Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions
Abstract
:1. Introduction
1.1. History of Photodynamic Therapy
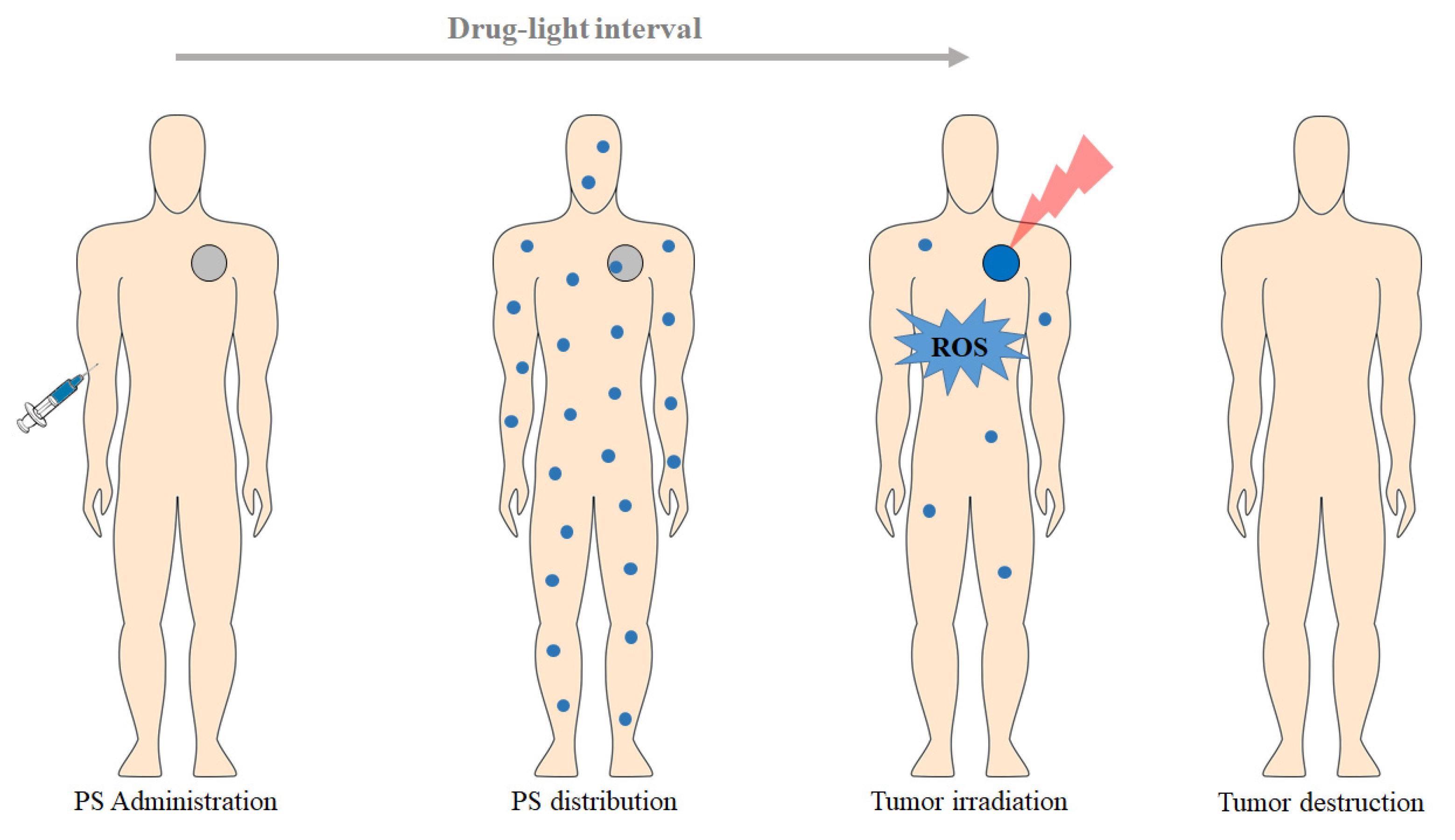
1.2. Principles of PDT
2. Photodynamic Reaction
3. PDT-Mediated Action Mechanisms
3.1. Apoptosis and Necrosis
3.2. Vascular Mechanisms
3.3. Immunological Mechanisms
4. PDT Essential Elements
4.1. Photosensitizers
- -
- Wash the area to be treated with soap and water;
- -
- Remove any residue and remaining oil with a gauze soaked in acetone or alcohol;
- -
- Apply the PS evenly over the entire area to be treated. Apply a second layer of PS after the first one has dried;
- -
- Allow the PS to incubate for 0.5–4 h;
- -
- Activate the PS with the appropriate light source;
- -
- Wash the treated area with soap and water to remove any residual PS;
- -
- Avoid any direct sunlight for 48 h;
- -
- Repeat as needed in 2–3 weeks.
