External Model Performance Evaluation of Twelve Infliximab Population Pharmacokinetic Models in Patients with Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. External Evaluation Data Set
2.2. Population Pharmacokinetic Models and Software
2.3. Model Performance Evaluation
3. Results
3.1. Characteristics of Published Population Pharmacokinetic Models of Infliximab in Patients with IBD
3.2. Eligible Population Pharmacokinetic Models for Evaluation
3.3. External Evaluation Data Set
| Publication | CD/UC | Patient Cohort | No. of Patients (Samples) | Sampling Times | Base Model | Covariates on CL | Covariates on Vc | IOV | Induction/ Maintenance 1 | Inclusion of ADA+ Patients | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ternant et al., 2008 | both | adults | 33 (478) | peak, trough | 2-CMT | ADA | sex, weight | - | both | yes (15%) | [44] |
| Fasanmade et al., 2009 * | UC | adults | 482 (4145) | peak, midpoint, trough | 2-CMT | ADA, alb, sex | sex, weight | - | both | yes (7%) | [23] |
| Fasanmade et al., 2011 (a) * | CD | adults | 580 (/) | peak, midpoint, trough | 2-CMT | ADA, alb, IMM, weight | weight 2 | CL | both | yes (11%) | [24] |
| Fasanmade et al., 2011 (c) | CD | children | 112 (/) | peak, midpoint, trough | 2-CMT | alb, weight | weight 2 | CL | both | yes (3%) | [24] |
| Fasanmade et al., 2011(a/c) * | CD | both | 692 (5757) | peak, midpoint, trough | 2-CMT | ADA, alb, IMM, weight | weight 2 | CL | both | yes (10%) | [24] |
| Xu et al., 2012 * | both | both | 655 3 (/) | / | 2-CMT | ADA, alb, weight 4 | weight 2 | - | / | yes (/) | [57] |
| Dotan et al., 2014 | both | adults | 54 (169) | trough | 2-CMT | ADA, alb, weight 4 | weight 2 | - | both | yes (31%) | [45] |
| Aubourg et al., 2015 * | CD | adults | 133 (/) | trough, peak | 2-CMT | sex | sex, weight | - | treatment initiation | no | [53] |
| Buurman et al., 2015 * | both | adults | 42 (188) | trough | 2-CMT | ADA, period 5, sex | HBI | - | both | yes (5%) | [54] |
| Ternant et al., 2015 | CD | adults | 111 (546) | throughout dosing interval | 1-CMT | FCGR3A-158V/V, hsCRP | - | - | maintenance | yes (2%) | [46] |
| Brandse et al., 2016 * | UC | adults | 19 (/) | throughout dosing interval | 2-CMT | ADA, alb | - | - | induction | yes (32%) | [55] |
| Passot et al., 2016 * | both | both | 79 6 (/) | trough | 1-CMT | CD/UC, sex, weight | CD/UC, sex, weight | - | both | no | [56] |
