The Therapeutic Effect of Second Near-Infrared Absorbing Gold Nanorods on Metastatic Lymph Nodes via Lymphatic Delivery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PEGylated Gold Nanorod Synthesis
2.3. Characterisation of AuNRs
2.4. Mice
2.5. FM3A-Luc Cell Culture
2.6. Metastasis Induction of PALN with Tumour Cells from the SiLN
2.7. In Vivo Evaluation of the Metastatic PALNs Treated with PAuNRs and Laser
2.8. PAuNR Biodistribution
2.9. Histological Analysis
2.10. Statistical Analysis
3. Results
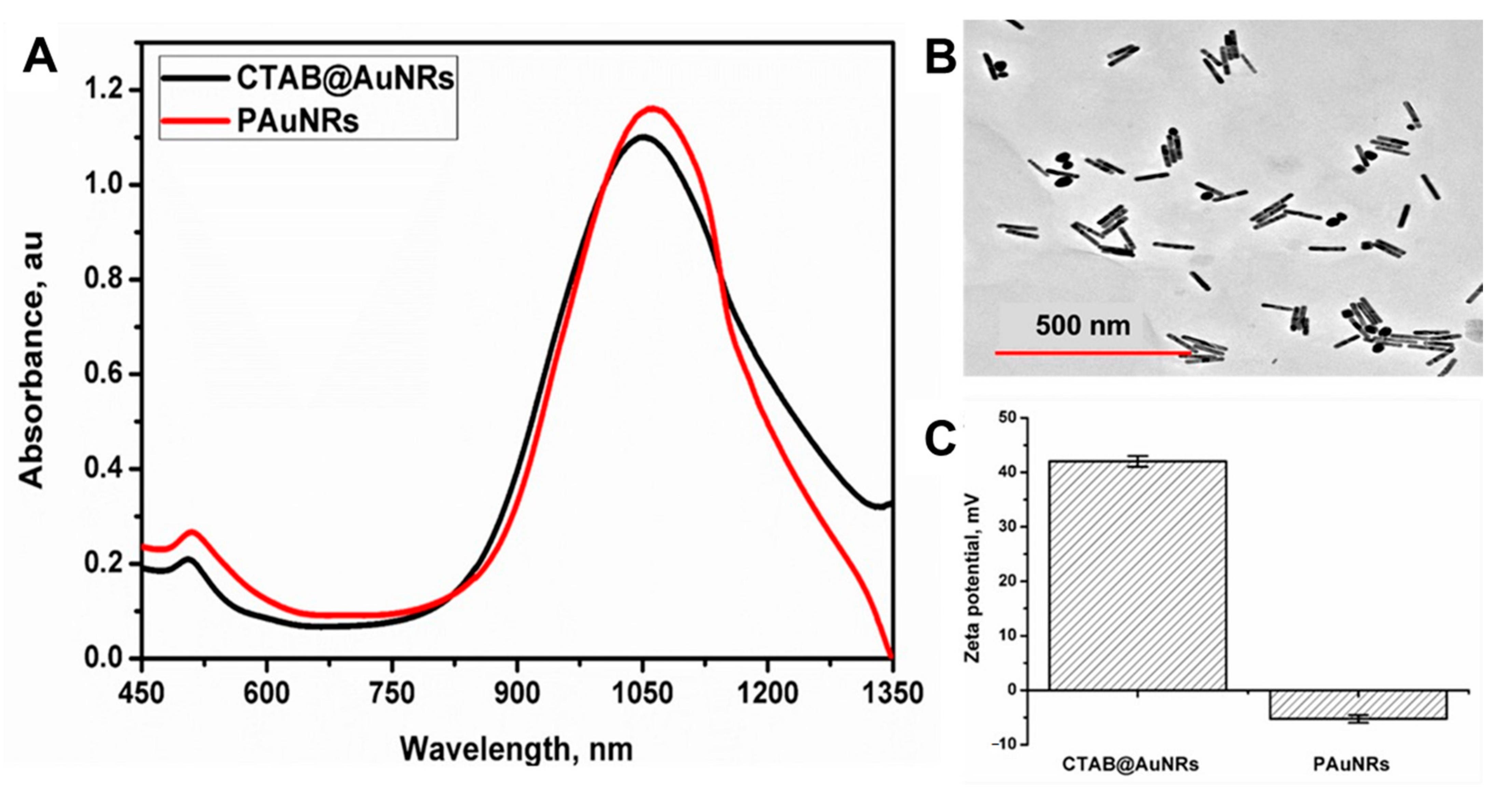
3.1. Synthesis and Characterisation of PAuNRs
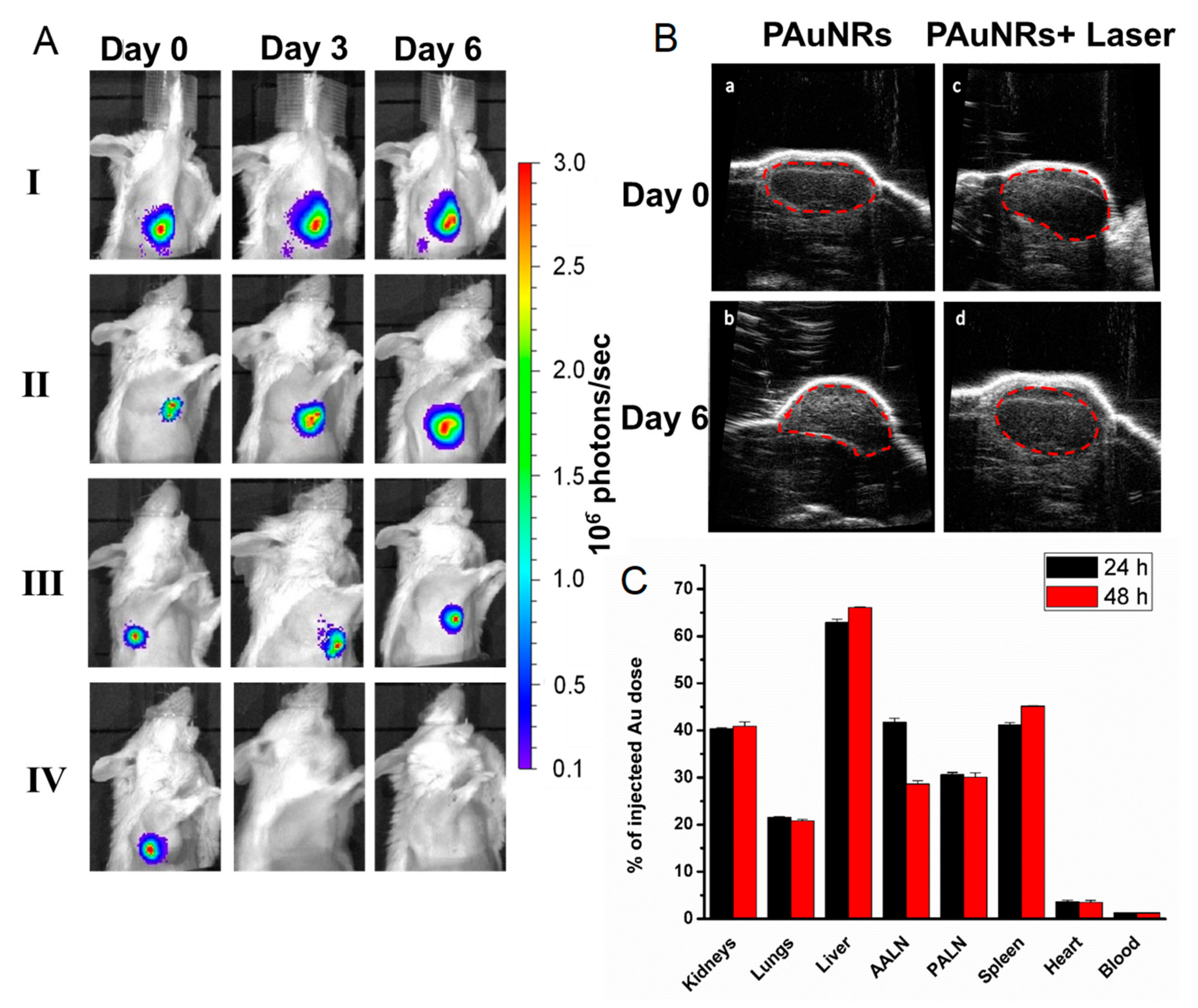
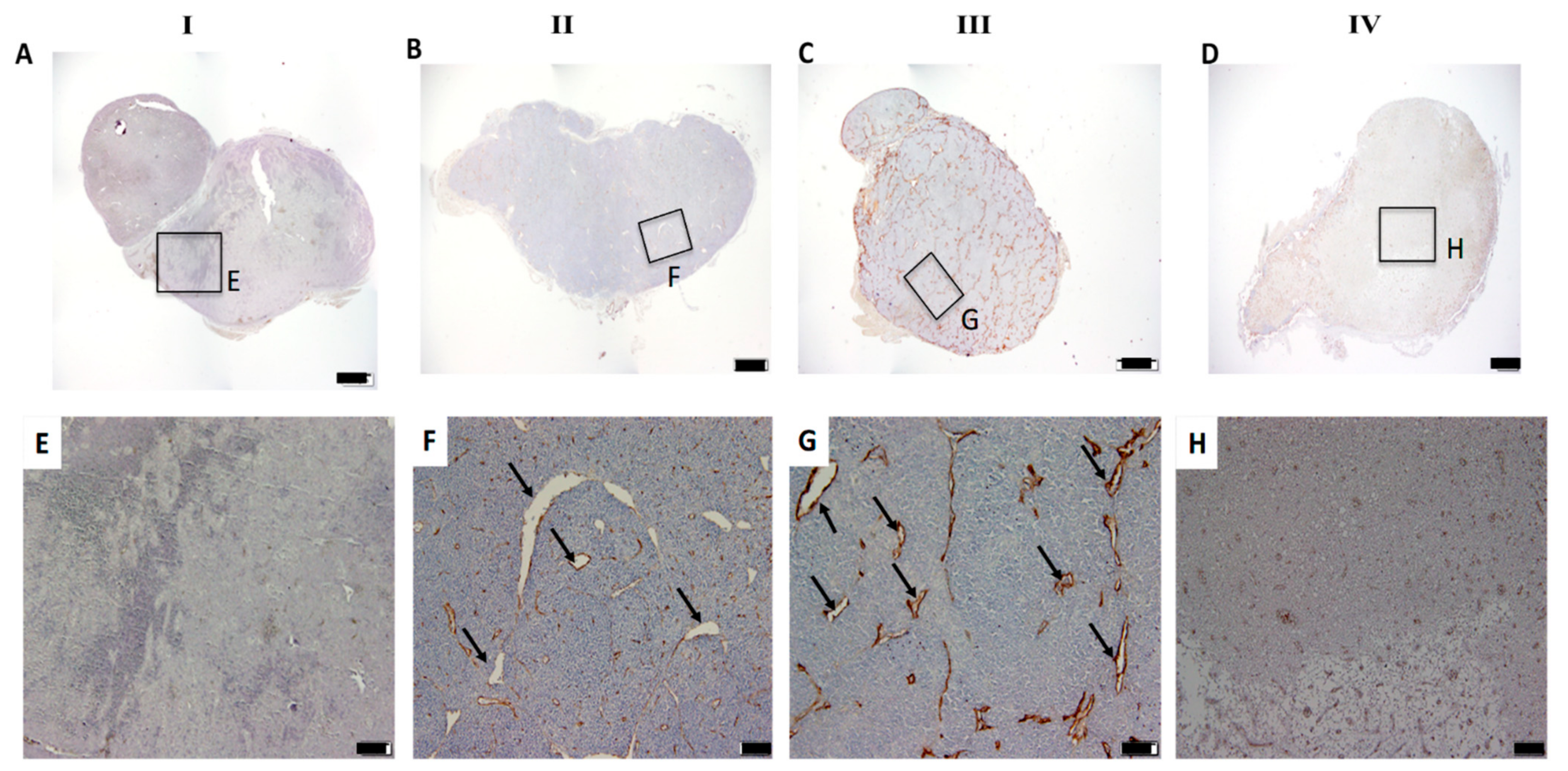
3.2. In Vivo Time-Mediated Anti-Tumour Therapy Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Wang, J.; Shi, W.; Chen, W.; Li, W.; Shu, Y.; Liu, P.; Lu, K. Prognostic significance of metastatic lymph nodes ratio in patients with gastric adenocarcinoma after curative gastrectomy. Chin. Med. J. 2014, 127, 1874–1878. [Google Scholar]
- Matsuki, D.; Adewale, O.; Horie, S.; Okajima, J.; Komiya, A.; Oluwafemi, O.; Maruyama, S.; Mori, S.; Kodama, T. Treatment of tumor in lymph nodes using near-infrared laser light-activated thermosensitive liposome-encapsulated doxorubicin and gold nanorods. J. Biophotonics 2017, 10, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Matsuki, D.; Okajima, J.; Komiya, A.; Mori, S.; Maruyama, S.; Kodama, T. Photothermal therapy of tumors in lymph nodes using gold nanorods and near-infrared laser light with controlled surface cooling. Nano Res. 2015, 8, 3842–3852. [Google Scholar] [CrossRef]
- Ryan, G.M.; Kaminskas, L.M.; Porter, C.J.H. Nano-chemotherapeutics: Maximising lymphatic drug exposure to improve the treatment of lymph-metastatic cancers. J. Control. Release 2014, 193, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Shirai, Y.; Motozono, C.; Kanzaki, H.; Mori, S.; Kodama, T. In vivo delivery of an exogenous molecule into murine T lymphocytes using a lymphatic drug delivery system combined with sonoporation. Biochem. Biophys. Res. Commun. 