4.2. Light
4.3. Oxygen
5. Advantages and Limitations of PDT
6. Applications of PDT
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamblin, M.R.; Huang, Y. Imaging in Photodynamic Therapy; Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Lee, C.-N.; Hsu, R.; Chen, H.; Wong, T.-W. Daylight photodynamic therapy: An update. Molecules 2020, 25, 5195. [Google Scholar] [CrossRef]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Li, X.; Lee, S.; Yoon, J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef]
- Mitton, D.; Ackroyd, R. A brief overview of photodynamic therapy in Europe. Photodiagnosis Photodyn. Ther. 2008, 5, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Photodynamic therapy for cancer: What’s past is prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, M.; Mehran, Y.Z.; Orthaber, A.; Saadat, H.H.; Weber, R.; Wojcik, M. Successful reduction of SARS-CoV-2 viral load by photodynamic therapy (PDT) verified by QPCR—A novel approach in treating patients in early infection stages. Med. Clin. Res. 2020, 5, 311–325. [Google Scholar]
- Weber, M. Intravenöse und interstitielle Lasertherapie: Eine neue Option in der Onkologie. Akupunkt. Aurikulomed. 2011, 37, 32–34. [Google Scholar]
- Rocha, L.G.B. Development of a Novel Photosensitizer for Photodynamic Therapy of Cancer. Ph.D. Thesis, University of Coimbra, Coimbra, Portugal, 2015. [Google Scholar]
- Fitzgerald, F. Photodynamic Therapy (PDT): Principles, Mechanisms and Applications; Nova Science Publishers, Inc.: New York, NY, USA, 2017. [Google Scholar]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M. Antifungal photodynamic therapy. Microbiol. Res. 2008, 163, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, J.M. Reactive oxygen species in photodynamic therapy: Mechanisms of their generation and potentiation. Adv. Inorg. Chem. 2017, 70, 343–394. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Allison, R.R.; Moghissi, K. Photodynamic therapy (PDT): PDT mechanisms. Clin. Endosc. 2013, 46, 24. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, J.M.; Arnaut, L.G. Photodynamic therapy (PDT) of cancer: From local to systemic treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Simões, J.C.S.; Sarpaki, S.; Papadimitroulas, P.; Therrien, B.; Loudos, G. Conjugated photosensitizers for imaging and PDT in cancer research. J. Med. Chem. 2020, 63, 14119–14150. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schäfer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic therapy of ovarian carcinoma cells with curcumin-loaded biodegradable polymeric nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef] [Green Version]
- Chizenga, E.P.; Abrahamse, H. Nanotechnology in modern photodynamic therapy of cancer: A review of cellular resistance patterns affecting the therapeutic response. Pharmaceutics 2020, 12, 632. [Google Scholar] [CrossRef]
- Li, T.; Yan, L. Functional polymer nanocarriers for photodynamic therapy. Pharmaceuticals 2018, 11, 133. [Google Scholar] [CrossRef] [Green Version]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Inorganic nanoparticles applied for active targeted photodynamic therapy of breast cancer. Pharmaceutics 2021, 13, 296. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017, 5, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, J.A.O. Therapy in Invasive Medical Devices with Image. Ph.D. Thesis, University of Minho, Braga, Portugal, 2019. [Google Scholar]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Ormond, A.; Freeman, H. Dye sensitizers for photodynamic therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef] [PubMed]
- FDA. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208081s000lbl.pdf (accessed on 3 August 2021).