| Brandse et al., 2017 | both | adults | 332 (997) | throughout dosing interval | 2-CMT | ADA, alb, previous exposure, weight 4 | weight 2 | - | both | yes (23%) | [47] |
| Edlund et al., 2017(I–III) *,7 | CD | adults | 68 (152) | midpoint, trough | 2-CMT | ADA 8, weight 4,9 | weight 2,9 | - | maintenance | yes (37%) | [43] |
| Kevans et al., 2018 | both | adults | 51 (/) | throughout dosing interval | 2-CMT | ADA, alb, weight 4, time-varying CL 10 | weight 2 | - | induction | yes (11%) | [48] |
| Petitcollin et al., 2018 * | CD | children | 20 (145) | trough | 1-CMT | alb, time-varying CL/risk of immunization 11 | - | - | both | yes (15%) | [25] |
| Dreesen et al., 2019 | UC | adults | 204 (583) | trough | 1-CMT | alb, CRP, Mayo | FFM, CS, panc. | CL | induction | yes (1%) 12 | [49] |
| Matsuoka et al., 2019 | CD | adults | 121 (832) | trough | 1-CMT | ADA, alb, weight | - | - | maintenance | yes (26%) | [50] |
| Petitcollin et al., 2019 | both | adults | 91 (607) | trough | 1-CMT | CD/UC, CRP, dose, Mayo, AZA, time-varying CL/risk of immunization 11, weight 13 | - | - | maintenance | yes (1%) | [51] |
| Bauman et al., 2020 | both | children | 135 (289) | trough | 2-CMT | ADA 14, alb, ESR, weight | weight 2 | - | maintenance | yes (62%) | [21] |
| Dreesen et al., 2020 | CD | adults | 116 (1329) | midpoint, trough | 2-CMT | ADA, alb, CDAI, fCal | - | - | both | yes (18%) | [27] |
| Grišić et al., 2020 | both | pregnant | 19 (172) | throughout dosing interval | 1-CMT | ADA, 2nd/3rd trimester | - | - | both | yes (30%) 12,15 | [52] |
| Kantasiripitak et al., 2021 | both | adults | 104 (272) | trough | 2-CMT | ADA, age, alb, CRP, FFM | - | - | induction | yes (13%) | [26] |
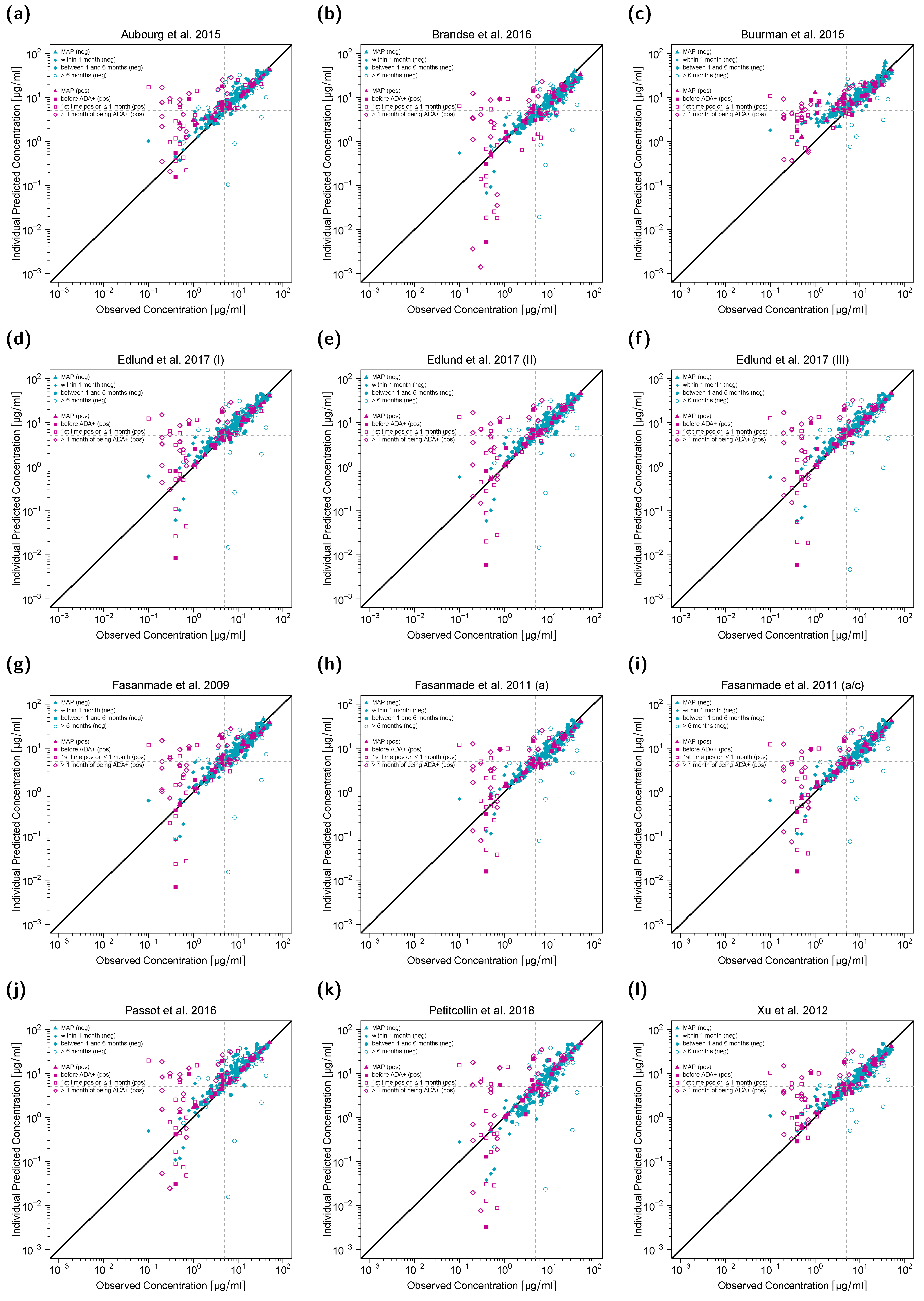
3.4. Predictive Model Evaluation Goodness-of-Fit Plots
3.5. Accuracy and Bias of Model Predictions
3.6. Predictions of “Need for Dose Escalation”
3.7. Prediction- and Variability-Corrected Visual Predictive Checks (pvcVPCs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Medicines Agency Remicade—EPAR. Product Information, Annex I—Summary of Product Characteristics—EMEA/H/C/000240. 2020, pp. 1–59. Available online: https://www.ema.europa.eu/en/documents/product-information/remicade-epar-product-information_en.pdf (accessed on 25 November 2020).