2020, 525, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Yoshiba, S.; Mori, S.; Kodama, T. Optimization of the delivery of molecules into lymph nodes using a lymphatic drug delivery system with ultrasound. Int. J. Pharm. 2021, 597, 120324. [Google Scholar] [CrossRef]
- Sukhbaatar, A.; Mori, S.; Saiki, Y.; Takahashi, T.; Horii, A.; Kodama, T. Lymph node resection induces the activation of tumor cells in the lungs. Cancer Sci. 2019, 110, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephen, Z.R.; Zhang, M. Recent Progress in the Synergistic Combination of Nanoparticle-Mediated Hyperthermia and Immunotherapy for Treatment of Cancer. Adv. Healthc. Mater. 2021, 10, 2001415. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Zhang, Y.; Zhao, Y.; Lu, S.; Peng, Y.; Lu, L.; Hu, X.; Zhan, M. Native Mitochondria-Targeting polymeric nanoparticles for mild photothermal therapy rationally potentiated with immune checkpoints blockade to inhibit tumor recurrence and metastasis. Chem. Eng. J. 2021, 424, 130171. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, M.; Hwang, J.H.; Nam, J.M. Golden Opportunities: Plasmonic Gold Nanostructures for Biomedical Applications based on the Second Near-Infrared Window. Small Methods 2017, 1, 1600032. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; He, H.; Tong, Z.; Xia, H.; Mao, Z.; Gao, C. The impact of size and surface ligand of gold nanorods on liver cancer accumulation and photothermal therapy in the second near-infrared window. J. Colloid Interface Sci. 2020, 565, 186–196. [Google Scholar] [CrossRef]
- Tang, H.; Xu, X.; Chen, Y.; Xin, H.; Wan, T.; Li, B.; Pan, H.; Li, D.; Ping, Y. Reprogramming the Tumor Microenvironment through Second-Near-Infrared-Window Photothermal Genome Editing of PD-L1 Mediated by Supramolecular Gold Nanorods for Enhanced Cancer Immunotherapy. Adv. Mater. 2021, 33, 2006003. [Google Scholar] [CrossRef] [PubMed]
