüe, B.; Caverzán, M.D.; Chesta, C.A.; Palacios, R.E.; López-Larrubia, P. Iron Oxide Incorporated Conjugated Polymer Nanoparticles for Simultaneous Use in Magnetic Resonance and Fluorescent Imaging of Brain Tumors. Pharmaceutics 2021, 13, 1258. https://doi.org/10.3390/pharmaceutics13081258
Arias-Ramos N, Ibarra LE, Serrano-Torres M, Yagüe B, Caverzán MD, Chesta CA, Palacios RE, López-Larrubia P. Iron Oxide Incorporated Conjugated Polymer Nanoparticles for Simultaneous Use in Magnetic Resonance and Fluorescent Imaging of Brain Tumors. Pharmaceutics. 2021; 13(8):1258. https://doi.org/10.3390/pharmaceutics13081258
Chicago/Turabian StyleArias-Ramos, Nuria, Luis Exequiel Ibarra, María Serrano-Torres, Balbino Yagüe, Matías Daniel Caverzán, Carlos Alberto Chesta, Rodrigo Emiliano Palacios, and Pilar López-Larrubia. 2021. "Iron Oxide Incorporated Conjugated Polymer Nanoparticles for Simultaneous Use in Magnetic Resonance and Fluorescent Imaging of Brain Tumors" Pharmaceutics 13, no. 8: 1258. https://doi.org/10.3390/pharmaceutics13081258