- Prescribing Information REMICADE® (Infliximab), Janssen Biotech, Inc. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf (accessed on 8 January 2021).
- Feagan, B.G.; Choquette, D.; Ghosh, S.; Gladman, D.D.; Ho, V.; Meibohm, B.; Zou, G.; Xu, Z.; Shankar, G.; Sealey, D.C.; et al. The challenge of indication extrapolation for infliximab biosimilars. Biologicals 2014, 42, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Dreesen, E.; Gils, A.; Vermeire, S. Pharmacokinetic Modeling and Simulation of Biologicals in Inflammatory Bowel Disease: The Dawning of a New Era for Personalized Treatment. Curr. Drug Targets 2018, 19, 757–776. [Google Scholar] [CrossRef]
- European Medicines Agency Remicade—EPAR. Summary for the Public, EMA/76495/2012. 2018, pp. 1–3. Available online: https://www.ema.europa.eu/en/documents/overview/remicade-epar-summary-public_en.pdf (accessed on 9 March 2020).
- Hemperly, A.; Vande Casteele, N. Clinical Pharmacokinetics and Pharmacodynamics of Infliximab in the Treatment of Inflammatory Bowel Disease. Clin. Pharmacokinet. 2018, 57, 929–942. [Google Scholar] [CrossRef]
- Klotz, U.; Teml, A.; Schwab, M. Clinical pharmacokinetics and use of infliximab. Clin. Pharmacokinet. 2007, 46, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Ryman, J.T.; Meibohm, B. Pharmacokinetics of Monoclonal Antibodies. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 576–588. [Google Scholar] [CrossRef]
- Buhl, S.; Dorn-Rasmussen, M.; Brynskov, J.; Ainsworth, M.A.; Bendtzen, K.; Klausen, P.H.; Bolstad, N.; Warren, D.J.; Steenholdt, C. Therapeutic thresholds and mechanisms for primary non-response to infliximab in inflammatory bowel disease. Scand. J. Gastroenterol. 2020, 55, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.W.; Sanderson, J.D.; Irving, P.M. Anti-infliximab antibodies in inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1078–1085. [Google Scholar] [CrossRef]
- Seow, C.H.; Newman, A.; Irwin, S.P.; Steinhart, A.H.; Silverberg, M.S.; Greenberg, G.R. Trough serum infliximab: A predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 2010, 59, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Maser, E.A.; Villela, R.; Silverberg, M.S.; Greenberg, G.R. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin. Gastroenterol. Hepatol. 2006, 4, 1248–1254. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Nguyen, G.C.; Kupfer, S.S.; Falck-Ytter, Y.; Singh, S.; Gerson, L.; Hirano, I.; Rubenstein, J.H.; Smalley, W.E.; Stollman, N.; et al. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017, 153, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Santacana, E.; Rodríguez-Alonso, L.; Padullés, A.; Guardiola, J.; Bas, J.; Rodríguez-Moranta, F.; Serra, K.; Morandeira, F.; Colom, H.; Padullés, N. Predictors of Infliximab Trough Concentrations in Inflammatory Bowel Disease Patients Using a Repeated-Measures Design. Ther. Drug Monit. 2020, 42, 102–110. [Google Scholar] [CrossRef]
- Papamichael, K.; Gils, A.; Rutgeerts, P.; Levesque, B.G.; Vermeire, S.; Sandborn, W.J.; Vande Casteele, N. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: Evolution in the definition and management of primary nonresponse. Inflamm. Bowel Dis. 2015, 21, 182–197. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Panés, J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: A review. Am. J. Gastroenterol. 2009, 104, 760–767. [Google Scholar] [CrossRef]
- Chirmule, N.; Jawa, V.; Meibohm, B. Immunogenicity to therapeutic proteins: Impact on PK/PD and efficacy. AAPS J. 2012, 14, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Jharap, B.; Neeraj, N.; Colombel, J.F. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin. Transl. Gastroenterol. 2016, 7, e135. [Google Scholar] [CrossRef]
- Mould, D.R.; Dubinsky, M.C. Dashboard systems: Pharmacokinetic/pharmacodynamic mediated dose optimization for monoclonal antibodies. J. Clin. Pharmacol. 2015, 55, S51–S59. [Google Scholar] [CrossRef]
- Wojciechowski, J.; Upton, R.N.; Mould, D.R.; Wiese, M.D.; Foster, D.J.R. Infliximab Maintenance Dosing in Inflammatory Bowel Disease: An Example for In Silico Assessment of Adaptive Dosing Strategies. AAPS J. 2017, 19, 1136–1147. [Google Scholar] [CrossRef]