- Austin, L.A.; Mackey, M.A.; Dreaden, E.C.; El-Sayed, M.A. The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery. Arch. Toxicol. 2014, 88, 1391–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, B. Gold nanorods: Near-infrared plasmonic photothermal conversion and surface coating. J. Mater. Sci. Chem. Eng. 2014, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Lebepe, T.C.; Parani, S.; Oluwafemi, O.S. Graphene Oxide-Coated Gold Nanorods: Synthesis and Applications. Nanomaterials 2020, 10, 2149. [Google Scholar] [CrossRef]
- Centi, S.; Tatini, F.; Ratto, F.; Gnerucci, A.; Mercatelli, R.; Romano, G.; Landini, I.; Nobili, S.; Ravalli, A.; Marrazza, G. In vitro assessment of antibody-conjugated gold nanorods for systemic injections. J. Nanobiotechnol. 2014, 12, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liopo, A.V.; Conjusteau, A.; Oraevsky, A.A. PEG-coated gold nanorod monoclonal antibody conjugates in preclinical research with optoacoustic tomography, photothermal therapy, and sensing. In Photons Plus Ultrasound: Imaging and Sensing 2012; Oraevsky, A.A., Wang, L.V., Eds.; SPIE: Washington, DC, USA, 2012; p. 822344. [Google Scholar]
- Liu, K.; Zheng, Y.; Lu, X.; Thai, T.; Lee, N.A.; Bach, U.; Gooding, J.J. Biocompatible gold nanorods: One-step surface functionalization, highly colloidal stability, and low cytotoxicity. Langmuir 2015, 31, 4973–4980. [Google Scholar] [CrossRef] [PubMed]
- Tebbe, M.; Kuttner, C.; Männel, M.; Fery, A.; Chanana, M. Colloidally stable and surfactant-free protein-coated gold nanorods in biological media. ACS Appl. Mater. Interfaces 2015, 7, 5984–5991. [Google Scholar] [CrossRef]
- Yasun, E.; Li, C.; Barut, I.; Janvier, D.; Qiu, L.; Cui, C.; Tan, W. BSA modification to reduce CTAB induced nonspecificity and cytotoxicity of aptamer-conjugated gold nanorods. Nanoscale 2015, 7, 10240–10248. [Google Scholar] [CrossRef] [Green Version]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef]