- Reinhold, U.; Petering, H.; Dirschka, T.; Rozsondai, A.; Gille, J.; Kurzen, H.; Ostendorf, R.; Ebeling, A.; Stocker, M.; Radny, P. Photodynamic therapy with a 5-ALA patch does not increase the risk of conversion of actinic keratoses into squamous cell carcinoma. Exp. Dermatol. 2018, 27, 1399–1402. [Google Scholar] [CrossRef]
- Privalov, V.A.; Lappa, A.V.; Kochneva, E.V. Five years experience of photodynamic therapy with new chlorin photosensitizer. In Therapeutic Laser Applications and Laser-Tissue Interactions II, Proceedings of the European Conference on Biomedical Optics, Munich, Germany, 12–16 June 2005; Proceedings SPIE 5863; SPIE: Bellingham, WA, USA, 2005; p. 586310. [Google Scholar]
- Nyman, E.S.; Hynninen, P.H. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2004, 73, 1–28. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef]
- Ozog, D.M.; Rkein, A.M.; Fabi, S.G.; Gold, M.H.; Goldman, M.P.; Lowe, N.J.; Martin, G.M.; Munavalli, G.S. Photodynamic therapy: A clinical consensus guide. Dermatol. Surg. 2016, 42, 804–827. [Google Scholar] [CrossRef]
- Morton, C.A.; Szeimies, R.-M.; Sidoroff, A.; Braathen, L.R. European guidelines for topical photodynamic therapy part 1: Treatment delivery and current indications—Actinic keratoses, Bowen’s disease, basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A.; Szeimies, R.-M.; Basset-Séguin, N.; Calzavara-Pinton, P.G.; Gilaberte, Y.; Hædersdal, M.; Hofbauer, G.F.L.; Hunger, R.E.; Karrer, S.; Piaserico, S.; et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 2: Emerging indications—Field cancerization, photorejuvenation and inflammatory/infective dermatoses. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 17–29. [Google Scholar] [CrossRef]
- Morton, C.A.; Szeimies, R.-M.; Basset-Seguin, N.; Calzavara-Pinton, P.; Gilaberte, Y.; Hædersdal, M.; Hofbauer, G.F.L.; Hunger, R.E.; Karrer, S.; Piaserico, S.; et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 1: Treatment delivery and established indications—Actinic keratoses, Bowen’s disease and basal cell carcinomas. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- Wezgowiec, J.; Derylo, M.B.; Teissie, J.; Orio, J.; Rols, M.-P.; Kulbacka, J.; Saczko, J.; Kotulska, M. Electric field-assisted delivery of photofrin to human breast carcinoma cells. J. Membr. Biol. 2013, 246, 725–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef]
- Soliman, N.; Sol, V.; Ouk, T.-S.; Thomas, C.M.; Gasser, G. Encapsulation of a Ru(II) polypyridyl complex into polylactide nanoparticles for antimicrobial photodynamic therapy. Pharmaceutics 2020, 12, 961. [Google Scholar] [CrossRef]
- Yanovsky, R.L.; Bartenstein, D.W.; Rogers, G.S.; Isakoff, S.J.; Chen, S.T. Photodynamic therapy for solid tumors: A review of the literature. Photodermatol. Photoimmunol. Photomed. 2019, 35, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Zheng, H.; Huang, Z.; Lin, H.; Ke, Z.; Xie, S.; Li, B. Light-emitting diode-based illumination system for in vitro photodynamic therapy. Int. J. Photoenergy 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013, 46, 7. [Google Scholar] [CrossRef]
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagnosis Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef]
- Kinsella, T.J.; Colussi, V.C.; Oleinick, N.L.; Sibata, C.H. Photodynamic therapy in oncology. Expert Opin. Pharmacother. 2001, 2, 917–927. [Google Scholar] [CrossRef]
- Chilakamarthi, U.; Giribabu, L. Photodynamic therapy: Past, present and future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef]
- Rezzoug, H.; Bezdetnaya, L.; A’amar, O.; Merlin, J.L.; Guillemin, F. Parameters affecting photodynamic activity of Foscan® or metatetra(hydroxyphenyl)chlorin (mTHPC) in vitro and in vivo. Lasers Med. Sci. 1998, 13, 119–125. [Google Scholar] [CrossRef]