- Bauman, L.E.; Xiong, Y.; Mizuno, T.; Minar, P.; Fukuda, T.; Dong, M.; Rosen, M.J.; Vinks, A.A. Improved Population Pharmacokinetic Model for Predicting Optimized Infliximab Exposure in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Vande Casteele, N.; Ferrante, M.; Van Assche, G.; Ballet, V.; Compernolle, G.; Van Steen, K.; Simoens, S.; Rutgeerts, P.; Gils, A.; Vermeire, S. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015, 148, 1320–1329.e3. [Google Scholar] [CrossRef]
- Fasanmade, A.A.; Adedokun, O.J.; Ford, J.; Hernandez, D.; Johanns, J.; Hu, C.; Davis, H.M.; Zhou, H. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur. J. Clin. Pharmacol. 2009, 65, 1211–1228. [Google Scholar] [CrossRef] [Green Version]
- Fasanmade, A.A.; Adedokun, O.J.; Blank, M.; Zhou, H.; Davis, H.M. Pharmacokinetic Properties of Infliximab in Children and Adults with Crohn’s Disease: A Retrospective Analysis of Data from 2 Phase III Clinical Trials. Clin. Ther. 2011, 33, 946–964. [Google Scholar] [CrossRef]
- Petitcollin, A.; Leuret, O.; Tron, C.; Lemaitre, F.; Verdier, M.C.; Paintaud, G.; Bouguen, G.; Willot, S.; Bellissant, E.; Ternant, D. Modeling Immunization to Infliximab in Children with Crohn’s Disease Using Population Pharmacokinetics: A Pilot Study. Inflamm. Bowel Dis. 2018, 24, 1745–1754. [Google Scholar] [CrossRef]
- Kantasiripitak, W.; Verstockt, B.; Alsoud, D.; Lobatón, T.; Thomas, D.; Gils, A.; Vermeire, S.; Ferrante, M.; Dreesen, E. The effect of aging on infliximab exposure and response in patients with inflammatory bowel diseases. Br. J. Clin. Pharmacol. 2021, 1–14. [Google Scholar] [CrossRef]
- Dreesen, E.; Berends, S.; Laharie, D.; D’Haens, G.; Vermeire, S.; Gils, A.; Mathôt, R. Modelling of the relationship between infliximab exposure, faecal calprotectin and endoscopic remission in patients with Crohn’s disease. Br. J. Clin. Pharmacol. 2020, 1–13. [Google Scholar] [CrossRef]
- Darwich, A.S.; Ogungbenro, K.; Vinks, A.A.; Powell, J.R.; Reny, J.-L.; Marsousi, N.; Daali, Y.; Fairman, D.; Cook, J.; Lesko, L.J.; et al. Why Has Model-Informed Precision Dosing Not Yet Become Common Clinical Reality? Lessons From the Past and a Roadmap for the Future. Clin. Pharmacol. Ther. 2017, 101, 646–656. [Google Scholar] [CrossRef]
- Gonzalez, D.; Rao, G.G.; Bailey, S.C.; Brouwer, K.L.R.; Cao, Y.; Crona, D.J.; Kashuba, A.D.M.; Lee, C.R.; Morbitzer, K.; Patterson, J.H.; et al. Precision Dosing: Public Health Need, Proposed Framework, and Anticipated Impact. Clin. Transl. Sci. 2017, 10, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Santacana Juncosa, E.; Rodríguez-Alonso, L.; Padullés Zamora, A.; Guardiola, J.; Rodríguez-Moranta, F.; Serra Nilsson, K.; Bas Minguet, J.; Morandeira Rego, F.; Colom Codina, H.; Padullés Zamora, N. Bayes-based dosing of infliximab in inflammatory bowel diseases: Short-term efficacy. Br. J. Clin. Pharmacol. 2021, 87, 494–505. [Google Scholar] [CrossRef]
- Strik, A.S.; Löwenberg, M.; Mould, D.R.; Berends, S.E.; Ponsioen, C.I.; van den Brande, J.M.H.; Jansen, J.M.; Hoekman, D.R.; Brandse, J.F.; Duijvestein, M.; et al. Efficacy of dashboard driven dosing of infliximab in inflammatory bowel disease patients; a randomized controlled trial. Scand. J. Gastroenterol. 2021, 56, 145–154. [Google Scholar] [CrossRef]
- Buclin, T.; Gotta, V.; Fuchs, A.; Widmer, N.; Aronson, J. Monitoring drug therapy. Br. J. Clin. Pharmacol. 2012, 73, 917–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frymoyer, A.; Piester, T.L.; Park, K.T. Infliximab Dosing Strategies and Predicted Trough Exposure in Children With Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 723–727. [Google Scholar] [CrossRef] [Green Version]
- Frymoyer, A.; Hoekman, D.R.; Piester, T.L.; de Meij, T.G.; Hummel, T.Z.; Benninga, M.A.; Kindermann, A.; Park, K.T. Application of Population Pharmacokinetic Modeling for Individualized Infliximab Dosing Strategies in Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 639–645. [Google Scholar] [CrossRef]
- Eser, A.; Primas, C.; Reinisch, S.; Vogelsang, H.; Novacek, G.; Mould, D.R.; Reinisch, W. Prediction of Individual Serum Infliximab Concentrations in Inflammatory Bowel Disease by a Bayesian Dashboard System. J. Clin. Pharmacol. 2018, 58, 790–802. [Google Scholar] [CrossRef] [PubMed]
- IDKmonitor® Infliximab Drug Level ELISA, Immundiagnostik AG. Available online: http://www.immundiagnostik.com/fileadmin/pdf/IDKmonitor_Infliximab_K9655.pdf (accessed on 20 January 2021).