- Aioub, M.; El-Sayed, M.A. A real-time surface enhanced raman spectroscopy study of plasmonic photothermal cell death using targeted gold nanoparticles. J. Am. Chem. Soc. 2016, 138, 1258–1264. [Google Scholar] [CrossRef]
- De Freitas, L.; Zanelatto, L.; Mantovani, M.; Silva, P.; Ceccini, R.; Grecco, C.; Moriyama, L.; Kurachi, C.; Martins, V.; Plepis, A. In Vivo photothermal tumour ablation using gold nanorods. Laser Phys. 2013, 23, 066003. [Google Scholar] [CrossRef]
- Oladipo, A.O.; Oluwafemi, O.S.; Songca, S.P.; Sukhbaatar, A.; Mori, S.; Okajima, J.; Komiya, A.; Maruyama, S.; Kodama, T. A novel treatment for metastatic lymph nodes using lymphatic delivery and photothermal therapy. Sci. Rep. 2017, 7, 45459. [Google Scholar] [CrossRef]
- Kato, S.; Mori, S.; Kodama, T. A novel treatment method for lymph node metastasis using a lymphatic drug delivery system with nano/microbubbles and ultrasound. J. Cancer 2015, 6, 1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar] [PubMed]
- Sato, T.; Mori, S.; Arai, Y.; Kodama, T. The combination of intralymphatic chemotherapy with ultrasound and nano-/microbubbles is efficient in the treatment of experimental tumors in mouse lymph nodes. Ultrasound Med. Biol. 2014, 40, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, R.; Sukhbaatar, A.; Sakamoto, M.; Mori, S.; Kodama, T. A model system for studying superselective radiotherapy of lymph node metastasis in mice with swollen lymph nodes. Clin. Transl. Radiat. Oncol. 2020, 20, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Kodama, T.; Hatakeyama, Y.; Kato, S.; Mori, S. Visualization of fluid drainage pathways in lymphatic vessels and lymph nodes using a mouse model to test a lymphatic drug delivery system. Biomed. Opt. Express. 2015, 6, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Fukumura, R.; Sukhbaatar, A.; Mishra, R.; Sakamoto, M.; Mori, S.; Kodama, T. Study of the physicochemical properties of drugs suitable for administration using a lymphatic drug delivery system. Cancer Sci. 2021, 112, 1735. [Google Scholar] [CrossRef]