- Coutier, S.; Mitra, S.; Bezdetnaya, L.N.; Parache, R.M.; Georgakoudi, I.; Foster, T.H.; Guillemin, F. Effects of fluence rate on cell survival and photobleaching in meta-tetra-(hydroxyphenyl)chlorin–photosensitized colo 26 multicell tumor spheroids. Photochem. Photobiol. 2001, 73, 297. [Google Scholar] [CrossRef]
- Bruscino, N.; Lotti, T.; Rossi, R. Photodynamic therapy for a hypertrophic scarring: A promising choice. Photodermatol. Photoimmunol. Photomed. 2011, 27, 334–335. [Google Scholar] [CrossRef] [PubMed]
- Hartl, B.A.; Hirschberg, H.; Marcu, L.; Cherry, S.R. Characterizing low fluence thresholds for in vitro photodynamic therapy. Biomed. Opt. Express 2015, 6, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, M.; Sandhu, S.; Sivamani, R. Clinical utility of daylight photodynamic therapy in the treatment of actinic keratosis—A review of the literature. Clin. Cosmet. Investig. Dermatol. 2019, 12, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Morton, C.A.; Braathen, L.R. Daylight photodynamic therapy for actinic keratoses. Am. J. Clin. Dermatol. 2018, 19, 647–656. [Google Scholar] [CrossRef]
- Calixto, G.; Bernegossi, J.; de Freitas, L.; Fontana, C.; Chorilli, M. Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: A review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Dos Santos, A.F.; De Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Lange, N.; Szlasa, W.; Saczko, J.; Chwiłkowska, A. Potential of cyanine derived dyes in photodynamic therapy. Pharmaceutics 2021, 13, 818. [Google Scholar] [CrossRef]
- Plaetzer, K.; Berneburg, M.; Kiesslich, T.; Maisch, T. New applications of photodynamic therapy in biomedicine and biotechnology. Biomed. Res. Int. 2013, 2013, 1–3. [Google Scholar] [CrossRef]
- Yoo, S.W.; Oh, G.; Ahn, J.C.; Chung, E. Non-oncologic applications of nanomedicine-based phototherapy. Biomedicines 2021, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Calzavara-Pinton, P.G.; Rossi, M.T.; Aronson, E.; Sala, R.; The Italian Group for Photodynamic Therapy. A retrospective analysis of real-life practice of off-label photodynamic therapy using methyl aminolevulinate (MAL-PDT) in 20 Italian dermatology departments. Part 1: Inflammatory and aesthetic indications. Photochem. Photobiol. Sci. 2013, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Stender, I.-M.; Na, R.; Fogh, H.; Gluud, C.; Wulf, H.C. Photodynamic therapy with 5-aminolaevulinic acid or placebo for recalcitrant foot and hand warts: Randomised double-blind trial. Lancet 2000, 355, 963–966. [Google Scholar] [CrossRef]
- Shin, H.T.; Kim, J.H.; Shim, J.; Lee, J.H.; Lee, D.Y.; Lee, J.H.; Yang, J.M. Photodynamic therapy using a new formulation of 5-aminolevulinic acid for wrinkles in Asian skin: A randomized controlled split face study. J. Dermatol. Treat. 2015, 26, 246–251. [Google Scholar] [CrossRef]
- Choi, Y.M.; Adelzadeh, L.; Wu, J.J. Photodynamic therapy for psoriasis. J. Dermatol. Treat. 2015, 26, 202–207. [Google Scholar] [CrossRef]
- Jerjes, W.; Upile, T.; Hamdoon, Z.; Mosse, C.A.; Akram, S.; Morley, S.; Hopper, C. Interstitial PDT for vascular anomalies. Lasers Surg. Med. 2011, 43, 357–365. [Google Scholar] [CrossRef]
- Comacchi, C.; Bencini, P.L.; Galimberti, M.G.; Cappugi, P.; Torchia, D. Topical photodynamic therapy for idiopathic hirsutism and hypertrichosis. Plast. Reconstr. Surg. 2012, 129, 1012e–1014e. [Google Scholar] [CrossRef] [Green Version]
- Linares-González, L.; Ródenas-Herranz, T.; Sáenz-Guirado, S.; Ruiz-Villaverde, R. Successful response to photodynamic therapy with 5-aminolevulinic acid nanoemulsified gel in a patient with universal alopecia areata refractory to conventional treatment. Dermatol. Ther. 2020, 33, e13416. [Google Scholar] [CrossRef]