- Prometheus Therapeutics & Diagnostics Prometheus Anser IFX Monohraph. Available online: www.anserifx.com/PDF/AnserIFX-Monograph.pdf (accessed on 8 January 2021).
- Wang, S.L.; Ohrmund, L.; Hauenstein, S.; Salbato, J.; Reddy, R.; Monk, P.; Lockton, S.; Ling, N.; Singh, S. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab levels in patient serum. J. Immunol. Methods 2012, 382, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Lindbom, L.; Ribbing, J.; Jonsson, E.N. Perl-speaks-NONMEM (PsN)—A Perl module for NONMEM related programming. Comput. Methods Programs Biomed. 2004, 75, 85–94. [Google Scholar] [CrossRef]
- Lindbom, L.; Pihlgren, P.; Jonsson, N. PsN-Toolkit—A collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed. 2005, 79, 241–257. [Google Scholar] [CrossRef]
- Abrantes, J.A.; Jönsson, S.; Karlsson, M.O.; Nielsen, E.I. Handling interoccasion variability in model-based dose individualization using therapeutic drug monitoring data. Br. J. Clin. Pharmacol. 2019, 85, 1326–1336. [Google Scholar] [CrossRef]
- Morley, S.K.; Brito, T.V.; Welling, D.T. Measures of Model Performance Based On the Log Accuracy Ratio. Sp. Weather 2018, 16, 69–88. [Google Scholar] [CrossRef]
- Edlund, H.; Steenholdt, C.; Ainsworth, M.A.; Goebgen, E.; Brynskov, J.; Thomsen, O.; Huisinga, W.; Kloft, C. Magnitude of Increased Infliximab Clearance Imposed by Anti-infliximab Antibodies in Crohn’s Disease Is Determined by Their Concentration. AAPS J. 2017, 19, 223–233. [Google Scholar] [CrossRef]
- Ternant, D.; Aubourg, A.; Magdelaine-Beuzelin, C.; Degenne, D.; Watier, H.; Picon, L.; Paintaud, G. Infliximab Pharmacokinetics in Inflammatory Bowel Disease Patients. Ther. Drug Monit. 2008, 30, 523–529. [Google Scholar] [CrossRef]
- Dotan, I.; Ron, Y.; Yanai, H.; Becker, S.; Fishman, S.; Yahav, L.; Ben Yehoyada, M.; Mould, D.R. Patient Factors That Increase Infliximab Clearance and Shorten Half-life in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2014, 20, 2247–2259. [Google Scholar] [CrossRef]
- Ternant, D.; Berkane, Z.; Picon, L.; Gouilleux-Gruart, V.; Colombel, J.F.; Allez, M.; Louis, E.; Paintaud, G. Assessment of the Influence of Inflammation and FCGR3A Genotype on Infliximab Pharmacokinetics and Time to Relapse in Patients with Crohn’s Disease. Clin. Pharmacokinet. 2015, 54, 551–562. [Google Scholar] [CrossRef]
- Brandse, J.F.; Mould, D.; Smeekes, O.; Ashruf, Y.; Kuin, S.; Strik, A.; van den Brink, G.R.; D’Haens, G.R. A Real-life Population Pharmacokinetic Study Reveals Factors Associated with Clearance and Immunogenicity of Infliximab in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 650–660. [Google Scholar] [CrossRef] [Green Version]
- Kevans, D.; Murthy, S.; Mould, D.R.; Silverberg, M.S. Accelerated clearance of infliximab is associated with treatment failure in patients with corticosteroid-refractory acute ulcerative colitis. J. Crohn’s Colitis 2018, 12, 662–669. [Google Scholar] [CrossRef] [Green Version]
- Dreesen, E.; Faelens, R.; Van Assche, G.; Ferrante, M.; Vermeire, S.; Gils, A.; Bouillon, T. Optimising infliximab induction dosing for patients with ulcerative colitis. Br. J. Clin. Pharmacol. 2019, 85, 782–795. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, K.; Hamada, S.; Shimizu, M.; Nanki, K.; Mizuno, S.; Kiyohara, H.; Arai, M.; Sugimoto, S.; Iwao, Y.; Ogata, H.; et al. Factors contributing to the systemic clearance of infliximab with long-term administration in Japanese patients with Crohn’s disease: Analysis using population pharmacokinetics. Int. J. Clin. Pharmacol. Ther. 2020, 58, 89–102. [Google Scholar] [CrossRef]