- Kodama, T.; Matsuki, D.; Tada, A.; Takeda, K.; Mori, S. New concept for the prevention and treatment of metastatic lymph nodes using chemotherapy administered via the lymphatic network. Sci. Rep. 2016, 6, 32506. [Google Scholar] [CrossRef] [Green Version]
- Shen, K.-H.; Lu, C.-H.; Kuo, C.-Y.; Li, P.-Y.; Yeh, Y.-C. Smart near infrared-responsive nanocomposite hydrogels for therapeutics and diagnostics. J. Mater. Chem. B 2021. [Google Scholar] [CrossRef]
- Shao, L.; Mori, S.; Yagishita, Y.; Okuno, T.; Hatakeyama, Y.; Sato, T.; Kodama, T. Lymphatic mapping of mice with systemic lymphoproliferative disorder: Usefulness as an inter-lymph node metastasis model of cancer. J. Immunol. Methods 2013, 389, 69–78. [Google Scholar] [CrossRef]
- Ye, X.; Zheng, C.; Chen, J.; Gao, Y.; Murray, C.B. Using binary surfactant mixtures to simultaneously improve the dimensional tunability and monodispersity in the seeded growth of gold nanorods. Nano Lett. 2013, 13, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Kato, S.; Hatakeyama, Y.; Okajima, J.; Maruyama, S.; Sakamoto, M.; Mori, S.; Kodama, T. Photothermal therapy of tumors in lymph nodes using gold nanorods and near-infrared laser light. J. Control. Release 2013, 172, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-M.; Fang, C.; Jia, H.; Huang, Y.; Cheng, C.H.; Ko, C.-H.; Chen, Z.; Wang, J.; Wang, Y.-X.J. Cellular uptake behaviour, photothermal therapy performance, and cytotoxicity of gold nanorods with various coatings. Nanoscale 2014, 6, 11462–11472. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, J.; Zhou, F.; Liu, Q.; Zhang, Z. Nanoparticle-based approaches to target the lymphatic system for antitumor treatment. Cell. Mol. Life Sci. 2021, 78, 5139–5161. [Google Scholar] [CrossRef] [PubMed]