- van Dijk, E.H.C.; Fauser, S.; Breukink, M.B.; Blanco-Garavito, R.; Groenewoud, J.M.M.; Keunen, J.E.E.; Peters, P.J.H.; Dijkman, G.; Souied, E.H.; MacLaren, R.E.; et al. Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy. Ophthalmology 2018, 125, 1547–1555. [Google Scholar] [CrossRef]
- Díaz-Dávalos, C.D.; Carrasco-Quiroz, A.; Rivera-Díez, D. Neovascularization corneal regression in patients treated with photodynamic therapy with verteporfin. Rev. Med. Inst. Mex. Seguro Soc. 2016, 54, 164–169. [Google Scholar]
- Houthoofd, S.; Vuylsteke, M.; Mordon, S.; Fourneau, I. Photodynamic therapy for atherosclerosis. The potential of indocyanine green. Photodiagn. Photodyn. Ther. 2020, 29, 101568. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Z.; Cheng, L.F.; Wang, Z.Q.; Gu, Y. Attempt of photodynamic therapy on esophageal varices. Lasers Med. Sci. 2009, 24, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Cosgarea, R.; Pollmann, R.; Sharif, J.; Schmidt, T.; Stein, R.; Bodea, A.; Auschill, T.; Sculean, A.; Eming, R.; Greene, B.; et al. Photodynamic therapy in oral lichen planus: A prospective case-controlled pilot study. Sci. Rep. 2020, 10, 1667. [Google Scholar] [CrossRef] [PubMed]
- Lee, B., II; Suh, Y.S.; Chung, Y.J.; Yu, K.; Park, C.B. Shedding light on Alzheimer’s β-amyloidosis: Photosensitized methylene blue inhibits self-assembly of β-amyloid peptides and disintegrates their aggregates. Sci. Rep. 2017, 7, 7523. [Google Scholar] [CrossRef] [Green Version]
- Gallardo-Villagrán, M.; Leger, D.Y.; Liagre, B.; Therrien, B. Photosensitizers used in the photodynamic therapy of rheumatoid arthritis. Int. J. Mol. Sci. 2019, 20, 3339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favre, L.; Borle, F.; Velin, D.; Bachmann, D.; Bouzourene, H.; Wagnieres, G.; van den Bergh, H.; Ballabeni, P.; Gabrecht, T.; Michetti, P.; et al. Low dose endoluminal photodynamic therapy improves murine T cell-mediated colitis. Endoscopy 2011, 43, 604–616. [Google Scholar] [CrossRef]
- Huang, L.; Dai, T.; Hamblin, M.R. Antimicrobial photodynamic inactivation and photodynamic therapy for infections. Methods Mol. Biol. 2010, 635, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Rapacka-Zdończyk, A.; Woźniak, A.; Michalska, K.; Pierański, M.; Ogonowska, P.; Grinholc, M.; Nakonieczna, J. Factors determining the susceptibility of bacteria to antibacterial photodynamic inactivation. Front. Med. 2021, 8, 617. [Google Scholar] [CrossRef]
- Almeida, A. Photodynamic therapy in the inactivation of microorganisms. Antibiotics 2020, 9, 138. [Google Scholar] [CrossRef] [Green Version]
- Tariq, R.; Khalid, U.A.; Kanwal, S.; Adnan, F.; Qasim, M. Photodynamic therapy: A rational approach toward COVID-19 management. J. Explor. Res. Pharmacol. 2021, 6, 44–52. [Google Scholar] [CrossRef]
- Fekrazad, R. Photobiomodulation and antiviral photodynamic therapy as a possible novel approach in COVID-19 management. Photobiomodul. Photomed. Laser Surg. 2020, 38, 255–257. [Google Scholar] [CrossRef]
- Shen, J.J.; Jemec, G.B.E.; Arendrup, M.C.; Saunte, D.M.L. Photodynamic therapy treatment of superficial fungal infections: A systematic review. Photodiagn. Photodyn. Ther. 2020, 31, 101774. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Chuang, W.-C.; Yu, K.-H.; Jheng, C.-P.; Lee, C.