- Petitcollin, A.; Brochard, C.; Siproudhis, L.; Tron, C.; Verdier, M.C.; Lemaitre, F.; Lucidarme, C.; Bouguen, G.; Bellissant, É. Pharmacokinetic Parameters of Infliximab Influence the Rate of Relapse After De-Escalation in Adults With Inflammatory Bowel Diseases. Clin. Pharmacol. Ther. 2019, 106, 605–615. [Google Scholar] [CrossRef]
- Grišić, A.M.; Dorn-Rasmussen, M.; Ungar, B.; Brynskov, J.; Ilvemark, J.F.K.F.; Bolstad, N.; Warren, D.J.; Ainsworth, M.A.; Huisinga, W.; Ben-Horin, S.; et al. Infliximab clearance decreases in the second and third trimesters of pregnancy in inflammatory bowel disease. United Eur. Gastroenterol. J. 2020, 9, 91–101. [Google Scholar] [CrossRef]
- Aubourg, A.; Picon, L.; Lecomte, T.; Bejan-Angoulvant, T.; Paintaud, G.; Ternant, D. A robust estimation of infliximab pharmacokinetic parameters in Crohn’s disease. Eur. J. Clin. Pharmacol. 2015, 71, 1541–1542. [Google Scholar] [CrossRef]
- Buurman, D.J.; Maurer, J.M.; Keizer, R.J.; Kosterink, J.G.W.; Dijkstra, G. Population pharmacokinetics of infliximab in patients with inflammatory bowel disease: Potential implications for dosing in clinical practice. Aliment. Pharmacol. Ther. 2015, 42, 529–539. [Google Scholar] [CrossRef]
- Brandse, J.F.; Mathôt, R.A.; van der Kleij, D.; Rispens, T.; Ashruf, Y.; Jansen, J.M.; Rietdijk, S.; Löwenberg, M.; Ponsioen, C.Y.; Singh, S.; et al. Pharmacokinetic Features and Presence of Antidrug Antibodies Associate With Response to Infliximab Induction Therapy in Patients With Moderate to Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2016, 14, 251–258.e2. [Google Scholar] [CrossRef]
- Passot, C.; Mulleman, D.; Bejan-Angoulvant, T.; Aubourg, A.; Willot, S.; Lecomte, T.; Picon, L.; Goupille, P.; Paintaud, G.; Ternant, D. The underlying inflammatory chronic disease influences infliximab pharmacokinetics. MAbs 2016, 8, 1407–1416. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Mould, D.R.; Hu, C.; Ford, J.; Keen, M.; Davis, H.M.; Zhou, H. Population pharmacokinetic analysis of infliximab in pediatrics using integrated data from six clinical trials. Clin. Pharmacol. Drug Dev. 2012, 1, 203. [Google Scholar]
- Xu, X.S.; Dunne, A.; Kimko, H.; Nandy, P.; Vermeulen, A. Impact of low percentage of data below the quantification limit on parameter estimates of pharmacokinetic models. J. Pharmacokinet. Pharmacodyn. 2011, 38, 423–432. [Google Scholar] [CrossRef]
- Janssen, E.J.H.; Välitalo, P.A.J.; Allegaert, K.; de Cock, R.F.W.; Simons, S.H.P.; Sherwin, C.M.T.; Mouton, J.W.; van den Anker, J.N.; Knibbe, C.A.J. Towards Rational Dosing Algorithms for Vancomycin in Neonates and Infants Based on Population Pharmacokinetic Modeling. Antimicrob. Agents Chemother. 2016, 60, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Smits, A.; De Cock, R.F.W.; Allegaert, K.; Vanhaesebrouck, S.; Danhof, M.; Knibbe, C.A.J. Prospective Evaluation of a Model-Based Dosing Regimen for Amikacin in Preterm and Term Neonates in Clinical Practice. Antimicrob. Agents Chemother. 2015, 59, 6344–6351. [Google Scholar] [CrossRef] [Green Version]