- Niidome, T.; Ohga, A.; Akiyama, Y.; Watanabe, K.; Niidome, Y.; Mori, T.; Katayama, Y. Controlled release of PEG chain from gold nanorods: Targeted delivery to tumor. Biorg. Med. Chem. 2010, 18, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cheng, Y.; Huang, Y.; Tian, Y.; Wang, S.; Chen, Y. PEGylated gold nanoprisms for photothermal therapy at low laser power density. RSC Adv. 2015, 5, 81682–81688. [Google Scholar] [CrossRef]
- Stacker, S.A.; Williams, S.P.; Karnezis, T.; Shayan, R.; Fox, S.B.; Achen, M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 2014, 14, 159–172. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oladipo, A.O.; Lebepe, T.C.; Ncapayi, V.; Tsolekile, N.; Parani, S.; Songca, S.P.; Mori, S.; Kodama, T.; Oluwafemi, O.S. The Therapeutic Effect of Second Near-Infrared Absorbing Gold Nanorods on Metastatic Lymph Nodes via Lymphatic Delivery System. Pharmaceutics 2021, 13, 1359. https://doi.org/10.3390/pharmaceutics13091359
Oladipo AO, Lebepe TC, Ncapayi V, Tsolekile N, Parani S, Songca SP, Mori S, Kodama T, Oluwafemi OS. The Therapeutic Effect of Second Near-Infrared Absorbing Gold Nanorods on Metastatic Lymph Nodes via Lymphatic Delivery System. Pharmaceutics. 2021; 13(9):1359. https://doi.org/10.3390/pharmaceutics13091359
Chicago/Turabian StyleOladipo, Adewale O., Thabang C. Lebepe, Vuyelwa Ncapayi, Ncediwe Tsolekile, Sundararajan Parani, Sandile P. Songca, Shiro Mori, Tetsuya Kodama, and Oluwatobi S. Oluwafemi. 2021. "The Therapeutic Effect of Second Near-Infrared Absorbing Gold Nanorods on Metastatic Lymph Nodes via Lymphatic Delivery System" Pharmaceutics 13, no. 9: 1359. https://doi.org/10.3390/pharmaceutics13091359
APA StyleOladipo, A. O., Lebepe, T. C., Ncapayi, V., Tsolekile, N., Parani, S., Songca, S. P., Mori, S., Kodama, T., & Oluwafemi, O. S. (2021). The Therapeutic Effect of Second Near-Infrared Absorbing Gold Nanorods on Metastatic Lymph Nodes via Lymphatic Delivery System. Pharmaceutics, 13(9), 1359. https://doi.org/10.3390/pharmaceutics13091359