-I. Sequential photodynamic therapy with phthalocyanine encapsulated chitosan-tripolyphosphate nanoparticles and flucytosine treatment against Candida tropicalis. Pharmaceutics 2019, 11, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janeth Rimachi Hidalgo, K.; Cabrini Carmello, J.; Carolina Jordão, C.; Aboud Barbugli, P.; de Sousa Costa, C.A.; Garcia de Oliveira Mima, E.; Pavarina, A.C. Antimicrobial photodynamic therapy in combination with nystatin in the treatment of experimental oral candidiasis induced by candida albicans resistant to fluconazole. Pharmaceuticals 2019, 12, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tortora, G.; Orsini, B.; Pecile, P.; Menciassi, A.; Fusi, F.; Romano, G. An ingestible capsule for the photodynamic therapy of helicobacter pylori infection. IEEE ASME Trans. Mechatron. 2016, 21, 1935–1942. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Amorim, R.; Silva, M.F.; Baltazar, F.; Wolffenbuttel, R.F.; Correia, J.H. Photodynamic therapy at low-light fluence rate: In vitro assays on colon cancer cells. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–6. [Google Scholar] [CrossRef]




| Trade Name (Class) | Molecular Formula | Excitation Wavelength (nm) | Quantum Yield | Molar Extinction Coefficient (M−1 cm−1) | Main Applications |
|---|---|---|---|---|---|
| Photofrin® (porphyrin) | C34H38N4NaO5+ | 630 | 0.01 in PBS | 3.0 × 103 in PBS | Esophageal, lung, and endobronchial cancers |
| Ameluz® (porphyrin) | C5H9NO3•HCl | 630 | - | - | Actinic keratosis and basal cell carcinoma |
| AlaCare® (porphyrin) | C5H9NO3 | 630 | - | - | Actinic keratosis |
| Levulan® (porphyrin) | C5H9NO3 | 635 | 0.56 | 5.0 × 103 | Actinic keratosis |
| Hexvix® (porphyrin) | C11H21NO3 | 635 | - | <1.0 × 103 | Bladder cancer |
| Foscan® (chlorine) | C44H32O4N4 | 652 | 0.43 in methanol | 3.0 × 104 in methanol | Head and neck cancers |
| Laserphyrin® (chlorine) | C38H37N5O9 | 664 | 0.77 in PBS | 4.0 × 104 in PBS | Lung and esophageal cancers and brain tumors |
| Metvix® (porphyrin) | C6H11NO3 | 570–670 | - | <1.0 × 103 | Basal cell carcinoma, Bowen’s disease, and actinic keratosis |
| Visudyne® (porphyrin) | C82H84N8O16 | 690 | 0.7 in methanol | 3.4 × 104 in methanol | Age-related macular degeneration |
| Redaporphine® (LUZ11) (bacteriochlorin) | C48H38F8N8O8S4 | 749 | 0.43 in ethanol | 140 × 103 in ethanol | Biliary tract cancer |
| Trade Name | Molecular Formula | Excitation Wavelength (nm) | Quantum Yield | Molar Extinction Coefficient (M−1 cm−1) | Main Applications |
|---|---|---|---|---|---|
| Radachlorin® (chlorine) | C34H36N4O6 C33H34N4O5 C33H34N4O6 | 662 | 0.52–0.62 | 3.42 × 104 | Skin cancer |
| Photochlor® (chlorins) | C39H48N4O4 | 664 | 0.48 in CH2Cl2 | 4.75 × 104 in 1% Tween-80 micelles | Head and neck cancer |
| Purlytin® (purpurin) | C37H42Cl2N4O2Sn | 664 | 0.7 in acetonitrile | 2.8 × 104 | Age-related macular degeneration |
| Fotolon® (chlorin) | C34H36N4O6 | 665 | 0.63 in dimethylformamide | 5.0 × 104 in diethyl ether | Nasopharyngeal sarcoma |
| Lutrin® (texaphyrin) | C52H72LuN5O14 | 732 | 4.2 × 104 in methanol | 0.11 in methanol | Coronary artery disease |
| TOOKAD® (WST09) (bacteriochlorin) | C37H41K2N5O9PdS | 762 | 0.99 in organic solvent | 8.85 × 104 | Prostate cancer |
| Advantages | Limitations |
|---|---|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. https://doi.org/10.3390/pharmaceutics13091332
Correia JH, Rodrigues JA, Pimenta S, Dong T, Yang Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics. 2021; 13(9):1332. https://doi.org/10.3390/pharmaceutics13091332
Chicago/Turabian StyleCorreia, José H., José A. Rodrigues, Sara Pimenta, Tao Dong, and Zhaochu Yang. 2021. "Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions" Pharmaceutics 13, no. 9: 1332. https://doi.org/10.3390/pharmaceutics13091332
APA StyleCorreia, J. H., Rodrigues, J. A., Pimenta, S., Dong, T., & Yang, Z. (2021). Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics, 13(9), 1332. https://doi.org/10.3390/pharmaceutics13091332