- Neely, M.; Philippe, M.; Rushing, T.; Fu, X.; van Guilder, M.; Bayard, D.; Schumitzky, A.; Bleyzac, N.; Goutelle, S. Accurately Achieving Target Busulfan Exposure in Children and Adolescents With Very Limited Sampling and the BestDose Software. Ther. Drug Monit. 2016, 38, 332–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krekels, E.H.J.; Tibboel, D.; de Wildt, S.N.; Ceelie, I.; Dahan, A.; van Dijk, M.; Danhof, M.; Knibbe, C.A.J. Evidence-Based Morphine Dosing for Postoperative Neonates and Infants. Clin. Pharmacokinet. 2014, 53, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Darwich, A.S.; Polasek, T.M.; Aronson, J.K.; Ogungbenro, K.; Wright, D.F.B.; Achour, B.; Reny, J.-L.; Daali, Y.; Eiermann, B.; Cook, J.; et al. Model-Informed Precision Dosing: Background, Requirements, Validation, Implementation, and Forward Trajectory of Individualizing Drug Therapy. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 225–245. [Google Scholar] [CrossRef]
- Zhao, W.; Kaguelidou, F.; Biran, V.; Zhang, D.; Allegaert, K.; Capparelli, E.V.; Holford, N.; Kimura, T.; Lo, Y.; Peris, J.; et al. External evaluation of population pharmacokinetic models of vancomycin in neonates: The transferability of published models to different clinical settings. Br. J. Clin. Pharmacol. 2013, 75, 1068–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santacana, E.; Rodríguez-Alonso, L.; Padullés, A.; Guardiola, J.; Rodríguez-Moranta, F.; Serra, K.; Bas, J.; Morandeira, F.; Colom, H.; Padullés, N. External Evaluation Of Population Pharmacokinetic Models Of Infliximab In Inflammatory Bowel Disease Patients. Ther. Drug Monit. 2017, 40, 120–129. [Google Scholar] [CrossRef]
- Santacana Juncosa, E.; Padullés Zamora, A.; Colom Codina, H.; Rodríguez Alonso, L.; Guardiola Capo, J.; Bas Minguet, J.; Padullés Zamora, N. Contribution of infliximab population pharmacokinetic model for dose optimization in ulcerative colitis patients. Rev. Esp. Enferm. Dig. 2016, 108, 104–105. [Google Scholar] [CrossRef] [Green Version]
- Candel, M.G.; Gascón Cánovas, J.J.; Espín, R.G.; Nicolás de Prado, I.; Redondo, L.R.; Sanz, E.U.; Navalón, C.I. Usefulness of population pharmacokinetics to optimize the dosage regimen of infliximab in inflammatory bowel disease patients. Rev. Esp. Enfermedades Dig. 2020, 112, 590–597. [Google Scholar] [CrossRef]
- Dave, M.B.; Dherai, A.J.; Desai, D.C.; Mould, D.R.; Ashavaid, T.F. Optimization of infliximab therapy in inflammatory bowel disease using a dashboard approach—An Indian experience. Eur. J. Clin. Pharmacol. 2021, 77, 55–62. [Google Scholar] [CrossRef]
- Eser, A.; Reinisch, W.; Schreiber, S.; Ahmad, T.; Boulos, S.; Mould, D.R. Increased Induction Infliximab Clearance Predicts Early Antidrug Antibody Detection. J. Clin. Pharmacol. 2020, 61, 224–233. [Google Scholar] [CrossRef]
- Landemaine, A.; Petitcollin, A.; Brochard, C.; Miard, C.; Dewitte, M.; Le Balc’h, E.; Grainville, T.; Bellissant, E.; Siproudhis, L.; Bouguen, G. Cumulative Exposure to Infliximab, But Not Trough Concentrations, Correlates With Rate of Infection. Clin. Gastroenterol. Hepatol. 2021, 19, 288–295.e4. [Google Scholar] [CrossRef]
- Magnusson, M.K.; Strid, H.; Sapnara, M.; Lasson, A.; Bajor, A.; Ung, K.; Öhman, L. Anti-TNF Therapy Response in Patients with Ulcerative Colitis Is Associated with Colonic Antimicrobial Peptide Expression and Microbiota Composition. J. Crohn’s Colitis 2016, 10, 943–952. [Google Scholar] [CrossRef]
- Seong, G.; Kim, N.; Joung, J.; Kim, E.R.; Chang, D.K.; Chun, J.; Hong, S.N.; Kim, Y.-H. Changes in the Intestinal Microbiota of Patients with Inflammatory Bowel Disease with Clinical Remission during an 8-Week Infliximab Infusion Cycle. Microorganisms 2020, 8, 874. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Ghozlane, A.; Hu, H.; Li, X.; Xiao, Y.; Li, D.; Yu, G.; Zhang, T. Characteristics of Faecal Microbiota in Paediatric Crohn’s Disease and Their Dynamic Changes During Infliximab Therapy. J. Crohn’s Colitis 2018, 12, 337–346. [Google Scholar] [CrossRef]


| Characteristic | Median or No. | Range | IQR |
|---|---|---|---|
| Patients, n | 105 | ||
| Sex, female, n (%) | 50 (48) | ||
| Patients with CD, n (%) | 76 (72) | ||
| Patients with UC, n (%) | 29 (28) | ||
| ADA-positive patient status, n (%) | 22 (21) | ||
| IMM 1, n (%) | 17 (16) | ||
| Nonsmoker, n (%) | 35 (33) | ||
| Smoker, n (%) | 41 (39) | ||
| Past smoker, n (%) | 28 (27) | ||
| Unknown smoking status, n (%) | 1 (1) | ||
| Body weight 1 [kg] | 70 | 47–115 | 59–80 |
| Height 1 [cm] | 171 | 155–190 | 165–178 |
| Albumin 1 [g/dL] | 4.35 | 2.53–5.08 | 4.12–4.54 |
| CRP 1 [mg/dL] | 0.29 | 0.02–7.49 | 0.11–0.49 |
| HBI 1 | 1 | 0–18 | 1–3 |
| Total serum samples, n | 336 | ||
| ADA-positive serum samples, n (%) | 49 (15) |
| ADA Negative | ADA Positive | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose Escalation Needed? (Cobs < 5 µg/mL) | Yes (n = 67) | No (n = 67) | Yes (n = 23) | No (n = 2) | ||||||
| Correctly Predicted? | Yes | No | Yes | No | Accuracy | Yes | No | Yes | No | Accuracy |
| Aubourg et al., 2015 | 48 | 19 | 63 | 4 | 82.8% | 13 | 10 | 2 | 0 | 60.0% |
| Brandse et al., 2016 | 62 | 5 | 39 | 28 | 75.4% | 18 | 5 | 0 | 2 | 72.0% |
| Buurman et al., 2015 | 38 | 29 | 62 | 5 | 74.6% | 19 | 4 | 1 | 1 | 80.0% |
| Edlund et al., 2017 (I) | 51 | 16 | 61 | 6 | 83.6% | 16 | 7 | 2 | 0 | 72.0% |
| Edlund et al., 2017 (II) | 50 | 17 | 63 | 4 | 84.3% | 15 | 8 | 1 | 1 | 64.0% |
| Edlund et al., 2017 (III) | 50 | 17 | 63 | 4 | 84.3% | 16 | 7 | 1 | 1 | 68.0% |
| Fasanmade et al., 2009 | 54 | 13 | 58 | 9 | 83.6% | 17 | 6 | 1 | 1 | 72.0% |
| Fasanmade et al., 2011 (a/c) | 60 | 7 | 53 | 14 | 84.3% | 19 | 4 | 0 | 2 | 76.0% |
| Fasanmade et al., 2011 (a) | 60 | 7 | 53 | 14 | 84.3% | 19 | 4 | 0 | 2 | 76.0% |
| Passot et al., 2016 | 44 | 23 | 64 | 3 | 80.6% | 13 | 10 | 2 | 0 | 60.0% |
| Petitcollin et al., 2018 | 62 | 5 | 48 | 19 | 82.1% | 15 | 8 | 0 | 2 | 60.0% |
| Xu et al., 2012 | 56 | 11 | 52 | 15 | 80.6% | 18 | 5 | 1 | 1 | 76.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schräpel, C.; Kovar, L.; Selzer, D.; Hofmann, U.; Tran, F.; Reinisch, W.; Schwab, M.; Lehr, T. External Model Performance Evaluation of Twelve Infliximab Population Pharmacokinetic Models in Patients with Inflammatory Bowel Disease. Pharmaceutics 2021, 13, 1368. https://doi.org/10.3390/pharmaceutics13091368
Schräpel C, Kovar L, Selzer D, Hofmann U, Tran F, Reinisch W, Schwab M, Lehr T. External Model Performance Evaluation of Twelve Infliximab Population Pharmacokinetic Models in Patients with Inflammatory Bowel Disease. Pharmaceutics. 2021; 13(9):1368. https://doi.org/10.3390/pharmaceutics13091368
Chicago/Turabian StyleSchräpel, Christina, Lukas Kovar, Dominik Selzer, Ute Hofmann, Florian Tran, Walter Reinisch, Matthias Schwab, and Thorsten Lehr. 2021. "External Model Performance Evaluation of Twelve Infliximab Population Pharmacokinetic Models in Patients with Inflammatory Bowel Disease" Pharmaceutics 13, no. 9: 1368. https://doi.org/10.3390/pharmaceutics13091368
APA StyleSchräpel, C., Kovar, L., Selzer, D., Hofmann, U., Tran, F., Reinisch, W., Schwab, M., & Lehr, T. (2021). External Model Performance Evaluation of Twelve Infliximab Population Pharmacokinetic Models in Patients with Inflammatory Bowel Disease. Pharmaceutics, 13(9), 1368. https://doi.org/10.3390/pharmaceutics13091368








